Introduction
Colorectal laterally spreading tumors (LSTs) of the
colon and rectum are defined as lesions >10 mm in diameter with
a low vertical axis that extend laterally along the luminal wall
(1). The clinical characteristics
of LSTs include a flat and superficial growth pattern. Although
LSTs are known as adenomas or ‘pre-existing cancers’, they have
been hypothesized to have malignant potential. LSTs are divided
into two macroscopic subtypes, flat (F)-type and protruded-type
(2). Despite the distinctive
biological behaviors of LSTs, a number of genetic alterations have
been reported, including K-ras and p53 mutations and
cyclooxygenase 2 overexpression (3–6).
Wang et al(7) observed that
the expression of E-cadherin, β-catenin, glycogen synthase
kinase-3β (GSK-3β), cyclin D1 and c-myc was positive in LSTs. Human
telomerase reverse transcriptase (hTERT/Hest2/TP2), the main
subunit involved in telomerase reverse transcription, is considered
as one of the most important proteins affecting telomerase activity
(8,9). However, hTERT expression in LSTs has
not been thoroughly studied. Thus, this study aimed to elucidate
the expression levels of hTERT in LSTs, colorectal tumors with a
high malignancy potential.
Materials and methods
LST cell line
The novel LST cell line was obtained from the
Department of Gastroenterology, Nan Fang Hospital, Affiliated
Hospital of Nan Fang Medical University, China. The LST cell line
was established from a human rectal villous adenoma obtained using
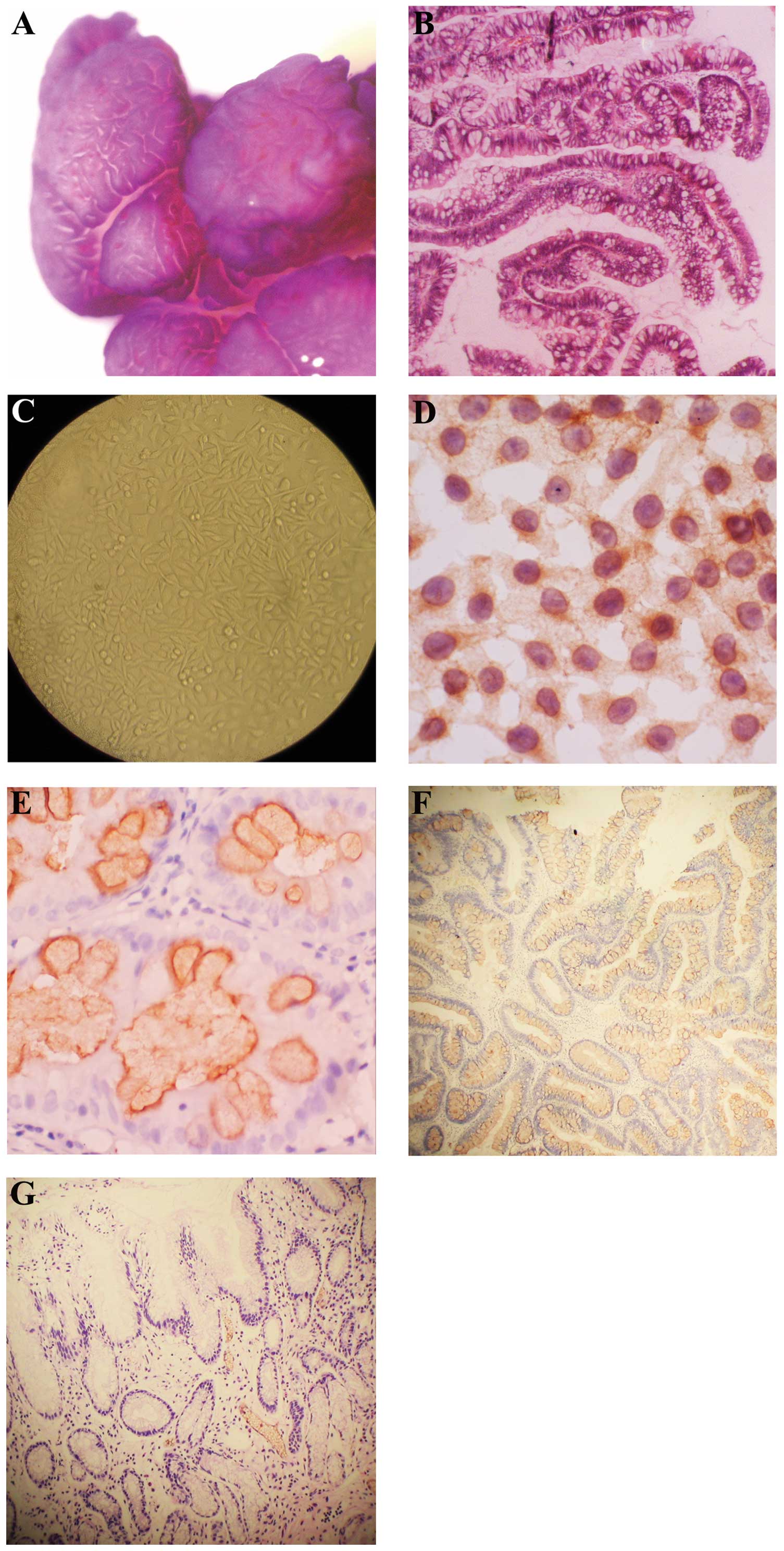
endoscopic resection. A magnifying endoscope showed a flat granular
tumor with nodes (70×60 mm) in the rectum (Fig. 1A). Pathological examination of the
biopsy specimen revealed that it had the characteristic morphology
of a villous adenoma accompanied by moderately severe atypical
hyperplasia (Fig. 1B). The LST
cell line was grown in RPMI-1640 medium (Invitrogen, Carlsbad, CA,
USA) containing 100 mol/l fetal calf serum and antibiotics. Cells
