Introduction
Pancreatic cancer is one of the most common types of
cancer with 300,000 mortalities every year worldwide. Morbidity and
mortality are gradually increasing (1). At present, the success of tumor
resection and the efficacy of chemotherapy and radiotherapy are
extremely low (2). In addition to
conventional cancer therapies, several alternative approaches for
limiting tumor progression are currently under investigation. These
strategies aim to reduce the expression of tumor-related genes, for
example, by the use of small interfering RNAs (siRNAs). The main
targets of these strategies are central regulatory genes which
control cell proliferation, cell death and angiogenesis, including
the apoptosis inhibitor survivin and vascular endothelial growth
factor (VEGF). Survivin, a member of the inhibitor of apoptosis
protein (IAP) family, has been demonstrated to be involved in the
regulation of apoptosis, cellular proliferation and angiogenesis in
cancer and has attracted growing attention as a potential target
for cancer therapy (3). VEGF is
the most effective and specific factor for the promotion of tumor
angiogenesis and is vital for tumor growth and metastasis (4). VEGF and survivin are overexpressed in
the majority of cancer types, including human pancreatic cancer
(5–8). It has been reported that antisense
oligodeoxynucleotides (AS-ODNs) or siRNAs, specifically directed at
survivin or VEGF, induced apoptosis and inhibited the proliferation
of tumor cells (9,10). However, the effects of combined
target gene silencing of survivin and VEGF on the proliferation,
apoptosis and angiogenesis of human pancreatic cancer cells have
not yet been reported.
The aim of the present study was to investigate the
effects of simultaneously targeting survivin and VEGF with short
hairpin RNA (shRNA) on the proliferation, apoptosis and
angiogenesis of human pancreatic cancer cells (Panc-1). Gene
therapy simultaneously targeting survivin and VEGF may be a potent
and attractive strategy for the treatment of pancreatic cancer.
Materials and methods
shRNA design and plasmid
construction
siRNA target design tools from oligo designer 3.0
were used to design survivin-, VEGF- and non-specific-shRNA
sequences. Four vectors were designed, which included 4 survivin-
and VEGF-specific siRNAs designated as S1, S2, S3 and S4 and V1,
V2, V3 and V4, respectively. The vectors including nonsense
sequences were designated Snc and Vnc. Sequences of siRNAs targeted
at survivin and VEGF and the nonsense control constructs are
presented in Table I. The
oligonucleotides were annealed and inserted into the pGPU6/GFP/Neo
expression vector according to the manufacturer’s instructions
(Genepharma, Shanghai, China). The recombinant vectors were
confirmed by digestion analysis using restriction endonucleases and
all inserted sequences were verified by DNA sequencing.
 | Table ISequences of shRNA against human
survivin and VEGF. |
Table I
Sequences of shRNA against human
survivin and VEGF.
| Vector | Target sequences | Sequences cloned into
the vector (5′-3′) |
|---|
| S1 |
GCGCTTTCCTTTCTGTCAAGA |
