Introduction
Myocardial ischemia reperfusion
(ischemia/reperfusion, I/R) injury significantly impacts the
recovery of heart function following ischemia and may even be
life-threatening. Consequently, the effective prevention and
treatment of myocardial I/R injury has become the focus of numerous
studies. Murry et al(1)
first proposed the myocardial ischemic preconditioning process in
1986. Following this, ischemic post-processing (2,3) and
remote ischemic post-processing (4)were suggested to be effective against
myocardial I/R injury and play a role in myocardial protection.
Previous studies have demonstrated that I/R of the
heart causes damage to the lungs and has a significant impact on
the treatment and prognosis of patients. Kitashiro et
al(5) carried out in
vivo experiments in dogs which showed that myocardial I/R
injury significantly increased lung water content. Tang et
al(4) demonstrated that the
remote postconditioning induced by brief occlusion and reperfusion
of the pulmonary artery could attenuate myocardial reperfusion
injury. The left pulmonary artery was blocked for 5 min followed by
a 5-min reperfusion, and the left anterior descending coronary
artery was occlude for 30 min with a 180-min reperfusion.
Furthermore, the mechanism of protection was associated with the
activation of endothelial nitric oxide synthase (eNOS). A study by
He et al(6) revealed that
the application of anti-human tissue factor antibodies may play a
protective role in intestinal I/R-induced lung injury. An increased
concentration of proinflammatory cytokines has been identified in
the plasma of acute kidney injury patients, which leads to an
increased incidence of respiratory complications (7). Following acute kidney injury in rats,
the lungs exhibited an increase in pulmonary capillary leakage and
lung myeloperoxidase activity (8),
suggesting that other remote organs with I/R may also cause lung
complications. Therefore, the occurrence and development of lung
injury and protective measures require attention. Lung injury
following myocardial I/R is mainly characterized by a significant
accumulation of polymorphonuclear leukocytes, which produce large
numbers of cytokines and oxygen free radicals that attack alveolar
cell mitochondria, leading to increased pulmonary capillary
permeability and the development of lung injury (5).
β-defensin-2 (BD-2) is a small cationic
antimicrobial peptide. Human BD-2 was initially identified in
epithelial tissue and the lungs and is secreted following
stimulation by bacterial infections and inflammation. In a previous
study recombinant adenovirus carrying an expression cassette of rat
BD-2 was administered intratracheally to Sprague-Dawley rats 48 h
prior to performing acute lung injury, which was induced by
Pseudomonas aeruginosa infection. The level of P.
aeruginosa in the lung with BD-2 overexpression declined
significantly. Overexpression of BD-2 reduced alveolar damage,
interstitial edema and infiltration of neutrophils in the model.
Furthermore, Pseudomonas aeruginosa infection in the lungs
of cystic fibrosis patients has been shown to be strongly
associated with the inactivation of BD-2 (9). Hu et al(10) developed a rat model that
excessively expressed human BD-2 through gene regulation, which
significantly prolonged the survival time of rats following
infection with Pseudomonas aeruginosa. This also reduced the
aggregation of bacteria in the lungs following infection and
regulated the expression of numerous types of cells/inflammatory
cytokines during the early stages of inflammation (including
interleukin-1β and TNF-α). BD-2 may be able to directly kill
microorganisms in the lung tissue and regulate the immune process.
Wu et al(11) showed that
acute lung injury and inflammation of the lungs induced by
different modes of mechanical ventilation affect BD-2 expression. A
number of studies have demonstrated that the BD-2 gene in lung
tissue is upregulated following intestinal I/R in rats (12). However, no previous studies have
investigated the changes in BD-2 expression in lung tissue
following myocardial I/R. Edaravone is a novel free radical
scavenger, which inhibits reactive oxygen species generated by I/R
and thus has a protective effect in heart, brain and liver damage
(13–15). However, the effects of edaravone on
myocardial I/R-induced lung injury have not been investigated.
Therefore, the aims of the present study were to
investigate BD-2 expression in the lung tissue following myocardial
I/R, observe the protective effects of edaravone in lung injury
following heart I/R processing and discuss whether this may have an
effect on BD-2 expression in lung tissue following myocardial
ischemia.