were cultured in a humidified 5% CO2 atmosphere. Cells
from the primary passage of the LST were cultured for 48 h and
fixed in ice-cold pure alcohol at -20°C (Fig. 1C).
Tissue specimens
A total of 48 LSTs were collected by endoscopic
resection at the Cancer Center of Sun Yat-Sen University between
August 2007 and July 2010. The histology of the tissue specimens
revealed that they were all adenomas. In total, 48 protruded-type
colorectal adenomas (PAs) and 48 normal mucosa samples were used as
negative controls. The study was performed in accordance with
institutional ethical guidelines and was approved by the Scientific
Committee of Cancer Center of Sun Yat-Sen University. Informed
consent was obtained from all patients.
Immunohistochemistry
Cells were grown on chamber slides and stained for
hTERT expression with a specific antibody (Beijing Zhongshan
Biology Technology Ltd., Beijing, China). The specimens were fixed
in 10% formalin solution and then embedded in paraffin. Serial
sections were cut and prepared for hematoxylin and eosin (H&E)
staining and immunohistochemistry, then rehydrated through graded
alcohol. Antigen retrieval was performed by microwaving at moderate
power for 3 min, followed by high power for 10 min in 0.01 M
citrate buffer (PH 6.0). Endogenous peroxide activity was quenched
by incubation with 3% hydrogen peroxide in methanol for 20 min. The
slide was then incubated with a mouse monoclonal whole-serum
antibody against hTERT (1:25 dilution; NCL-hTERT; Novo Castra,
Newcastle upon Tyne, UK) at room temperature for 2 h.
HRP-conjugated secondary antibody (anti-mouse, EnVision System kit;
Dako, Carpinteria, CA, USA) was then applied for 60 min. Slides
were rinsed in TBST twice. 3,3′-Diaminobenzidine was used as the
chromogen. Slides were counterstained with hematoxylin, dehydrated
in a graded series of ethanol, mounted on slides and cover slipped
(10). For negative controls,
normal mouse serum was added instead of the primary antibody.