S:CACCGCGCTTTCCTTTCTGTCAAGATTCAAGAGATCTTGACAGAAAGGAAAGCGCTTTTTTG |
| | A:
GATCCAAAAAAGCGCTTTCCTTTCTGTCAAGATCTCTTGAATCTTGACAGAAAGGAAAGCGC |
| S2 |
GACAGAGAAAGAGCCAAGAAC | S:
CACCGACAGAGAAAGAGCCAAGAACTTCAAGAGAGTTCTTGGCTCTTTCTCTGTCTTTTTTG |
| | A:
GATCCAAAAAAGACAGAGAAAGAGCCAAGAACTCTCTTGAAGTTCTTGGCTCTTTCTCTGTC |
| S3 |
GCACCACTTCCAGGGTTTATT | S:
CACCGCACCACTTCCAGGGTTTATTTCAAGAGAATAAACCCTGGAAGTGGTGCTTTTTTG |
| | A:
GATCCAAAAAAGCACCACTTCCAGGGTTTATTCTCTTGAAATAAACCCTGGAAGTGGTGC |
| S4 |
GCACTTCAGACCCACTTATTT | S:
CACCGCACTTCAGACCCACTTATTTCAAGAGAATAAGTGGGTCTGAAGTGCTTTTTTG |
| | A:
GATCCAAAAAAGCACTTCAGACCCACTTATTCTCTTGAAATAAGTGGGTCTGAAGTGC |
| Snc |
GTTCTCCGAACGTGTCACGTC | S:
CACCGTTCTCCGAACGTGTCACGTCAAGAGATTACGTGACACGTTCGGAGAATTTTTTG |
| | A:
GATCCAAAAAATTCTCCGAACGTGTCACGTAATCTCTTGACGTGACACGTTCGGAGAAC |
| V1 |
GCAGATTATGCGGATCAAACC | S:
CACCGCAGATTATGCGGATCAAACCTTCAAGAGAGGTTTGATCCGCATAATCTGCTTTTTTG |
| | A:
GATCCAAAAAAGCAGATTATGCGGATCAAACCTCTCTTGAAGGTTTGATCCGCATAATCTGC |
| V2 |
GCGCAAGAAATCCCGGTATAA | S:
CACCGCGCAAGAAATCCCGGTATAATTCAAGAGATTATACCGGGATTTCTTGCGCTTTTTTG |
| | A:
GATCCAAAAAAGCGCAAGAAATCCCGGTATAATCTCTTGAATTATACCGGGATTTCTTGCGC |
| V3 |
GCGAGGCAGCTTGAGTTAAAC | S:
CACCGCGAGGCAGCTTGAGTTAAACTTCAAGAGAGTTTAACTCAAGCTGCCTCGCTTTTTTG |
| | A:
GATCCAAAAAAGCGAGGCAGCTTGAGTTAAACTCTCTTGAAGTTTAACTCAAGCTGCCTCGC |
| V4 |
GCCAGCACATAGGAGAGATGA | S:
CACCGCCAGCACATAGGAGAGATGATTCAAGAGATCATCTCTCCTATGTGCTGGCTTTTTTG |
| | A:
GATCCAAAAAAGCCAGCACATAGGAGAGATGATCTCTTGAATCATCTCTCCTATGTGCTGGC |
| Vnc |
GTTCTCCGAACGTGTCACGTC | S:
CACCGTTCTCCGAACGTGTCACGTCAAGAGATTACGTGACACGTTCGGAGAATTTTTT G |
| | A:
GATCCAAAAAATTCTCCGAACGTGTCACGTAATCTCTTGACGTGACACGTTCGGAGAAC |
Cell culture and transfection
The human pancreatic cancer cell line, Panc-1
(American Type Culture Collection, Manassas, VA, USA), was cultured
in Dulbecco’s modified Eagle’s medium (DMEM; Invitrogen Life
Technologies, Carlsbad, CA, USA) containing 10% fetal calf serum in
a 37°C incubator with a 5% CO2-humidified atmosphere.
Panc-1 cells were seeded in 6-well plates at 4-5×104
cells/well and cultured overnight to 70% confluence prior to
transfection. Transfection was performed using Lipofectamine™ 2000
and the cells were transfected with the vectors according to the
manufacturer’s instructions (Invitrogen Life Technologies,
Carlsbad, CA, USA). Following this, assays were performed using
transfectants.
Real-time PCR analysis of mRNA
expression
Total cellular RNA was isolated using TRIzol reagent
according to the manufacturer’s instructions (Invitrogen Life
Technologies). Real-time PCR was performed using total RNA (2 mg)
using oligo(dT)18 primers at 42°C for 60 min and 70°C
for 10 min. The primer sequences used were as follows: survivin
(136 bp), 5′-accgcatctctacattcaag-3′ (forward) and
5′-ttgaagcagaagaaacactg-3′ (reverse); VEGF (136 bp),
5′-actgaggagtccaacatcac-3′ (forward) and 5′-gtctgcattcacatttgttg-3′
(reverse); β-actin (208 bp), 5′-cattaaggagaagctgtgct-3′ (forward)
and 5′-gttgaaggtagtttcgtgga-3′ (reverse). The relative
quantification of the target gene expression was performed using
the 2−ΔΔCt method. Each experiment was performed at
least three times.
