Introduction
Oxygen therapy is an important clinical treatment
for hypoxemia (1). However, long
exposure to a high concentration of oxygen causes the production of
oxygen-free radicals and their derivatives in the lung tissue
(2). Once over the body’s
compensation limit, hyperoxia lung injury can occur (3).
The Wnt signaling pathway is composed of Wnt
proteins, receptors and regulatory factors (4). Previous studies have shown that
external factors interfere with the expression and normal
transduction of this signaling pathway, and thus cause abnormal
lung development (5).
An increased number of studies have shown that the
repair of lung injury completely depends on the proliferation and
differentiation of alveolar type II epithelial cells (AEC IIs). The
proliferation and differentiation of AEC IIs not only repairs the
normal alveolar structure, but also improves alveolar function.
Therefore, AEC IIs are regarded as the stem cells of the alveolae
(6).
In the present study, premature rat AEC IIs were
exposed in vitro to a low oxygen fraction of 0.4 (40%
O2), which is normally used for oxygen therapy in the
clinic, and were compared with cells exposed to a high oxygen
fraction of >0.95 (95% O2), and to room air (21%
O2), to investigate the growth and apoptosis of AEC IIs.
Moreover, the effect of ROS on the Wnt signaling pathway in the
high oxygen fraction (>0.95) (95% O2) and the room
air groups is discussed.
Materials and methods
Reagents
Adult clean-grade Sprague-Dawley rats were purchased
from the Experimental Animal Center of Chongqing Medical University
(Chongqing, China). Trypsin, DNase I, Dulbecco’s modified Eagle’s
medium/F12 (DMEM/F12) and fetal calf serum (FCS) were purchased
from Gibco (Grand Island, NY, USA). Collagenase I, MTT and dimethyl
sulfoxide (DMSO) were purchased from Sigma-Aldrich (St. Louis, MO,
USA), while the Annexin V-FITC-labeled apoptosis kit was from
Nanjing KeyGen Biotech. Co., Ltd. (Nanjing, China). The DCFH-DA
molecular probe was obtained from the Shanghai Beyotime Institute
of Biotechnology (Shanghai, China). TRIzol and chloral hydrate were
obtained from Tiangen Biotech. Co., Ltd. (Beijing, China). Reverse
transcription system kits and PCR kits were purchased from
Fermentas (Burlington, ON, Canada), and the BCA protein assay kit
was from Pierce Biotechnology, Inc. (Rockford, IL, USA). The study
was approved by the ethics committee of Chongqing Medical
University.
Methods
Isolation and culture of fetal rat AEC
IIs
Fetal rat AEC IIs were isolated at the canalicular
stage (19–20 days gestation) by modification of a method previously
described (7). Briefly, a pregnant
rat at 19–20 days of gestation was anesthetized by intraperitoneal
injection of 10% chloral hydrate (1 ml/100 g) and fetal rats were
extracted following the onset of adequate anesthesia. Fetal lungs
were minced and digested with 0.125% trypsin and 10 mg/ml DNase for
20 min at 37°C. The trypsin reaction was stopped with DMEM/F12 with
10% FCS, and the cell suspension was centrifuged at 1,500 × g for 5
min. Supernatants were removed and cell pellets were resuspended in
collagenase and incubated for 15 min at 37°C. The collagenase
reaction was stopped by adding serum followed by centrifugation,
and cell pellets were resuspended and plated into 6-well plates for
differential adherence to remove fibroblasts. Purified cells were
plated in 6-well plates at a seeding density of 1×106
cells/ml and grown to 70–80% confluence over 20–24 h in DMEM/F12
supplemented with 10% fetal bovine serum (FBS). The purity of AEC
II cells was found to be >90% by modified Papanicolaou staining,
and the cell survival rate was >85% by trypan blue staining.
Hyperoxia model and experimental
groups
AEC IIs were inoculated into 6-well plates, grew to
70–80% confluence, and were then randomly divided into three
groups: i) the room air group, where cells were placed into an
incubator with 5% CO2 at 37°C; the low oxygen fraction
(0.4) group, where cells were exposed to 40% O2 with 5%
CO2 at 37°C; and the high oxygen fraction (>0.95)
group, where cells were exposed to 95% O2 with 5%
CO2 at 37°C. The last two groups were placed in two
modular chambers, and the chambers were flushed with a gas mixture
of 40% O2/5% CO2/55% N2 and 95%
O2/5% CO2, respectively, until equilibrium.