Epithelium and glands with a normal appearance in each section
provided positive internal controls for binding of the primary
antibodies.
Positive expression of hTERT in epithelial tissue
cells revealed a primarily cytoplasmic or nuclear pattern with
yellow or brown staining. All sections were blindly and
independently assessed microscopically by two trained pathologists.
The intensity and distribution of immunohistochemical staining for
hTERT were evaluated (7). The
intensity of staining was assessed with a semi-quantitative scoring
system as follows: 0, negative staining; 1, weak staining; 2,
moderate staining; and 3, strong staining. The distribution of
staining was graded by the percentage of stained cells in the
region of interest as follows: 0, <10% of tumor cells were
positive; 1, 10–50% of tumor cells were positive; 2, 50–75% of
tumor cells were positive; and 3, >75% of tumor cells were
positive. An overall score was obtained by combining the intensity
and distribution of positive staining. Cases with 0 points were
considered to be negative (0), cases with a final score of 1–3 as
weakly positive (1+), cases with a final score of 4–7 as moderately
positive (2+) and cases with a final score of >7 as strongly
positive (3+).
Statistical analysis
Student’s t-test was used to compare age, gender and
tumor size features between the PAs and LSTs. A Chi-square test was
used for comparing the remaining parameters between these two
groups. To evaluate differences in the expression of hTERT proteins
among LSTs, PAs and normal mucosa, the Kruskal-Wallis test was
used, and further analysis with a Bonferroni test was used to
compare any two groups. All statistical tests were two-sided and
P<0.05 was considered to indicate a statistically significant
difference. The statistical analyses were performed using the SPSS
13.0 software package (SPSS, Chicago, IL, USA).
Results
Patient characteristics
The clinicopathological features of LSTs and PAs are
summarized in Table I. The mean
age of patients with PAs was 62.3 years, whereas the mean age of
those with LSTs was 64.1 years. No significant difference was
identified in age (P=0.163), gender (P=0.142), histology (P=0.137)
or grade of intraepithelial neoplasia (P=0.150) between the LSTs
and PAs. With regard to the tumor size, the mean size of PAs was
15.5 mm, whereas the mean size of LSTs was 28.2 mm and a
significant difference was identified (P=0.032). In total, 43.5% of
LSTs were located in the proximal colon; however, only 12.5% of PAs
were located in the proximal colon. Additionally, PAs were
identified more frequently in the distal colon (62.5%), whereas
only (8.7%) of LSTs were located in the distal colon
(P<0.001).
 | Table IPatient characteristics. |
Table I
Patient characteristics.
| Characteristics | PAs | LSTs | P-valuea |
|---|
| Age (years) | | | 0.163 |
| Mean ± SD | 62.3±11 | 64.1±11.8 | |
| Gender | | | 0.142 |
| Male/female | 36/12 | 28/20 | |
| Tumor size (mm) | | 0.032 | |
| Mean ± SD | 15.5±6.1 | 28.2±16.3 | |
| Location, n (%) | | | <0.001 |
| Proximal colon | 6 (12.5) | 20 (41.7) | <0.001 |
| Distal colon | 30 (62.5) | 6 (12.5) | <0.001 |
| Rectum | 12 (25.0) | 22 (45.8) | 0.106 |
| Histology, n (%) | | | 0.137 |
| Tubular | 18 (37.5) | 12 (25) | |
| Tubulovillous | 26 (54.2) | 34 (70.8) | |
| Villotubular | 4 (8.3) | 2 (4.2) | |
| Grade, n (%) | | | 0.150 |
| Low | 28 (58.3) | 20 (41.7) | |
| High | 20 (41.7) | 28 (58.3) | |
Immunohistochemical staining in the LST
cell line
As shown in Fig.