Western blot analysis of target protein
expression
Untransfected or stably transfected Panc-1 cells
were lysed in lysis buffer and the lysates were cleared by
centrifuging. Proteins were separated by 10% sodium dodecyl
sulfate-polyacrylamide gele electrophoresis (SDS-PAGE),
electroblotted onto a nitrocellulose membrane, blocked by 5%
skimmed milk and probed with anti-survivin, -VEGF and -GAPDH
antibodies (Sigma-Aldrich, St. Louis, MO, USA). Following
incubation with secondary antibody, immunoblots were visualized by
chemiluminescence using a chemiluminescence kit and the specific
bands were recorded on X-ray film. GAPDH protein levels were used
as a control to verify equal protein loading.
Cell proliferation assay
Panc-1 cells and HUVECs (American Type Culture
Collection) were seeded in the collected culture medium of each
group and cell viability was measured by
3-(4,5-dimethylthazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT)
assay (Sigma-Aldrich). Panc-1 cells or HUVECs (1×104
cells/well) were seeded into seven 96-well culture plates and each
group consisted of 3 parallel wells. MTT was added to each well and
the cells were incubated at 37°C. The reaction was then stopped by
lysing the cells with 150 ml DMSO for 5 min. Optical densities were
determined using a Versamax microplate reader (Molecular Devices,
Sunnyvale, CA, USA) at 570 nm.
Apoptosis detection
The apoptosis of Panc-1 cells and HUVECs seeded in
the collected culture medium of each group was assessed 72 h
following transfection by staining cells with Annexin V/propidium
iodide (PI) and analyzed using flow cytometry (FCM).
Statistical analysis
All statistical analyses were performed using SPSS
13.0 (SPSS, Inc., Chicago, IL, USA). Comparisons among all groups
were performed using the one-way analysis of variance (ANOVA) test
and Student Newman Keuls method. P<0.05 was considered to
indicate a statistically significant difference.
Results
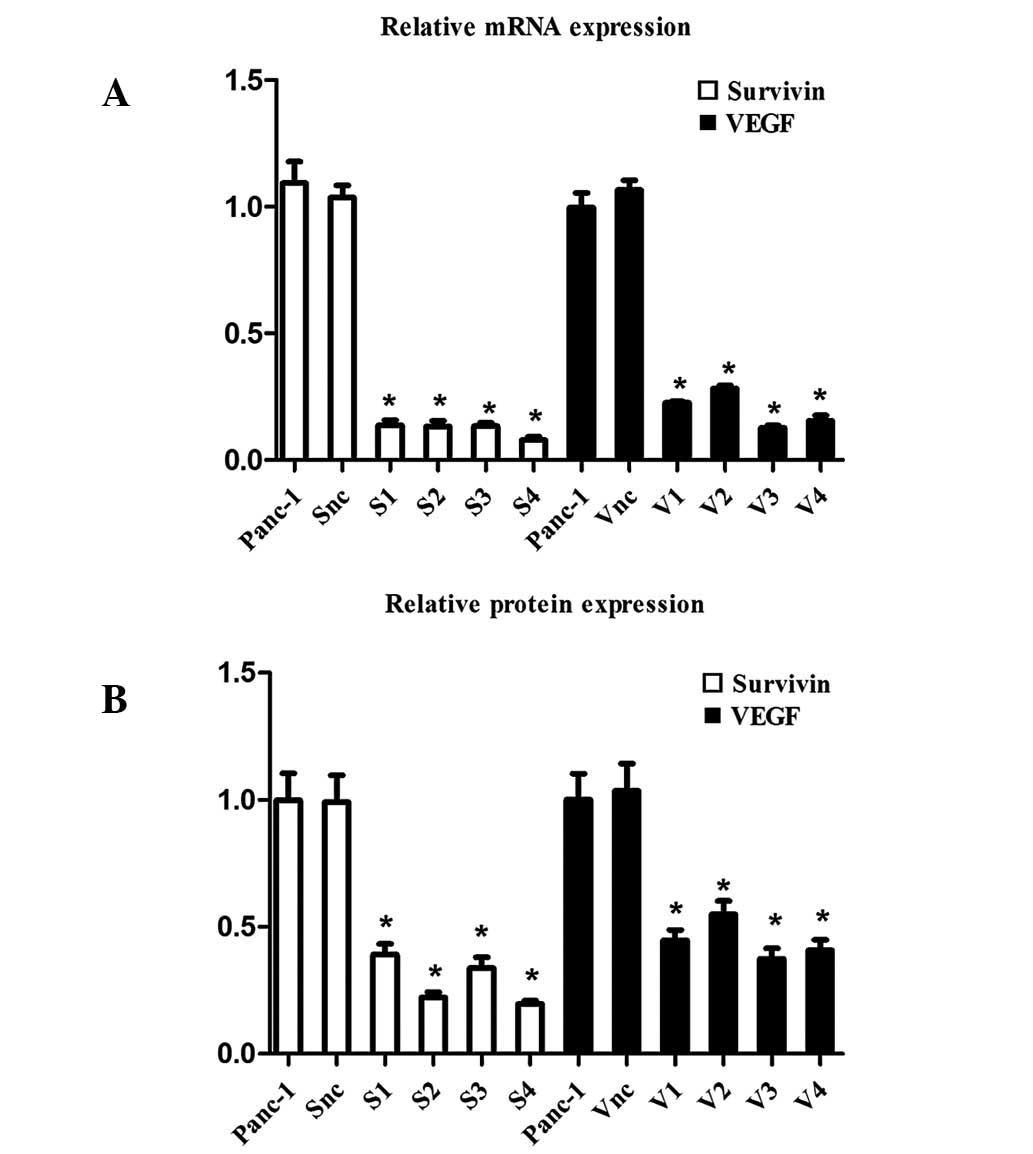
Inhibitive effect of specific shRNA