Materials and methods
Materials
A one-step total RNA extraction kit and DNA
polymerase were purchased from Beijing TransGen Biotech Co., Ltd.
(Beijing, China). RNA enzyme inhibitors, murine leukemia virus
reverse transcriptase, deoxynucleotide triphosphates (dNTPs) and a
direct cloning carrier of PCR products vector system were from
Fermentas (Toronto, ON, Canada). Mouse-derived β-actin (sc-47778),
sheep-derived TNF-α (sc-1348) and rabbit-derived β-defensin
antibodies (bs-1296R) were purchased from Beijing Biosynthesis
Biotechnology Co., Ltd. (Beijing, China).
Animals
Healthy and clean male Wistar rats (weight, 250–300
g; n=24) were provided by the Department of Physiology of Shanxi
Medical Laboratory Animal Center (Taiyuan, China). The rats were
randomly divided into 4 groups (n=6/group); the sham operation (S
group), myocardial I/R control (C group) and edaravone-treated
groups (E1 and E2 groups; different doses).
The study was approved by the ethics committee of Shanxi Provincial
People’s Hospital, Taiyuan, China.
Construction of the rat model
The myocardial I/R model was constructed according
to previously described methods (16). The rats were weighed and
anesthetized with an intraperitoneal injection of urethane (25%; 3
mg/kg). A HX-300 animal ventilator was used for breathing
assistance following tracheal cannulation and the standard limb
lead ECG was traced. A thoracotomy was performed to expose the
heart, and the left anterior descending branch (LAD) was separated
between the left atrial appendage and pulmonary cone. A 7/0
non-invasive suture was used through the myocardium surface, 2 mm
below the root of the left atrial appendage, and the needle was
withdrawn beside the pulmonary conus. The electrocardiogram was
observed for 15 min to allow the heart to become stable, then the
LAD was ligated. Elevation of the ST-segment in lead-II and the
darkening of the corresponding region on the surface of the heart
indicated the successful induction of ischemia. The ligature was
untied after 45 min. When the ECG ST-segment had declined >1/3
and the surface of the heart was recovered to a red color, this
indicated that the myocardium had recovered from the reperfusion
and further reperfusion was continued for 3 h. The rats in the
E1 and E2 groups were intravenously injected
with 3 or 10 mg/kg edaravone (2010031; Boda Pharmaceutical Co.,
Ltd., Jilin, China), respectively, 1 min before reperfusion via the
right femoral vein and reperfusion was performed for an additional
3 h. The myocardial I/R rat model for the C group was constructed
according to the method described above, while threading without
ligation was performed in rats of the S group.
Sample collection
After 3 h of reperfusion, the rats were sacrificed
using rapid abdominal aorta phlebotomy. Arterial blood samples (5
ml) were obtained by bloodletting, allowed to stand at room
temperature for 60 min and centrifuged at 12,000 × g for 10 min.
The supernatant was allowed to stand for a further 30 min and
centrifuged at 12,000 × g for 10 min. The resulting supernatant was
loaded into Eppendorf tubes, which were sealed and placed in a
−70°C refrigerator. Subsequently, serum CK-MB activity was detected
using the CCX automatic biochemical analyzer (Abbott, Abbott Park,
IL, USA); the kit was provided by Boehringer (Mannheim,
Germany).
After the rats were sacrificed, the sternum was
dissected along the midline to expose the lungs. A curved hemostat
was used to block the right lung hilum and a V-shaped incision
tracheostomy was made at the bottom of the tracheotomy spot. A
sterile infusion tube (inner diameter, ~2 mm) was inserted through
this incision into the left bronchus and the sterile thin tube was
fixed above the carina using suture ligation. Sterile saline (2 ml)
was fractionally injected through the thin tube using a syringe
five times and the left lung was lavaged with 20 cm H2O
pressure (1 cm H2O=0.098 kPa). The lavage fluid was
drawn back into the syringe and this was slowly repeated 4–5 times;
the lavage fluid was collected in dry and sterile glass tubes. The
duration of the complete lung lavage process was ~20 min. The
lavage fluid was maintained at room temperature for 30 min and
centrifuged at 10,000 × g for 10 min. The supernatant was sealed in
an Eppendorf tube and stored in a −70°C refrigerator. The Coomassie
brilliant blue staining method was used for protein quantification
and the lavage fluid protein contents were determined according to
the manufacturer’s instructions (Nanjing Jiancheng Bioengineering
Institute, Nanjing, China). The obtained data were used to
determine the lung permeability index (PPI), the protein
concentration in the lavage fluid and the serum protein
concentration ratio.