The cells were cultured for 12, 24 and 48 h.
Survival rate assessment using MTT
assay
The cells were plated in 96-well plates at a seeding
density of 1×106 cells/ml. The room air, the low oxygen
fraction (0.4) and the high oxygen fraction (>0.95) groups were
each seeded into four wells to be cultured for 12, 24 and 48 h.
Following exposure to oxygen, the medium was removed from each
well, and 20 μl of 5-mg/ml MTT solution and 180 μl of medium were
added to each well. The medium was immediately removed after 4 h of
exposure, and 150 μl of DMSO were added to each well and mixed for
10 min. A multi-detection microplate reader was used to detect
absorbance at 492 nm (A492). Empty wells were used for blanking;
the higher the absorbance value, the more the living cells. The
formula used to calculate the cell survival rate was: cell survival
rate = experimental well A492/control well A492 ×100%.
Apoptosis assessment using flow
cytometry
Cell apoptosis and death were detected using Annexin
V and propidium iodide (PI) staining with an Annexin V-FITC
apoptosis detection kit according to the manufacturer’s
instructions.
Intracellular ROS assessment using
flow cytometry
The DCFH-DA molecular probe was used to detect the
level of intracellular ROS. AEC IIs were washed with DMEM and
cultured with 10 μM DCFH-DA at 37°C for 20 min. The cells were then
harvested, and the deposit was washed twice with ice-cold
phosphate-buffered saline (PBS). The fluorescent signal intensity
of DCF was detected using flow cytometry (excitation at 488 nm and
emission at 610 nm).
Detection of Wnt5α expression using
reverse transcriptase-polymerase chain reaction (RT-PCR)
Total RNA was extracted from AEC IIs using a total
RNA isolation system kit (Fermentas) for the detection of Wnt5α
mRNA expression by RT-PCR. cDNA was synthesized with the reverse
transcription system kit. The following primers were used: Wnt5α
(amplicon length, 364 bp), 5′-CCCACTCCCAGGACCCA CATA-3′ and
5′-CTTTCACCAGGATACCACCCA-3′; β-actin (amplicon length, 293 bp),
5′-ACCCACACTGTGCCCATC TATG-3′ and 5′-CATCGGAACCGCTCATTGCCGA-3′. PCR
was performed as follows: holding for 3 min at 94°C followed by
amplification of cDNA for 30 cycles with melting for 40 sec at
94°C, annealing for 40 sec at 60°C and extension for 60 sec at
72°C. The relative gene expression was determined using the β-actin
gene as an endogenous internal standard with a Bio-Rad Quantity One
software (Bio-Rad, Hercules, CA, USA).
Non-phosphorylated β-catenin protein
assay using western blot analysis
The cells were washed once with ice-cold PBS at the
appropriate time, and lysed in 0.10 ml lysis buffer [20 mM
Tris/HCl, pH 7.4, 100 mM NaCl, 10 mM sodium pyrophosphate, 5 mM
EDTA, 50 mM NaF, 1 mM sodium vanadate, 0.1% (w/v) SDS, 10% (w/v)
glycerol, 1% (v/v) Triton X-100, 1% (w/v) sodium deoxycholate]
containing 1 μM leupeptin, 0.1 μM aprotinin, 1 mM
phenylmethanesulfonyl fluoride and 1 μM pepstatin]. Protein
concentration was calculated using a BCA protein assay kit. Protein
(60 μg) was loaded onto a 10% SDS/polyacrylamide gel and
electrophoretically transferred to nitrocellulose membranes (Pall
Corporation, East Hill, NY, USA), analyzed with antibodies
according to the manufacturer’s instructions, and visualized with
peroxidase and an enhanced chemiluminescence system (ECL kit;
Pierce Biotechnology, Inc.).
Statistical analysis
Data were presented as the means ± standard
deviation (SD). P<0.05 was considered to indicate a
statistically significant difference. Repeated measurement data
analysis of variance was used to test the significant differences
in measured variables between groups. SPSS 17.0 software was used
for all the statistical analyses.