1D, the positive expression of hTERT was localized to the
cytoplasm or nuclei in LST cultured cells.
Immunohistochemical expression in
paraffin sections
The expression of hTERT localized to the cytoplasm
among PAs, LSTs and normal mucosa is shown in Fig. 1E-G. The expression of hTERT was
shown to be significantly different among the three groups
(P<0.001). The positive expression levels of hTERT in the LSTs,
PAs and normal mucosa were 60.4, 22.9 and 10%, respectively. The
weak nuclear expression of hTERT was demonstrated as 18.8% in LSTs
(9/48) and 16.7% in PAs (8/48). Further analysis with the
Bonferroni test revealed that differences in the expression of
hTERT occurred between LSTs, normal mucosa samples (P<0.001) and
PAs (P<0.001), but not between PAs and normal mucosa (P=0.201;
Table II).
 | Table IIExpression of hTERT localized to the
cytoplasm in PAs and LSTs. |
Table II
Expression of hTERT localized to the
cytoplasm in PAs and LSTs.
| | hTERTa | |
|---|
| |
| |
|---|
| Category | No. | 0 | 1+ | 2+ | 3+ | P valueb |
|---|
| LSTs | 48 | 19 | 9 | 14 | 6 | <0.001 |
| PAs | 48 | 37 | 8 | 2 | 1 | <0.001 |
| Normal mucosa | 20 | 18 | 2 | 0 | 0 | 0.021 |
Discussion
Since the discovery of the telomerase enzyme in
humans, it has become one of the most notable tumor markers and its
significance in diagnostic, prognostic and therapeutic applications
has been revealed. Extensive investigation has identified three
core components of the enzyme, of which the catalytic subunit hTERT
appears to be the most significant. More than 85% of tumors express
telomerase, which provides an effective tool for the diagnosis,
prognosis and treatment of malignancy. Telomerase activity is
readily detected in ~3000 human malignant tumors, including breast,
prostate, lung, liver, pancreatic and colon cancer. Telomerase
activity is absent in differentiated somatic tissues (11–20);
however, in the majority of tumors, overexpression of hTERT is
accompanied by an increase in telomerase activity. The presence of
hTERT in tissue samples and cells lines may be beneficial for
identifying the stage and grade of the tumor. Depending on the type
of test sample (fixed tissue, whole cells or tissue lysates), hTERT
may also be detected by fluorescence in situ hybridization
(FISH) (21). In a number of
cases, chronic conditions of specific tissues (e.g., liver
cirrhosis) exhibit weak telomerase activity (22). In certain tissues, two types of
hTERT distribution patterns are observed, diffused and focal
(21). In others, a perinuclear
and/or cytoplasmic staining pattern is observed (22). The hTERT-positive cells with
diffuse expression tend to have a higher proliferative index and
the tumors are smaller in size compared with the focally
distributed cells. hTERT-positive cells with focal distribution
have a reduced survival time. The type of hTERT distribution in
certain cases correlates with the clinicopathological parameters,
while in others there is no association between hTERT expression
and the proliferative index, grade or stage of the tumor.
To estimate the changes in hTERT expression between
LSTs and PAs, we examined the expression of hTERT using
immunohistochemical staining of the cell line and tumor tissues.
The hTERT protein is mainly expressed in the cytoplasm of cells,
with a lower level of expression in the nucleus. The accumulation
of hTERT within the cytoplasm and nucleus is associated with
colorectal cancer, in addition to other types of cancer. We
observed that the cytoplasmic expression of hTERT was significantly
higher in LSTs than in PAs (60.4 vs. 22.9%). Our data demonstrated
that LSTs, as a type of precancerous lesion, may have the potential
for malignancy. Kawanishi-Tabata et al(23) examined 122 colorectal carcinomas,
including 52 cases of colon cancer and 70 cases of rectum cancer,
and 80% of these carcinomas were positive for telomerase activity.
