Introduction
Lung adenocarcinoma accounts for approximately 40%
of all lung cancer cases (1). A
diverse range of genetic abnormalities are observed in lung cancer
cells, but less common genetic changes are observed in lung
adenocarcinoma (2,3). Understanding the molecular processes
underlying these cellular phenotypic changes is critical in order
to develop novel therapeutic strategies. Key to mediating these
changes are the interactions between cell surface receptors and
their cognate ligands, which, through intracellular signaling,
induce alterations in gene expression (4).
High mobility group protein B1 (HMGB1) is a highly
conserved nuclear protein, acting as a chromatin-binding factor
that binds DNA and promotes access to transcriptional protein
assemblies on specific DNA targets (5–7). In
addition to its nuclear role, HMGB1 also functions as an
extracellular signaling molecule during inflammation, cell
migration and tumor metastasis (8). The overexpression of HMGB1 has been
associated with each of the hallmarks of cancer, including
unlimited replicative potential, the ability to develop blood
vessels, evasion of programmed cell death, tissue invasion and
metastasis (9,10).
In this study, by analyzing the expression of HMGB1
in lung adenocarcinoma tissues and lung cancer cell lines, we
demonstrate that HMGB1 plays a role in the development of lung
adenocarcinoma. Furthermore, we provide evidence suggesting that
HMGB1 is involved in the regulation of angiogenesis, invasion and
metastasis of lung adenocarcinoma cells.
Materials and methods
Lung cancer cell lines and patients
The normal human bronchial epithelial (HBE) cell
line and 8 human lung cancer cell lines (95-D, A549, NCI-H1299,
NCI-H1975, NCI-H661, NCI-H446, NCI-H1395 and Calu-3) were purchased
from the American Type Culture Collection (Manassas, VA, USA).
SPCA-1 and LTEP-α-2 cell lines were obtained from Shanghai
Institutes for Biological Sciences (Shanghai, China). Primary lung
adenocarcinoma and corresponding non-cancerous tissues were
obtained during surgery from 24 patients who were treated at
Kunshan First People’s Hospital affiliated to Jiangsu University
(Kunshan, China). The materials to be analyzed were selected by a
pathologist to ensure that macroscopically the samples were
entirely cancerous and from an area devoid of necrotic tissue.
Cancerous and non-cancerous tissues were stored at −80°C until
protein extraction. Patients provided written informed consent, and
the study was approved by the ethics committee of Jiangsu
University.
Cell culture and reagents
The human lung cancer cell lines, 95-D, A549,
NCI-H1299, NCI-H1975, NCI-H661, NCI-H446, NCI-H1395, Calu-3,
SPCA-1, LTEP-α-2, and the HBE cell line were maintained in
Dulbecco’s modified Eagle’s medium (DMEM) or RPMI-1640 supplemented
with 10% fetal calf serum, L-glutamine (5 mmol/l), non-essential
amino acids (5 mmol/l), penicillin (100 U/ml) and streptomycin (100
U/ml) (Invitrogen, Carlsbad, CA, USA), at 37°C in a humidified 5%
CO2 atmosphere.
Plasmids and transfection
The HMGB1 eukaryotic expression vector construction
and plasmid transfection using Lipofectamine were performed as
previously described (11). The
expression of HMGB1 was determined by western blot analysis.
Cell viability assay
Cell proliferation was performed by
3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT)
viability assay, the most common assay for determining cell growth
and death. The MTT survival assay has been described in detail in
previous studies (12). Briefly,
exponentially growing cells were recultured (5,000 cells/well)
overnight in 96-well tissue culture plates. Up to 20 μl of MTT
(Sigma Aldrich, St. Louis, MO, USA) was added to the medium in each
well, with a final concentration of 2 mg/ml. After 4 h of
incubation, the medium containing MTT was discarded, and 120 μl of
DMSO were added followed by incubation for 10 min. The absorbance
was measured using an ELISA reader at 570 nm, with the absorbance
at 630 nm taken as the background correction. Cell viability was
expressed as a percentage of the untreated controls. All
experiments were performed at least 3 times.