Results
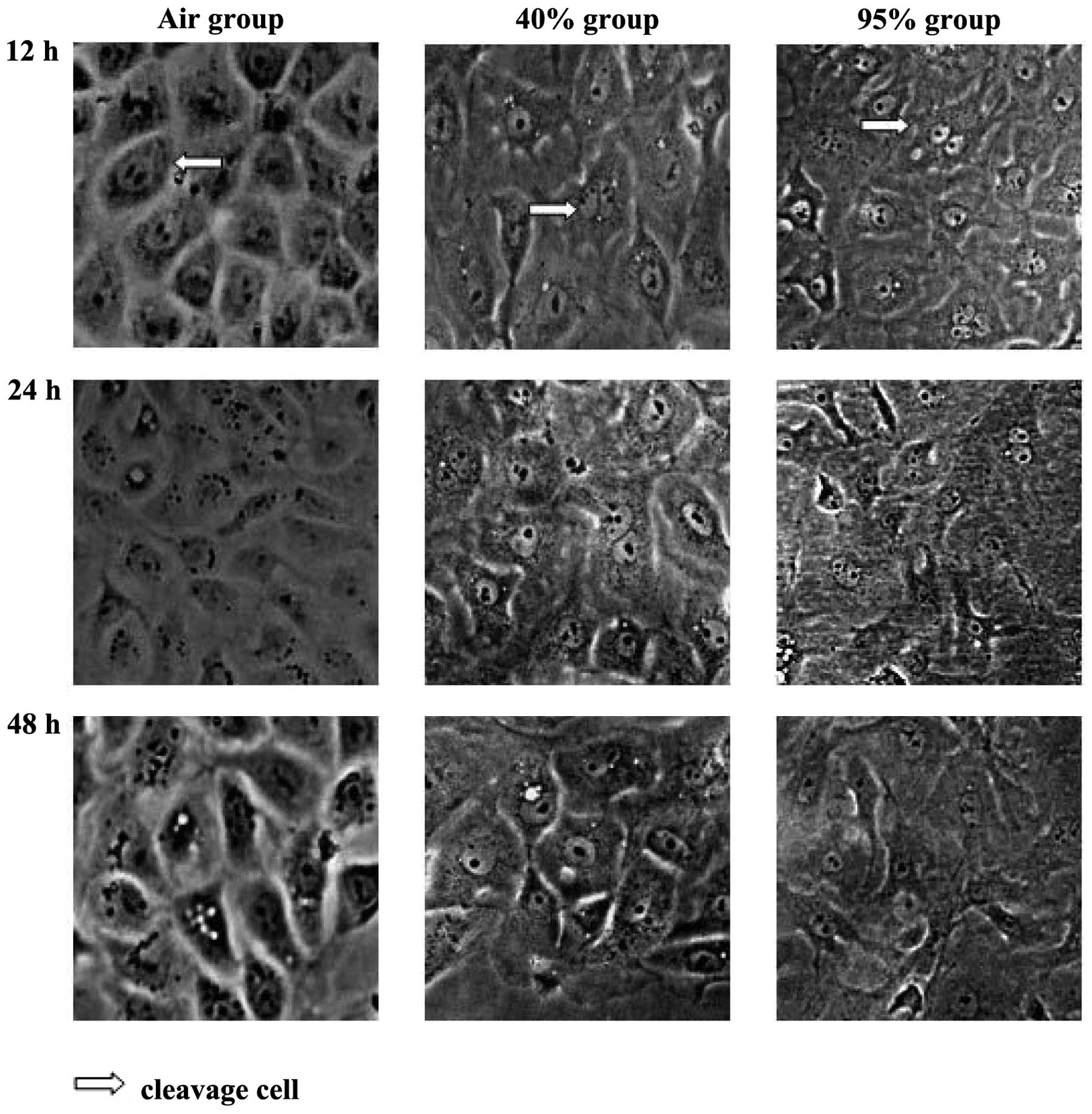
Morphological changes of each group
observed using an inverted microscope
AEC IIs in the room air group grew as islands at the
early time-points. The nucleus was large and deeply stained.
Dividing cells were occasionally observed. As the dutation of
exposure increased, the gap between cells increased, the color of
the cytoplasm faded, the number of lamellar bodies decreased and
vacuoles appeared around the nucleus and cytoplasm.
The above-mentioned structural changes were more
significant after 12 and 24 h of exposure in the high oxygen
fraction (>0.95) group. Parts of the cells increased or
decreased in size and intracellular vacuoles were more obvious,
while the number of floating dead cells increased. After 48 h, the
majority of adherent cells lost their normal basic structure.