vectors on survivin and VEGF expression in Panc-1 cells and
selection of the most effective specific shRNA vector
mRNA and protein expression levels of survivin and
VEGF, inhibited by specific-shRNAs in the Panc-1 cells, were
analyzed by real-time PCR and western blot analysis. As
demonstrated in Fig. 1, real-time
PCR revealed that the expression of survivin was inhibited in the
S1, S2, S3 and S4 groups (13.63, 13.14, 13.27 and 7.81%,
respectively, compared with the normal and positive controls;
P<0.05) and the expression of VEGF was inhibited in the V1, V2,
V3 and V4 groups (22.51, 28.27, 12.69 and 15.46% respectively,
compared with the normal and positive controls; P<0.05). No
significant difference was identified between survivin and the VEGF
positive and normal controls (P>0.05). Western blot analysis
revealed that survivin and VEGF protein expression was
significantly inhibited, consistent with the real-time PCR results.
S4 and V3, directed at survivin and VEGF, respectively, were
selected as the most effective inhibitors for investigation in the
latter experiments.
Individual and combined inhibitive effect
of siRNA on survivin and VEGF expression in Panc-1 cells
Real-time PCR and western blot analysis (Fig. 2) demonstrated that the expression
of survivin and VEGF by Panc-1 cells was inhibited in the S4 and V3
groups, respectively, at the mRNA and protein levels, and the
expression levels of survivin and VEGF mRNA and protein were
significantly reduced simultaneously in the S4+V3 group compared
with the control (P<0.05).
Proliferation assay
Cell growth curves of Panc-1 cells and HUVECs
determined by MTT for 48 h are presented in Fig. 3 and revealed that the viability of
the Panc-1 cells and HUVECs was inhibited in a time-dependent
manner and the highest inhibitory rates were 81.2 ±0.95 and
78.7±1.06%, respectively, at 48 h. Compared with control cells, the
viability of Panc-1 cells and HUVECs in the S4 or V3 groups was
reduced and the reduction was greater in the S4+V3 group
(P<0.05).