Wang et al(7) reported that
the expression of hTERT in colorectal carcinomas was 77.8%. Ohnishi
et al(10) showed that the
immunohistochemical expression of hTERT in the peripheral vein of
colorectal cancer was 81%. However, in our study, the expression
levels of hTERT in LSTs and PAs were lower than in the
above-mentioned studies. By contrast, it has been reported that the
expression of hTERT or telomerase activity does not always
correlate with cancer progression in colon carcinoma, or even in
bladder carcinoma (23).
Additionally, telomerase activity has been shown to be regulated by
hormones, growth factors and inflammatory mediators (24–26).
In this study, the expression of hTERT was localized to the
cytoplasm in LST cultured cells and hTERT immunoreactivity was
positive. We may explain this result by speculating that the LST
cell line was already in the malignant transformation process to a
certain extent. It has been reported that tumor cell lines Lan-1,
HeLa and Co115 are telomerase-positive (27). A study describing the establishment
of the LST cell line demonstrated that the histopathology of a nude
mouse xenograft tumor was a poorly differentiated adenocarcinoma
and cells cultured from the xenograft tumor had the same properties
as the original cell line (28).
Thus, the LST cell line has higher malignancy than normal adenomas.
To the best of our knowledge, this is the first study estimating
telomerase activity in LSTs, including hTERT immunoreactivity in
tissue samples and LST cultured cells.
In conclusion, the present study showed that
activation of the hTERT protein appears to be higher in LSTs than
in PAs and normal mucosa samples. The immunoreactivity expression
of hTERT in the LST cultured cells was positive. It may revealed
that there are some correlation between malignant tendency and LST
cells. Although hTERT subunit expression appears to be a useful
indicator for the prognosis of LST disease, the biological
behaviors involved remain to be clarified.
Acknowledgements
This study was supported by the Natural Science Fund
of Guangdong Province in China (No. S2011010002941). The authors
thank Professor Bo Jiang and Professor Hong-Quan Zhang for their
suggestions for this study.
References
|
1
|
Uraoka T, Saito Y, Matsuda T, et al:
Endoscopic indications for endoscopic mucosal resection of
laterally spreading tumours in the colorectum. Gut. 55:1592–1597.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Takahashi T, Nosho K, Yamamoto H, et al:
Flat-type colorectal advanced adenomas (laterally spreading tumors)
have different genetic and epigenetic alterations from
protruded-type advanced adenomas. Mod Pathol. 20:139–147. 2007.
View Article : Google Scholar
|
|
3
|
Hiraoka S, Kato J, Tatsukawa M, et al:
Laterally spreading type of colorectal adenoma exhibits a unique
methylation phenotype and K-ras mutations. Gastroenterology.
131:379–389. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Mukawa K, Fujii S, Takeda J, et al:
Analysis of K-ras mutations and expression of cyclooxygenase-2 and
gastrin protein in laterally spreading tumors. J Gastroenterol
Hepatol. 20:1584–1590. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Noro A, Sugai T, Habano W and Nakamura S:
Analysis of Ki-ras and p53 gene mutations in laterally spreading
tumors of the colorectum. Pathol Int. 53:828–836. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Kusaka T, Fukui H, Sano Y, Ueda Y, Chiba T
and Fujimori T: Analysis of K-ras codon 12 mutations and p53
overexpression in colorectal nodule-aggregating tumors. J
Gastroenterol Hepatol. 15:1151–1157. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Wang J, Wang X, Gong W, Mi B, Liu S and
Jiang B: Increased expression of beta-catenin, phosphorylated
glycogen synthase kinase 3beta, cyclin D1, and c-myc in laterally
spreading colorectal tumors. J Histochem Cytochem. 57:363–371.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Frías C, Morán A, de Juan C, et al:
Telomere function in colorectal cancer. World J Gastrointest Oncol.