Transwell invasion assay
The invasion assay was performed using Transwell
filters (Millipore, Billerica, MA, USA) as previously described
(13). The Transwell filter
surfaces (8 μm pore size) were uniformly coated with 25 mg Matrigel
overnight at 4°C prior to the experiment. The lower chamber was
filled with culture medium containing 10% fetal calf serum, and the
subconfluent proliferating cells were carefully transferred onto
the coated upper surface of the chamber. After 24 h of incubation,
the filter was gently removed, and the upper surface of the filter
was wiped using a cotton swab to remove all attached cells. The
cells that invaded the Matrigel and attached to the lower surface
of the filter were fixed with 4% paraformaldehyde, stained with
Giemsa, and then counted in 15 randomly selected microscopic fields
per filter. Finally, the invasion rate was calculated and compared.
Experiments were performed independently at least 3 times.
Western blot and
immunoprecipitation/immunoblot analysis
Western blot analysis was performed as previously
described (14). The following
primary antibodies were used for immunoblotting: β-actin (C-4),
HMGB1 (E-1), nuclear factor (NF)-κB (P65A) and secondary antibody
horseradish peroxidase (HRP)-labeled goat anti-mouse (GAM-007),
goat anti-rabbit (SC-2004) IgG, as well as phospho-ERK1/2
(T202/Y204), ERK1/2, phospho-p38 (T180/Y182) and p38 (Cell
Signaling Technology, Danvers, MA, USA, 1:1,000 dilution). The
protein bands were quantified by densitometry using QuantityOne
software (Bio-Rad, Hercules, CA, USA), and the values were
expressed relative to β-actin (control for loading and transfer).
At least 3 independent experiments were performed for each cell
type examined.
Semi-quantitative reverse
transcription-PCR (RT-PCR) analysis
mRNA expression was determined by RT-PCR. PCR
reaction conditions and cycle numbers were rigorously adjusted so
that each reaction occurred within the linear range of
amplification. Detailed methods for RNA isolation, cDNA synthesis
and RT-PCR analyses have been previously described (15). The primers for gene-specific PCR
were as follows: GAPDH sense, 5′-CAA CTA CAT GGT CTA CAT GTT CC-3′
and antisense, 5′-CAA CCT GGT CCT CAG TGT AG-3′; and HMGB1 sense,
5′-TTA GAT AGC CCT GTC CTG GTG G-3′ and antisense, 5′-TGA ATG TGG
CAT CTT TGT TTG A-3′. PCR products were analyzed by electrophoresis
using 1% agarose gels containing 0.1 mg/ml ethidium bromide and the
gels were photographed under ultraviolet light. mRNA expression
levels were quantified by densitometry of the cDNA bands using
QuantityOne software (Bio-Rad). At least 3 independent experiments
were performed for each cell type examined.
Statistical analysis
The data are presented as the means ± SD.
Statistical comparisons of the experimental results between the
treated group and the control group were made using the two-tailed
Student’s t-test. All statistical tests were performed using SPSS
version 17.0 software. P≥0.01 was considered to indicate a
statistically significant difference.
Results
Increased expression of HMGB1 in lung
adenocarcinoma specimens
In order to elucidate the role of HMGB1 in lung
cancer, we examined the level of HMGB1 expression in lung
adenocarcinoma cells by western blot analysis using clinical
samples. A total of 24 lung adenocarcinoma cases were examined. In
each patient, the level of HMGB1 expression normalized to β-actin
expression in cancerous tissue was compared to that observed in
non-cancerous tissue. In 87.5% (21/24) of the specimens, HMGB1
expression increased in the cancerous tissues compared to the
non-cancerous tissues. The protein bands were quantified using
QuantityOne software, and the augmentation was deemed to be
statistically significant (P<0.01) (Fig. 1). Therefore, we hypothesized that
the overexpression of HMGB1 contributes to the development of human
lung adenocarcinoma. However, due to the fact that we analyzed only
a small number of specimens, future analysis with a much larger
number of specimens is required.