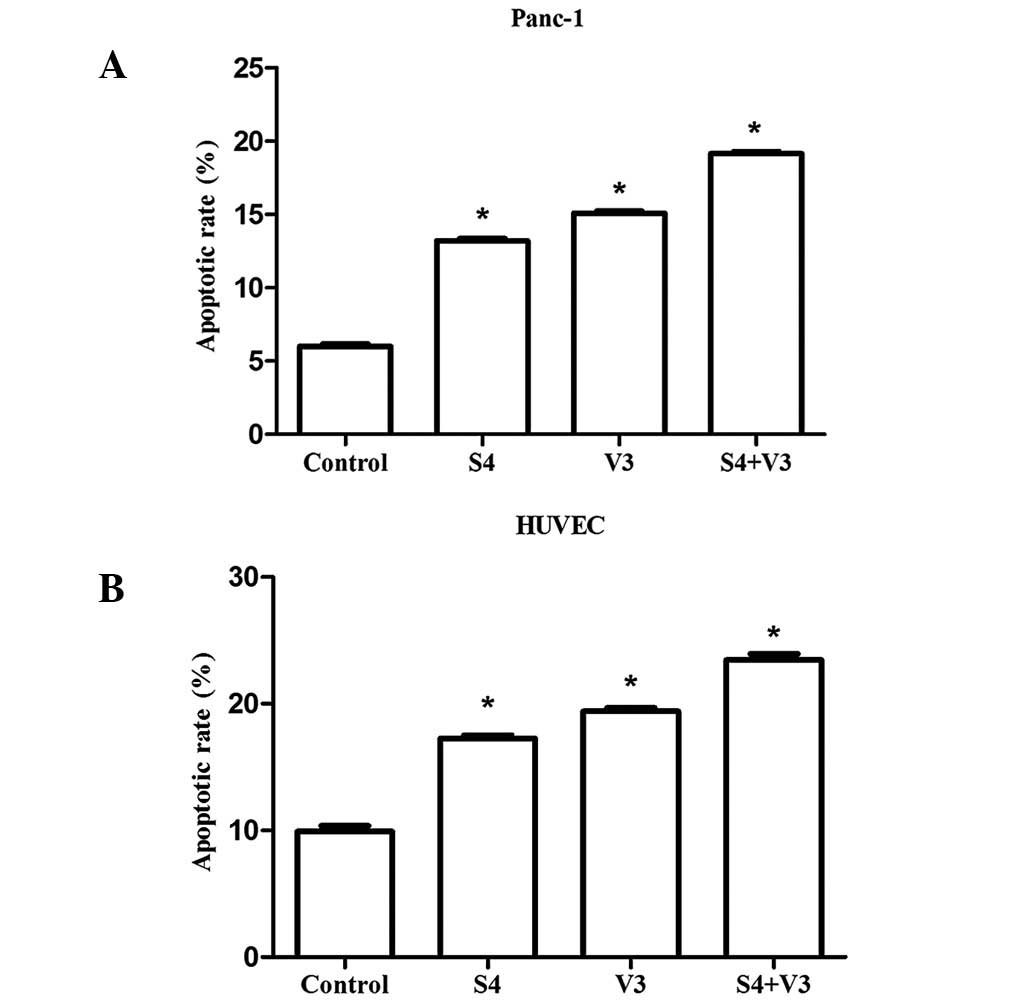
Apoptosis of Panc-1 cells and HUVECs
detected by Annexin V-FITC and PI staining
Apoptosis was assessed following transfection by
staining cells with Annexin V/PI and analyzed using FCM (Fig. 4). The strongest apoptotic signals
were identified in the Panc-1 cells and HUVECs of the S4+V3 group
and the percentages of apoptotic cells were 19.17±0.09 and
23.45±0.49%, respectively. The results indicate that the apoptosis
rates of Panc-1 cells and HUVECs in the S4 and V3 groups were
higher than those in the control. This increase was higher in the
S4+V3 group (P<0.05).
Discussion
Abnormal proliferation and angiogenesis and
resistance to apoptosis are hallmarks of various forms of cancer
and commonly lead to the failure of cancer therapy. Survivin is a
novel human IAP family member containing a single baculoviral IAP
repeat domain. Survivin inhibits caspases and blocks the apoptotic
pathway and its α-helix structure interacts with microtubules and
interfers with mitosis (11). In
addition, survivin is involved in tumor angiogenesis by inhibition
of vascular endothelial cell apoptosis (12). VEGF, a vascular permeability
factor, is a highly specific endothelial cell mitogen which
inhibits apoptosis and promotes the survival of vascular
endothelial cells (13,14). VEGF is secreted by malignant tumor
cells and plays a critical role in angiogenesis by binding
receptors on vascular endothelial cells (15,16).