BD-2 and TNF-α protein level
determination
A thoracotomy was performed on the rats following
sacrifice to obtain right and lower lung tissue samples. The
samples were placed in a −70°C refrigerator for the extraction of
the total RNA and protein. BD-2 and TNF-α protein levels were
detected using western blot analysis; the proteins were extracted
with protein lysate (RIPA lysis buffer), diluted with 2X SDS buffer
to the same concentration and boiled for 5 min. The proteins were
separated using SDS-PAGE gel electrophoresis and transferred to
nitrocellulose (PVDF) membranes. The membranes were blocked
overnight in a 5% bovine serum albumin (BSA) solution at 4°C. For
the primary antibody incubation, sc-47778 and sc-1348 were diluted
at a ratio of 1:1,000 (TBST-diluted solution) and bs-1296R was
diluted at a ratio of 1:200; these were maintained at 4°C
overnight. Anti-mouse, anti-goat and anti-rabbit secondary
antibodies were diluted at a ratio of 1:1,000 and incubated at room
temperature for 2 h. The grayscale value was used to determine BD-2
and TNF-α protein expression following enhanced chemiluminescence
(ECL) coloring and scanning.
mRNA expression level determination
Total RNA was extracted from lung tissue samples
(100 mg) using the TransZol one step method. β-actin was used as an
internal standard to detect the BD-2 mRNA expression levels using a
semi-quantitative RT-PCR assay. β-actin and BD-2 primers were
designed in our laboratory and synthesized by Shanghai Sangon
Biological Engineering Technology and Services Co., Ltd. (Shanghai,
China). The primer sequences used were as follows: β-actin
upstream, 5′-TGAACGGGAAGCTCACTGG-3′ and downstream,
5′-TCCACCACCCTGTTGCTGTA-3′ (product fragment length, 307 bp); and
BD-2 upstream, 5′-TGCCTCCTTTTC TCCTATGC-3′ and downstream,
5′-ATGGGAAACAGGTAC CCACA-3′ (product fragment length, 216 bp).
Total RNA (6 μg) was used to clone cDNA with the TaqMan
reverse transcription kit (Fermentas) and this was stored at −20°C.
The PCR cycling conditions were as follows: 94°C for 20 sec, 57°C
for 30 sec and 72°C for 30 sec (36 cycles). RT-PCR products (10 μl)