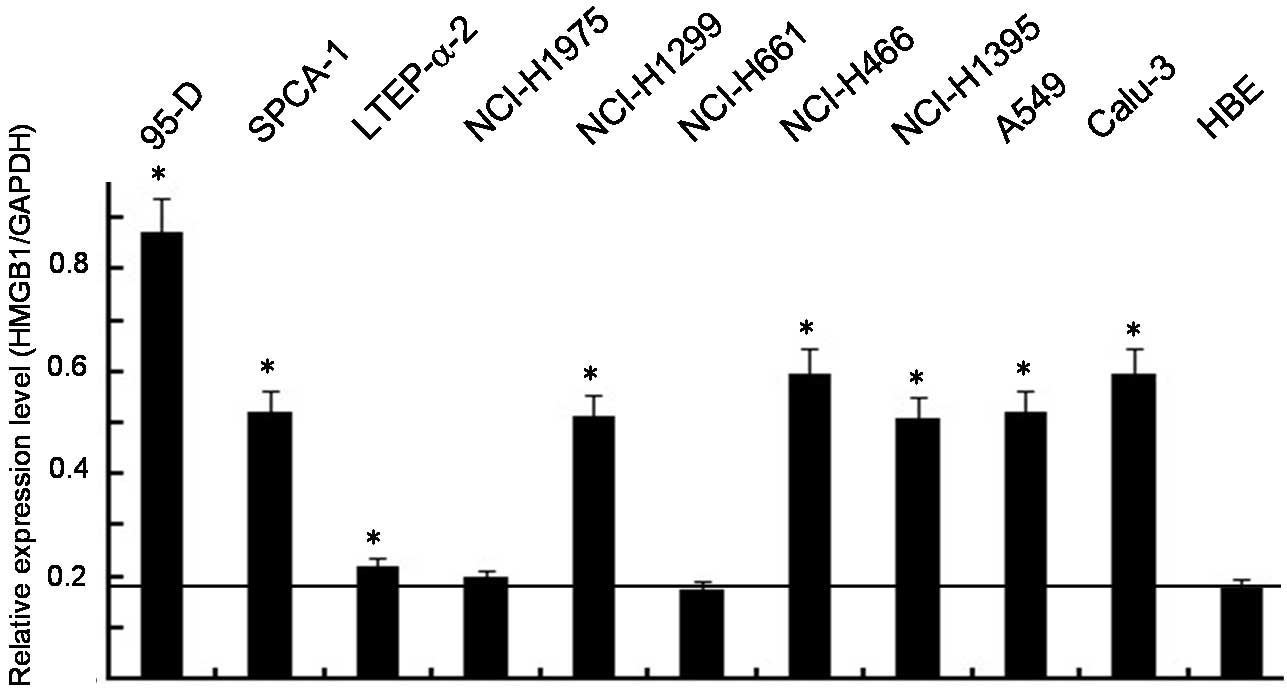
HMGB1 overexpression in lung cancer cell
lines
To determine the effect of HMGB1 on the
proliferation and metastasis of lung cancer cells, the HMGB1
expression levels in the 10 lung cancer cell lines and the HBE cell
line were analyzed by RT-PCR. As shown in Fig. 2, the HMGB1 protein level increased
in 8 of the lung cancer cell lines compared to the HBE cell line
and there was little or no change in the expression levels of GAPDH
in these cell lines. In vitro data confirmed that TOB1
overexpression is an important event in lung cancer.