The changes occurred at an intermediate level
between the other two groups in the low oxygen fraction (0.4) group
over the same period of time (Fig.
1).
Survival and apoptosis rate of each
group
Compared with the room air group, the survival rate
of the cells in the high oxygen fraction (>0.95) group was
significantly lower after 12, 24 and 48 h (P<0.05).
Additionally, the rate of apoptosis was significantly higher in the
high oxygen fraction (>0.95) group when compared with the room
air group after 12, 24 and 48 h of exposure.
Compared with the room air group, the survival rate
of the cells in the low oxygen fraction (0.4) group was not
significantly different after 12 and 24 h, while it was
significantly lower after 48 h of exposure (P< 0.05). Similarly,
the apoptosis rate of the low oxygen fraction (0.4) group was not
significantly different from the room air group at 12 and 24 h,
while it was significantly higher after 48 h of exposure (Table I).
 | Table ISurvival and apoptosis rates of AEC
IIs in each group at different exposure times. |
Table I
Survival and apoptosis rates of AEC
IIs in each group at different exposure times.
| Survival rate
(%) | Apoptosis rate
(%) |
|---|
|
|
|
|---|
| Group | 12 h | 24 h | 48 h | 12 h | 24 h | 48 h |
|---|
| Air | 100.00±2.013 | 87.09±3.851a | 71.17±1.655a | 4.35±1.516 | 5.67±0.301 | 8.17±1.479 |
| 40%
O2 | 93.87±0.389 | 87.73±4.505 | 65.74±1.479c | 4.46±2.110 | 12.24±1.256 | 28.33±6.189c |
| 95%
O2 | 67.64±1.929b,c | 51.86±6.724b,c | 37.99±1.215b,c | 14.76±2.265b,c | 25.28±8.353b,c | 28.78±6.681c |
Intracellular ROS of each group
After 12 h, the ROS content of the cells in the high
oxygen fraction (>0.95) group was significantly higher compared
with that of cells in the room air group, which continued to rise
after 24 h of exposure (Table
II).
 | Table IIReactive oxygen species (ROS) levels
in cells in each group after 12 and 24 h of exposure. |
Table II
Reactive oxygen species (ROS) levels
in cells in each group after 12 and 24 h of exposure.
| ROS |
|---|
|
|
|---|
| Group | 12 h | 24 h |
|---|
| Air | 36.54±2.338 | 95.57±5.259 |
| 95%
O2 | 321.14±15.976a | 504.63±30.982a,b |
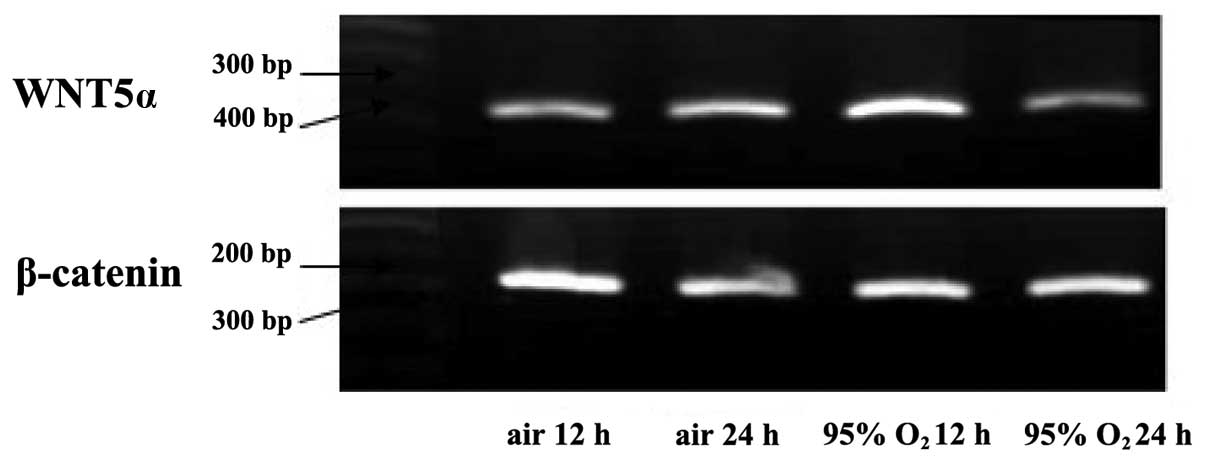
Wnt5α mRNA and non-phosphorylated
β-catenin protein expression
Wnt5α mRNA and non-phosphorylated β-catenin protein
expression reached their maximum and were significantly higher in
the cells of the high oxygen fraction (>0.95) group compared
with the cells of the room air group after 12 h of exposure. By
contrast, after 24 h, the Wnt5α mRNA and non-phosphorylated
β-catenin protein expression decreased in the room air and high
oxygen fraction (>0.95) groups, and was significantly lower in
the high oxygen fraction (>0.95) group compared with the room
air group (P<0.05) (Figs.