Therefore, simultaneous inhibition of expression of survivin and
VEGF in pancreatic cancer cells may inhibit proliferation and
angiogenesis and induce apoptosis more effectively than individual
inhibition.
Survivin and VEGF are upregulated in various
malignancies, including pancreatic cancer, and are associated with
aggressive tumor behavior and recurrence (5–8).
Previous studies have reported that inhibition of survivin in
pancreatic cancer cells by AS-ODNs or siRNA reduces tumor cell
growth and induces apoptosis (17). It has also been reported that
inhibition of survivin in the endothelial cells may induce the
apoptosis of endothelial cells and reduce tumor-associated
angiogenesis (18,19). In addition, inhibition of VEGF in
tumor cells by AS-ODNs or siRNA has been identified to reduce tumor
cell growth, induce apoptosis and affect tumor angiogenesis
(20,21). However, studies concerning the
effect of simultaneous targeting of survivin and VEGF on the
proliferation, apoptosis and angiogenesis of human pancreatic
cancer cells have not been performed to date.
RNA interference (RNAi) is a powerful
post-transcriptional gene silencing technique and is characterized
by high efficiency and specificity and low toxicity. At present,
the technique is widely utilized in gene therapy and has became a
powerful tool for studies on gene function (22). To explore the potential of survivin
and VEGF as effective therapeutic targets, RNAi was performed to
silence endogenous survivin and VEGF expression in Panc-1
cells.
In the present study, mRNA and protein expression
levels of survivin and VEGF in Panc-1 cells were markedly
downregulated. Consistent with this downregulation, MTT assay and
FCM revealed increased levels of cell apoptosis and inhibition of
cell growth. This effect on proliferation and apoptosis was higher
in the combined survivin and VEGF inhibition group. In addition,
due to the downregulation of survivin and VEGF in the culture
medium of the Panc-1 cells transfected by siRNA, cell apoptosis
rate was also observed to be increased and cell growth was
inhibited in HUVECs. Again, this effect was more apparent in the
combined survivin and VEGF inhibition group. However, the molecular
mechanism by which these effects are mediated in HUVECs remain
unknown. Further studies are required to validate survivin and VEGF
as pharmaceutical targets for anti-tumorigenesis in pancreatic
cancer in vivo.
In summary, the results of the current study
indicate that survivin and VEGF are associated with the development
of pancreatic cancer and the anti-tumorigenic effects of
simultaneous shRNA-targeted survivin and VEGF are considerably
greater than those of a single inhibitor. Through investigation of
the anti-tumorigenic mechanisms of simultaneous inhibition of
survivin and VEGF in Panc-1 cells and HUVECs, we hypothesize that
combined therapy with survivin and VEGF inhibition should be
analyzed further as a potential therapeutic strategy for human
pancreatic cancer.
Acknowledgements
The authors thank members of the Department of
Clinical Laboratory (Xiangya Hospital, Central South University)
for their assistance and technical support.
References
|
1
|
Saif MW, Sviglin H and Carpenter M: Impact
of ethnicity on outcome in pancreatic carcinoma. JOP. 6:246–254.
2005.PubMed/NCBI
|
|
2
|
Rodriguez JA, Li M, Yao Q, Chen C and
Fisher WE: Gene overexpression in pancreatic adenocarcinoma:
diagnostic and therapeutic implications. World J Surg. 29:297–305.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Kawasaki H, Toyoda M, Shinohara H, et al:
Expression of survivin correlates with apoptosis, proliferation and
angiogenesis during human colorectal tumorigenesis. Cancer.
91:2026–2032. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Ikeda N, Nakajima Y, Sho M, et al: The
association of K-ras gene mutation and vascular endothelial growth
factor gene expression in pancreatic carcinoma. Cancer. 92:488–499.