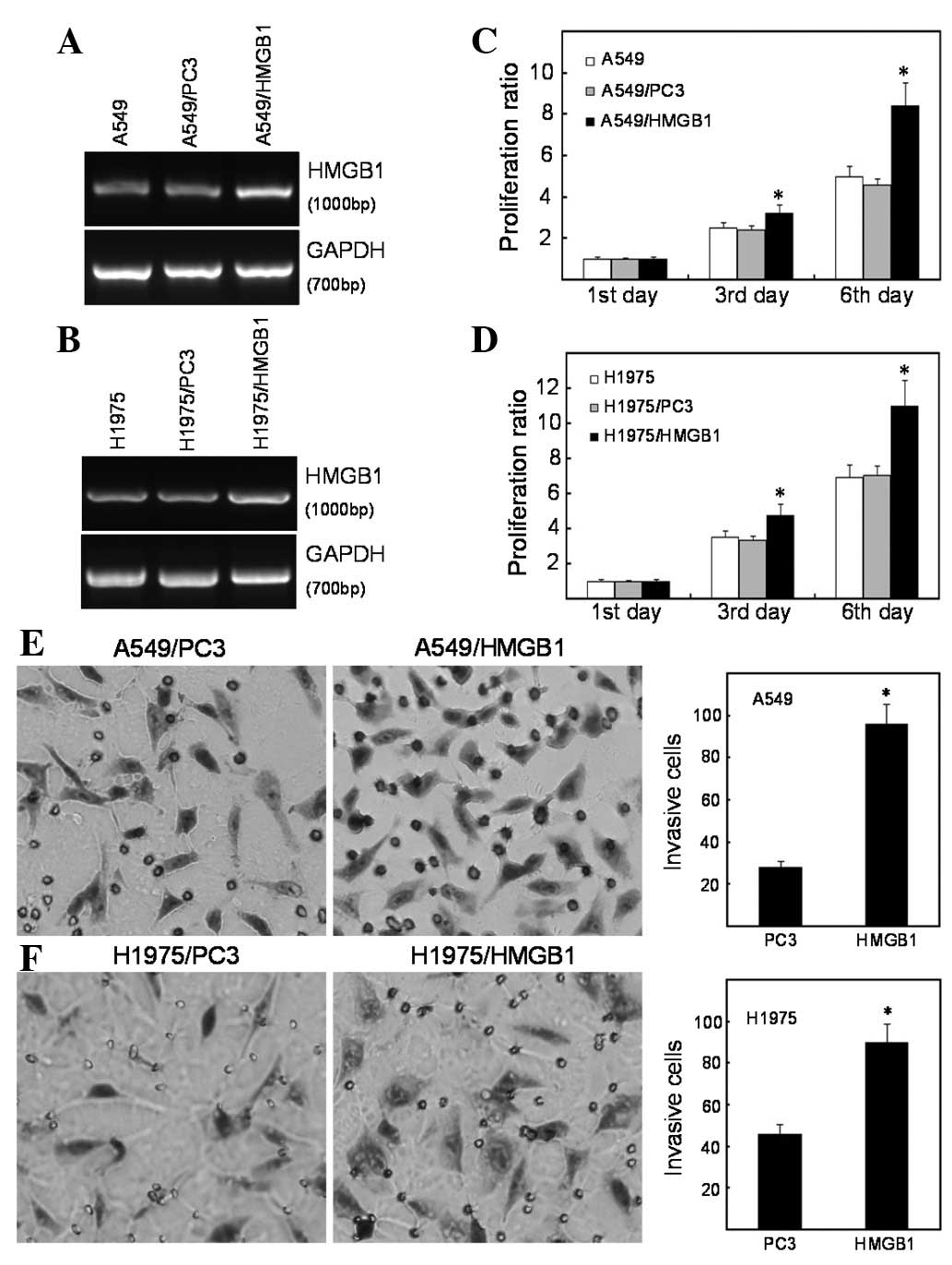
Exogenous expression of HMGB1 enhances
the proliferation and invasion of lung cancer cells
Cell proliferation and invasion are indispensable
for cancer metastasis. Due to the fact that HMGB1 expression was
increased in the lung cancer tissues and cell lines, we
hypothesized that HMGB1 regulates the progression of lung cancer.
According to gain-of-function approaches, A549 and NCI-H1975 cells
were selected as model systems, as they are lung adenocarcinoma
cell lines that express almost undetectable levels of HMGB1.