1:3–11. 2009.
|
|
9
|
Kim NW, Piatyszek MA, Prowse KR, et al:
Specific association of human telomerase activity with immortal
cells and cancer. Science. 266:2011–2015. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Ohnishi T, Watanabe T, Nozawa H, Kitayama
J and Nagawa H: Telomerase activity of blood samples and recurrence
of colorectal cancer. Hepatogastroenterology. 55:1513–1518.
2008.PubMed/NCBI
|
|
11
|
Tomás-Loba A, Flores I, Fernández-Marcos
PJ, et al: Telomerase reverse transcriptase delays aging in
cancer-resistant mice. Cell. 135:609–622. 2008.PubMed/NCBI
|
|
12
|
González-Suárez E, Geserick C, Flores JM
and Blasco MA: Antagonistic effects of telomerase on cancer and
aging in K5-mTert transgenic mice. Oncogene. 24:2256–2270.
2005.PubMed/NCBI
|
|
13
|
Shay JW and Wright WE: Telomeres and
telomerase: implications for cancer and aging. Radiat Res.
155:188–193. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Rudolph KL, Chang S, Lee HW, et al:
Longevity, stress response, and cancer in aging
telomerase-deficient mice. Cell. 96:701–712. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Matthews P and Jones CJ: Clinical
implications of telomerase detection. Histopathology. 38:485–498.
2001. View Article : Google Scholar
|
|
16
|
Yoshida K, Sugino T, Goodison S, et al:
Detection of telomerase activity in exfoliated cancer cells in
colonic luminal washings and its related clinical implications. Br
J Cancer. 75:548–553. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Chatziantoniou VD: Telomerase: biological
function and potential role in cancer management. Pathol Oncol Res.
7:161–170. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Stewart SA and Bertuch AA: The role of
telomeres and telomerase in cancer research. Cancer Res.
70:7365–7371. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Hahn WC: Role of telomeres and telomerase
in the pathogenesis of human cancer. J Clin Oncol. 21:2034–2043.
2003. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Goldman MA: The role of telomeres and
telomerase in cancer. Drug Discov Today. 8:294–296. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Falchetti ML, Pallini R, D’Ambrosio E, et
al: In situ detection of telomerase catalytic subunit mRNA in
glioblastoma multiforme. Int J Cancer. 88:895–901. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Harada K, Yasoshima M, Ozaki S, Sanzen T
and Nakanuma Y: PCR and in situ hybridization studies of telomerase
subunits in human non-neoplastic livers. J Pathol. 193:210–217.
2001. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Kawanishi-Tabata R, Lopez F, Fratantonio
S, et al: Telomerase activity in stage II colorectal carcinoma.
Cancer. 95:1834–1839. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Yang H, Kyo S, Takatura M and Sun L:
Autocrine transforming growth factor beta suppresses telomerase
activity and transcription of human telomerase reverse
transcriptase in human cancer cells. Cell Growth Differ.
12:119–127. 2001.
|
|
25
|
Engelhardt M, Drullinsky P, Guillem J and
Moore MA: Telomerase and telomere length in the development and
progression of premalignant lesions to colorectal cancer. Clin
Cancer Res. 3:1931–1941. 1997.PubMed/NCBI
|
|
26
|
Salvia R, Bassi C, Festa L, et al:
Clinical and biological behavior of pancreatic solid
pseudopapillary tumors: report on 31 consecutive patients. J Surg
Oncol. 95:304–310. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Guilleret I and Benhattar J: Demethylation
of the human telomerase catalytic subunit (hTERT) gene promoter
reduced hTERT expression and telomerase activity and shortened
telomeres. Exp Cell Res. 289:326–334. 2003. View Article : Google Scholar
|
|
28
|
Wang XY, Lai ZS, Yeung CM, et al:
Establishment and characterization of a new cell line derived from
human colorectal laterally spreading tumor. World J Gastroenterol.
14:1204–1211. 2008. View Article : Google Scholar : PubMed/NCBI
|