2001. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Satoh K, Kaneko K, Hirota M, Masamune A,
Satoh A and Shimosegawa T: Expression of survivin is correlated
with cancer cell apoptosis and is involved in the development of
human pancreatic duct cell tumors. Cancer. 92:271–278. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Lee MA, Park GS, Lee HJ, et al: Survivin
expression and its clinical significance in pancreatic cancer. BMC
Cancer. 5:1272005. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Itakura J, Ishiwata T, Friess H, et al:
Enhanced expression of vascular endothelial growth factor in human
pancreatic cancer correlates with local disease progression. Clin
Cancer Res. 3:1309–1316. 1997.
|
|
8
|
Ikeda N, Adachi M, Taki T, et al:
Prognostic significance of angiogenesis in human pancreatic cancer.
Br J Cancer. 79:1553–1563. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Olie RA, Simoes-Wust AP, Baumann B, et al:
A novel antisense oligonucleotide targeting survivin expression
induces apoptosis and sensitizes lung cancer cells to chemotherapy.
Cancer Res. 60:2805–2809. 2000.
|
|
10
|
Ciardiello F, Bianco R, Damiano V, et al:
Antiangiogenic and antitumor activity of anti-epidermal growth
factor receptor C225 monoclonal antibody in combination with
vascular endothelial growth factor antisense oligonucleotide in
human GEO colon cancer cells. Clin Cancer Res. 6:3739–3747.
2000.
|
|
11
|
Yonesaka K, Tamura K, Kurata T, et al:
Small interfering RNA targeting survivin sensitizes lung cancer
cell with mutant p53 to adriamycin. Int J Cancer. 118:812–820.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Mita AC, Mita MM, Nawrocki ST and Giles
FJ: Survivin: key regulator of mitosis and apoptosis and novel
target for cancer therapeutics. Clin Cancer Res. 14:5000–5005.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Dvorak HF, Nagy JA, Feng D, Brown LF and
Dvorak AM: Vascular permeability factor/vascular endothelial growth
factor and the significance of microvascular hyperpermeability in
angiogenesis. Curr Top Microbiol Immunol. 237:97–132. 1999.
|
|
14
|
Jones A and Fujiyama C: Angiogenesis in
urological malignancy: prognostic indicator and therapeutic target.
BJU Int. 83:535–555. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Hayashibara T, Yamada Y, Miyanishi T, et
al: Vascular endothelial growth factor and cellular chemotaxis: a
possible autocrine pathway in adult T-cell leukemia cell invasion.
Clin Cancer Res. 7:2719–2726. 2001.PubMed/NCBI
|
|
16
|
Xie K, Wei D and Huang S: Transcriptional
anti-angiogenesis therapy of human pancreatic cancer. Cytokine
Growth Factor Rev. 17:147–156. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Jiang C, Tan T, Yi XP, Shen H and Li YX:
Lentivirus-mediated shRNA targeting XIAP and survivin inhibit
SW1990 pancreatic cancer cell proliferation in vitro and in vivo.
Mol Med Rep. 4:667–674. 2011.PubMed/NCBI
|
|
18
|
O’Connor DS, Schechner JS, Adida C, et al:
Control of apoptosis during angiogenesis by survivin expression in
endothelial cells. Am J Pathol. 156:393–398. 2000.
|
|
19
|
Mesri M, Morales-Ruiz M, Ackermann EJ, et
al: Suppression of vascular endothelial growth factor-mediated
endothelial cell protection by survivin targeting. Am J Pathol.
158:1757–1765. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Takei Y, Kadomatsu K, Yuzawa Y, Matsuo S
and Muramatsu T: A small interfering RNA targeting vascular
endothelial growth factor as cancer therapeutics. Cancer Res.
64:3365–3370. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Forster Y, Meye A, Krause S and Schwenzer
B: Antisense-mediated VEGF suppression in bladder and breast cancer
cells. Cancer Lett. 212:95–103. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Meister G, Landthaler M, Dorsett Y and
Tuschl T: Sequence-specific inhibition of microRNA- and
siRNA-induced RNA silencing. RNA. 10:544–550. 2004. View Article : Google Scholar : PubMed/NCBI
|