Moreover, A549 cells are epidermal growth factor receptor (EGFR)
wild-type and NCI-1975 cells are EGFR mutated, and EGFR status is
known to modulate proliferation and metastasis. Using Lipofectamine
and G418-mediated plasmid stable transfection, multiple clones
transfected with HMGB1 were selected and confirmed by RT-PCR
(Fig. 3A and B). The dynamics of
A549, A549/PC3 and A549/HMGB1 cell growth were determined by MTT
assay. Following a 6-day period, the overexpression of HMGB1
enhanced the proliferative ability of the A549 cells, compared to
the control groups. The marked decrease in cell viability was
observed on day 3 (Fig. 3C). We
examined the effect of HMGB1 expression on the invasive ability of
A549 cells using a Transwell assay. The results demonstrated that,
compared to the parental cells, the overexpression of HMGB1
enhances the ability of the A549 cells to invade through the
Matrigel-coated filter. As shown in Fig. 3E, the invasion rate of A549/HMGB1
cells increased by >60% corresponding to the vector-transfected
cells (P<0.01). In the EGFR-mutated NCI-H1975 cells, the
overexpression of HMGB1 also promoted cell proliferation and
invasion (Fig. 3D and F).
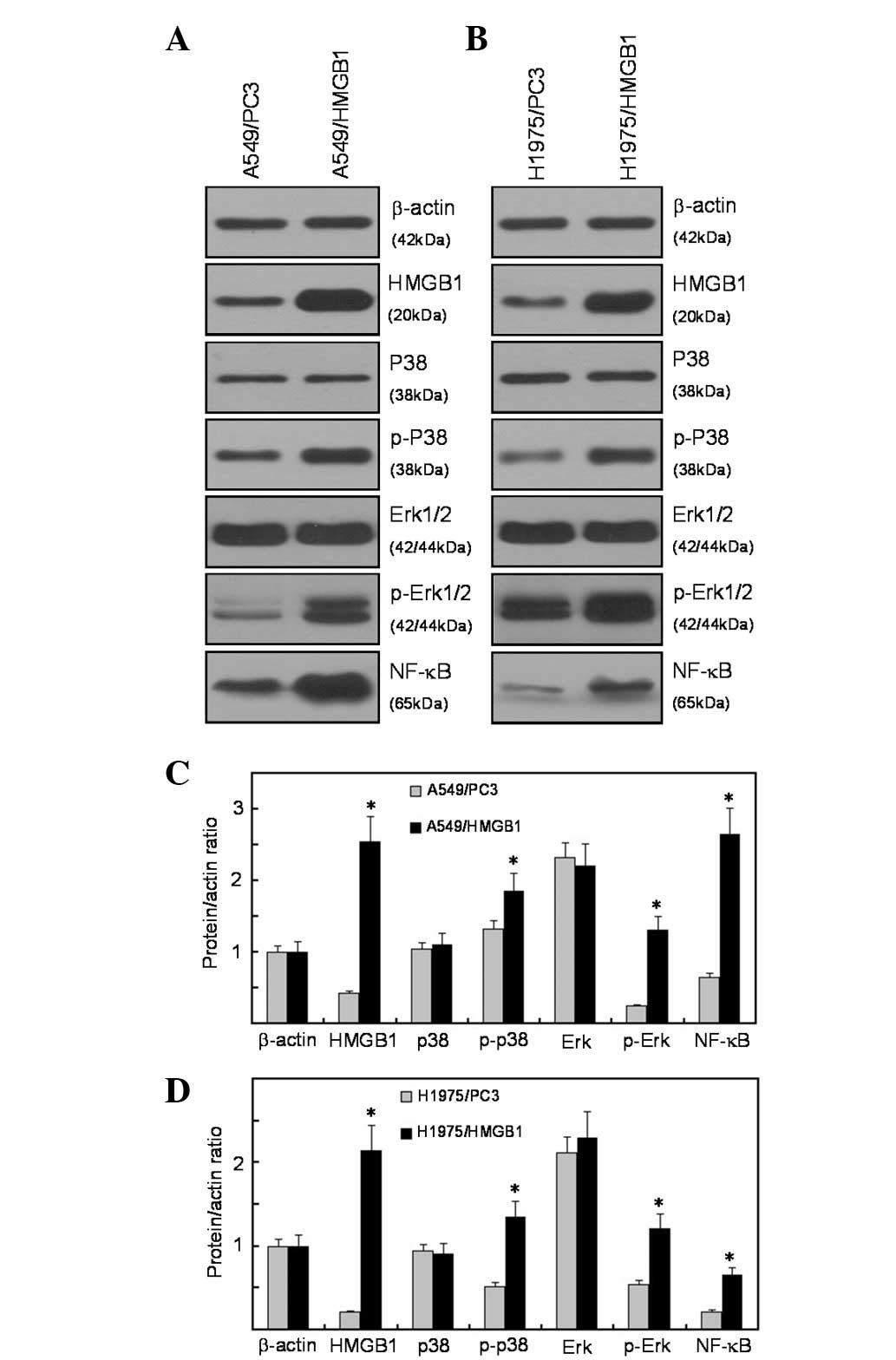
HMGB1 activates the ERK and p38
mitogen-activated protein kinase (MAPK) signaling pathway
To determine whether plasmid transfection induces
the activation of Erk1/2 and p38 MAPK, we transfected the A549
cells with HMGB1 recombinant plasmid and then immunoblot analysis
was performed to determine the phosphorylation levels of Erk1/2 and
p38. The results demonstrated that HMGB1 overexpression enhanced
the expression levels of phosphorylated Erk1/2 and p38; however,
the levels of total Erk1/2 and p38 remained unaffected. These data
suggest that Erk1/2 and p38 MAPK are activated in HMGB1-transfected
lung cancer cells. The MAPKs integrate a wide range of upstream
signals to determine patterns of downstream gene expression through
the regulation of transcription factors. NF-κB has been well
characterized as a molecule regulated by the MAPK signaling pathway
(16). In this study, the