2–5).
Discussion
Prolonged exposure to oxygen may cause ROS
accumulation in vivo. A high concentration of ROS may cause
irreversible toxicity to DNA and proteins, and speed up the damage
and death of the cell (7,8). Previous studies have shown that
oxygen toxicity to the lung differs at different oxygen
concentrations, patient age and durations of inhalation (9). Oxygen therapy is widely used in acute
oxygen deficiency disease (10).
However, high oxygen exposure may cause acute damage to the lung
(11). Consequently, as long as
the blood gas analysis returns to normal, oxygen therapy should be
administered for a short time and with a low oxygen concentration
(12). There is no clear
indication as yet regarding the proper duration of inhalation and
oxygen concentration (11,13).
In the present study, 40% O2, which is a
concentration that is commonly used for oxygen therapy in clinical
practice, was selected (14). The
results showed that the survival and apoptosis rates of the low
oxygen fraction (0.4) group were better compared with those of the
high oxygen fraction (>0.95) group. However, as the exposure
time increased, the degree of oxidative injury to the cells in the
low oxygen fraction (0.4) group was also increased. After 48 h, the
increase in the apoptosis rate and the decrease in the survival
rate of cells in the low oxygen fraction (0.4) group were
statistically significant compared with the room air group.
Therefore, as the duration of exposure to oxidative stress
increased, oxidative damage also appeared in the cells that were
stimulated with a low concentration of oxygen (40%) after 48 h.
Wnt signaling consists of the classical and
non-classical pathways. The Wnt/β-catenin signaling pathway is the
classical pathway, while the non-classical pathway includes the
Wnt/Ca2+ and Wnt/planar cell polarity (PCP) pathways.
Numerous factors in the Wnt signaling pathway participate in the
regulation and control of lung development, and are important in
the proliferation and differentiation of cells (15), the formation of lung steric
configuration and the development of the distal end of the lung. It
has been reported that Wnt/β-catenin plays a critical role in the
early stages of lung development (16). β-catenin protein is degraded in the
cytoplasm by the accumulation of phosphorylated and
non-phosphorylated β-catenin protein in the cytoplasm and its entry
into the cell nucleus to activate the late steps of the Wnt
pathway. Therefore, detection of the non-phosphorylated β-catenin
protein in the cell nucleus provides knowledge regarding the
activation status of the Wnt signaling pathway (17). The Wnt5α gene is considered to be
involved in the development of embryonic pulmonary parenchyma and
lung vessels (18).
As the duration of exposure increased, the ROS
levels in the cells of the room air and the high oxygen fraction
(>0.95) groups were elevated, and the levels of ROS in the high
oxygen fraction (>0.95) group were significantly higher compared
with those in the room air group after 12 and 24 h. Wnt5α mRNA and
nuclear β-catenin protein expression were higher in the high oxygen
fraction (>0.95) group compared with the room air group after 12
h, while they were lower compared with the room air group after 24
h. The present study showed that a higher oxygen fraction
stimulates Wnt signaling pathway activation at an early stage in
AEC IIs, causing a premature expression of Wnt5α mRNA and
accelerated translocation of β-catenin protein into the nucleus. We
demonstrated that the Wnt signaling pathway participates as an
early factor in the development of hyperoxia-induced lung injury,
and that ROS created by AEC IIs after oxidative stress stimulated
the premature expression of components of the Wnt pathway.
In most cells (19), the Wnt signaling pathway causes
proliferation to some extent, while in our experimental results,
prolonged oxidative stress caused an inhibition of Wnt pathway
expression. This finding may be due to the fact that the high
oxygen fraction caused cell degeneration and death. Some studies
have demonstrated that following the treatment of cells with
H2O2, a lower concentration of ROS at an
early stage stimulates the expression of the Wnt signaling pathway.