expression of NF-κB was upregulated in HMGB1-overexpressed A549
cells (Fig. 4A and C). As for
EGFR-mutated NCI-H1975 cells, the overexpression of HMGB1 activated
the ERK and p38 MAPK signaling pathways when compared with the
control cells, implying that HMGB1 activated the ERK and p38 MAPK
signlaing pathways through an EGFR-independent pathway (Fig. 4B and D).
Discussion
Although lung cancer has the highest mortality rate
of all cancers worldwide, a number of developments indicate future
clinical benefits (17,18). Perhaps the most significant advance
in the treatment of this disease has been the introduction of
molecularly targeted therapies, a term that currently includes
monoclonal antibodies and small-molecule tyrosine kinase
inhibitors. The development of effective targeted therapeutics
requires knowledge of the genes and pathways involved and the
mechanisms by which they affect the biological behavior of lung
cancer (4).
In this study, we identified the increased
expression of HMGB1 protein in lung adenocarcinoma tissues and lung
cancer cell lines, which suggests that the expression of HMGB1 is
biologically significant in lung cancer development. We further
demonstrated that the enforced overexpression of HMGB1 modulated
the proliferation and invasion of 2 lung adenocarcinoma cell lines,
A549 and NCI-H1975, in vitro. These observations support our
hypothesis that HMGB1 plays an oncogenic role in lung cancer
development. Additionally, using western blot analysis, we
identified the activation of the Erk1/2 and p38 cascade, as
evidenced by the phosphorylation of ERK1/2 and p38, revealing the
role of HMGB1 in the regulation of the ERK1/2 and p38 MAPK pathway.
Consistently, ERK1/2 and p38 activation leads to the
transcriptional regulation of NF-κB, resulting in increased cell
proliferation and invasion, suggesting that MAPK/NF-κB is an
oncogenic mechanism by which HMGB1 contributes to lung cancer
development.
In conclusion, lung adenocarcinoma cell growth and
survival is impaired by the inactivation of HMGB1, suggesting that
HMGB1 plays an oncogenic role and is a potentially novel
therapeutic target.