However, the Wnt pathway expression is inhibited with increasing
time. These effects may be associated with the intracellular
antioxidant system and the interaction of the Wnt signaling pathway
itself with ROS. Further studies are needed for the investigation
of the underlying mechanism.
References
|
1
|
Bent S, Bertoglio K, Ashwood P, Nemeth E
and Hendren RL: Brief report: hyperbaric oxygen therapy (HBOT) in
children with autism spectrum disorder: a clinical trial. J Autism
Dev Disord. 42:1127–1132. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Zorov DB, Juhaszova M and Sollott SJ:
Mitochondrial ROS-induced ROS release: an update and review.
Biochim Biophys Acta. 1757:509–517. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Wu CY, Yue F, Li M, Zhang LP, Wang J and
Guo XY: Effects of hyperoxia on inflammatory response in lung of
infantile rats. Zhongguo Wei Zhong Bing Ji Jiu Yi Xue. 22:389–392.
2010.(In Chinese).
|
|
4
|
Katoh M: WNT signaling in stem cell
biology and regenerative medicine. Curr Drug Targets. 9:565–570.
2008. View Article : Google Scholar
|
|
5
|
Coant N, Ben MS, Pedruzzi E, et al: NADPH
oxidase 1 modulates WNT and NOTCH1 signaling to control the fate of
proliferative progenitor cells in the colon. Mol Cell Biol.
30:2636–2650. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Gomperts BN and Strieter RM: Stem cells
and chronic lung disease. Annu Rev Med. 58:285–298. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Hengartner MO: The biochemistry of
apoptosis. Nature. 407:770–776. 2000. View
Article : Google Scholar : PubMed/NCBI
|
|
8
|
Kulkarni AC, Kuppusamy P and Parinandi N:
Oxygen, the lead actor in the pathophysiologic drama: enactment of
the trinity of normoxia, hypoxia, and hyperoxia in disease and
therapy. Antioxid Redox Signal. 9:1717–1730. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Liu HC, Chang LW, Rong ZH, et al: Relation
of insulin-like growth factor binding protein-2 with
hyperoxia-induced lung injury in term and premature neonatal rats.
Zhongguo Wei Zhong Bing Ji Jiu Yi Xue. 20:331–334. 2008.(In
Chinese).
|
|
10
|
Diringer MN, Aiyagari V, Zazulia AR,
Videen TO and Powers WJ: Effect of hyperoxia on cerebral metabolic
rate for oxygen measured using positron emission tomography in
patients with acute severe head injury. J Neurosurg. 106:526–529.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Altemeier WA and Sinclair SE: Hyperoxia in
the intensive care unit: why more is not always better. Curr Opin
Crit Care. 13:73–78. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Tarpy SP and Celli BR: Long-term oxygen
therapy. N Engl J Med. 333:710–714. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Saugstad OD, Ramji S and Vento M: Oxygen
for newborn resuscitation: how much is enough. Pediatrics.
118:789–792. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Oh S, Lee E, Lee J, Lim Y, Kim J and Woo
S: Comparison of the effects of 40% oxygen and two atmospheric
absolute air pressure conditions on stress-induced premature
senescence of normal human diploid fibroblasts. Cell Stress
Chaperones. 13:447–458. 2008.
|
|
15
|
Van Scoyk M, Randall J, Sergew A, Williams
LM, Tennis M and Winn RA: Wnt signaling pathway and lung disease.
Transl Res. 151:175–180. 2008.PubMed/NCBI
|
|
16
|
Cohen JC, Larson JE, Killeen E, Love D and
Takemaru K: CFTR and Wnt/beta-catenin signaling in lung
development. BMC Dev Biol. 8:702008. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Korswagen HC: Regulation of the
Wnt/beta-catenin pathway by redox signaling. Dev Cell. 10:687–688.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Li C, Xiao J, Hormi K, Borok Z and Minoo
P: Wnt5a participates in distal lung morphogenesis. Dev Biol.
248:68–81. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Dasgupta C, Sakurai R, Wang Y, et al:
Hyperoxia-induced neonatal rat lung injury involves activation of
TGF-β and Wnt signaling and is protected by rosiglitazone. Am J
Physiol Lung Cell Mol Physiol. 296:L1031–L1041. 2009.PubMed/NCBI
|