Acknowledgements
This study was supported by grants from the Natural
Science Foundation of Jiangsu Province (SZ126821), the Social
Development Projects of Kunshan City (KS1224) and the Priority
Academic Program Development of Jiangsu Higher Education
Institutions (PAPD).
References
|
1
|
Jemal A, Bray F, Center MM, et al: Global
cancer statistics. CA Cancer J Clin. 2:69–90. 2011. View Article : Google Scholar
|
|
2
|
Singhal S, Miller D, Ramalingam S and Sun
SY: Gene expression profiling of non-small cell lung cancer. Lung
Cancer. 60:313–324. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Li C, Fan S, Owonikoko TK, et al:
Oncogenic role of EAPII in lung cancer development and its
activation of the MAPK-ERK pathway. Oncogene. 35:3802–3812. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Larsen JE, Cascone T, Gerber DE, et al:
Targeted therapies for lung cancer. Cancer J. 6:512–527. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Lotze MT and Tracey KJ: High-mobility
group box 1 protein (HMGB1): nuclear weapon in the immune arsenal.
Nat Rev Immunol. 5:331–342. 2005. View
Article : Google Scholar : PubMed/NCBI
|
|
6
|
Muller S, Scaffidi P, Degryse B, et al:
New EMBO members’ review: the double life of HMGB1 chromatin
protein: architectural factor and extracellular signal. EMBO J.
20:4337–4340. 2001.
|
|
7
|
Tang DL, Kang R, Zeh HR III and Lotze MT:
High-mobility group box 1 and cancer. Biochim Biophys Acta.
1799:131–140. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Lotze MT and DeMarco RA: Dealing with
death: HMGB1 as a novel target for cancer therapy. Curr Opin
Investig Drugs. 4:1405–1409. 2003.PubMed/NCBI
|
|
9
|
Ito I, Fukazawa J and Yoshida M:
Post-translational methylation of high mobility group box 1 (HMGB1)
causes its cytoplasmic localization in neutrophils. J Biol Chem.
282:16336–16344. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Taguchi A, Blood DC, del Toro G, et al:
Blockade of RAGE-amphoterin signalling suppresses tumour growth and
metastases. Nature. 405:354–360. 2000. View
Article : Google Scholar : PubMed/NCBI
|
|
11
|
Jiao Y, Wang HC and Fan SJ: Growth
suppression and radiosensitivity increase by HMGB1 in breast
cancer. Acta Pharmacol Sin. 28:1957–1967. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Li XL, Meng QH and Fan SJ:
Adenovirus-mediated expression of UHRF1 reduces the
radiosensitivity of cervical cancer HeLa cells to
gamma-irradiation. Acta Pharmacol Sin. 30:458–466. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Wu QF, Liu C, Tai MH, et al: Knockdown of
FoxM1 by siRNA interference decreases cell proliferation, induces
cell cycle arrest and inhibits cell invasion in MHCC-97H cells in
vitro. Acta Pharmacol Sin. 31:361–366. 2010. View Article : Google Scholar
|
|
14
|
Jiao Y, Ge CM, Meng QH, et al:
Adenovirus-mediated expression of Tob1 sensitizes breast cancer
cells to ionizing radiation. Acta Pharmacol Sin. 28:1628–1636.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Jiao Y, Ge CM, Meng QH, et al:
Dihydroartemisinin is an inhibitor of ovarian cancer cell growth.
Acta Pharmacol Sin. 28:1045–1056. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Jiang R, Wang NP, Tanaka KA, et al: Factor
Xa induces tissue factor expression in endothelial cells by P44/42
MAPK and NF-κB-dependent pathways. J Surg Res. 169:319–327.
2011.PubMed/NCBI
|
|
17
|
Ray MR, David J and He B: Lung cancer
therapeutics that target signaling pathways: an update. Expert Rev
Respir Med. 4:631–645. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Palmer RH, Vernersson E, Grabbe C and
Hallberg B: Anaplastic lymphoma kinase: signalling in development
and disease. Biochem J. 420:345–361. 2009. View Article : Google Scholar : PubMed/NCBI
|