were used to carry out agarose gel electrophoresis for 2 h
(voltage, 128 V; current, 70 mA; power, 9 W), then stained with
ethidium bromide. The RT-PCR amplification products were observed
under an ultraviolet lamp, images were captured and an image
analyzer was used to analyze the amplification products. The
absorbance ratios of the target gene and β-actin were used to
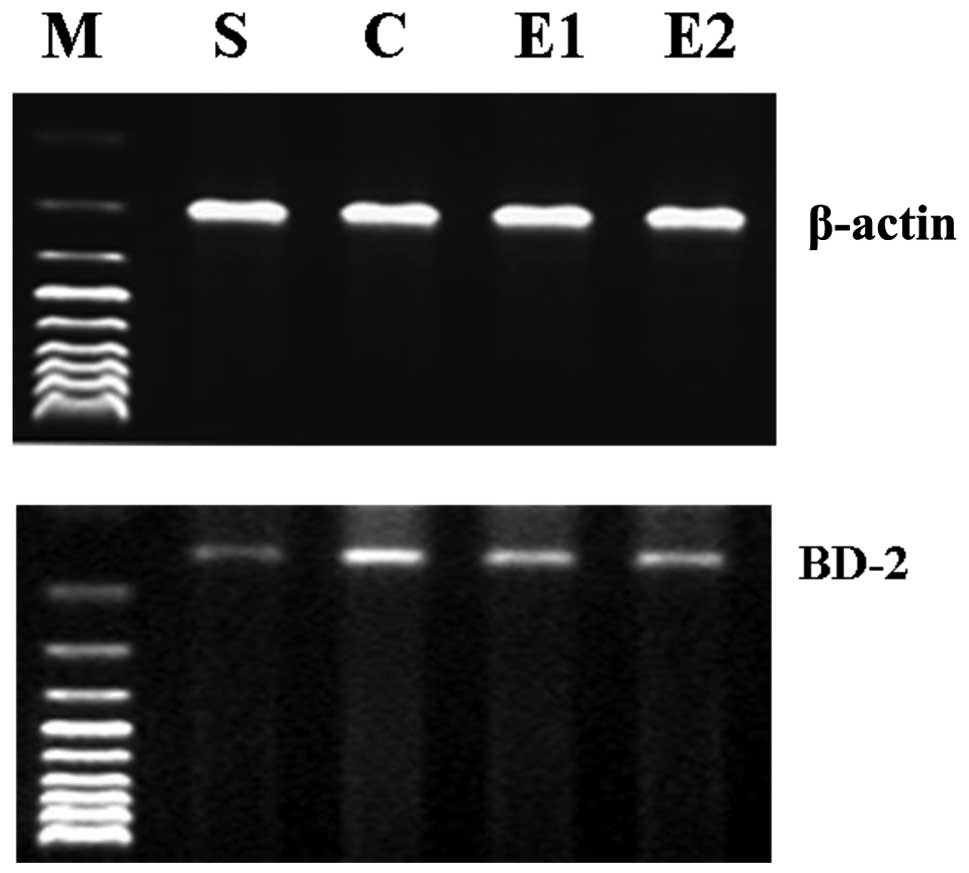
determine the BD-2 mRNA expression levels in the lung tissue.
Statistical analysis
SPSS 15.0 software was used for data analysis.
Measurement data are expressed as the mean ± standard deviation
(SD). The groups were compared using one-way ANOVA, and pairwise
comparisons were performed using the SNK test (a=0.05). The BD-2
and TNF-α protein content of lung tissues in the C, E1
and E2 groups were analyzed using Pearson’s correlation
analysis. P<0.05 was considered to indicate a statistically
significant difference.
Results
The CK-MB and PPI values of the C, E1 and
E2 groups were significantly increased compared with
those of the S group (P<0.01). Furthermore, the CK-MB and PPI
values were decreased in the E1 and E2 groups
compared with those of the C group and were also significantly
decreased in the E2 group compared with those of the
E1 group (P<0.01; Table
I).
 | Table IComparison of the CK-MB and PPI values
and BD-2 and TNF-α content levels with BD-2 mRNA expression in the
four groups (n=6/group). |
Table I
Comparison of the CK-MB and PPI values
and BD-2 and TNF-α content levels with BD-2 mRNA expression in the
four groups (n=6/group).
| Groups | |
|---|
|
| |
|---|
| Indicators | S | C | E1 | E2 | F-value |
|---|
| CK-MB (IU/l) | 13.667±8.477 |
2847.167±408.040a |
1306.667±89.122a,b |
1062.500±57.085a–c | 184.715 |
| PPI (%) | 0.685±0.168 | 4.287±1.778a | 2.780±0.604a,b | 1.550±0.386a–c | 30.456 |
| BD-2 protein | 0.292±0.088 | 1.132±0.117a | 0.782±0.098a,b | 0.613±0.083a–c | 77.469 |
| TNF-α protein | 0.377±0.158 | 1.127±0.024a | 0.918±0.163a,b | 0.727±0.114a–c | 37.392 |
| BD-2 mRNA | 0.402±0.697 | 0.817±0.091a | 0.712±0.054a,b | 0.622±0.044a–c | 41.549 |
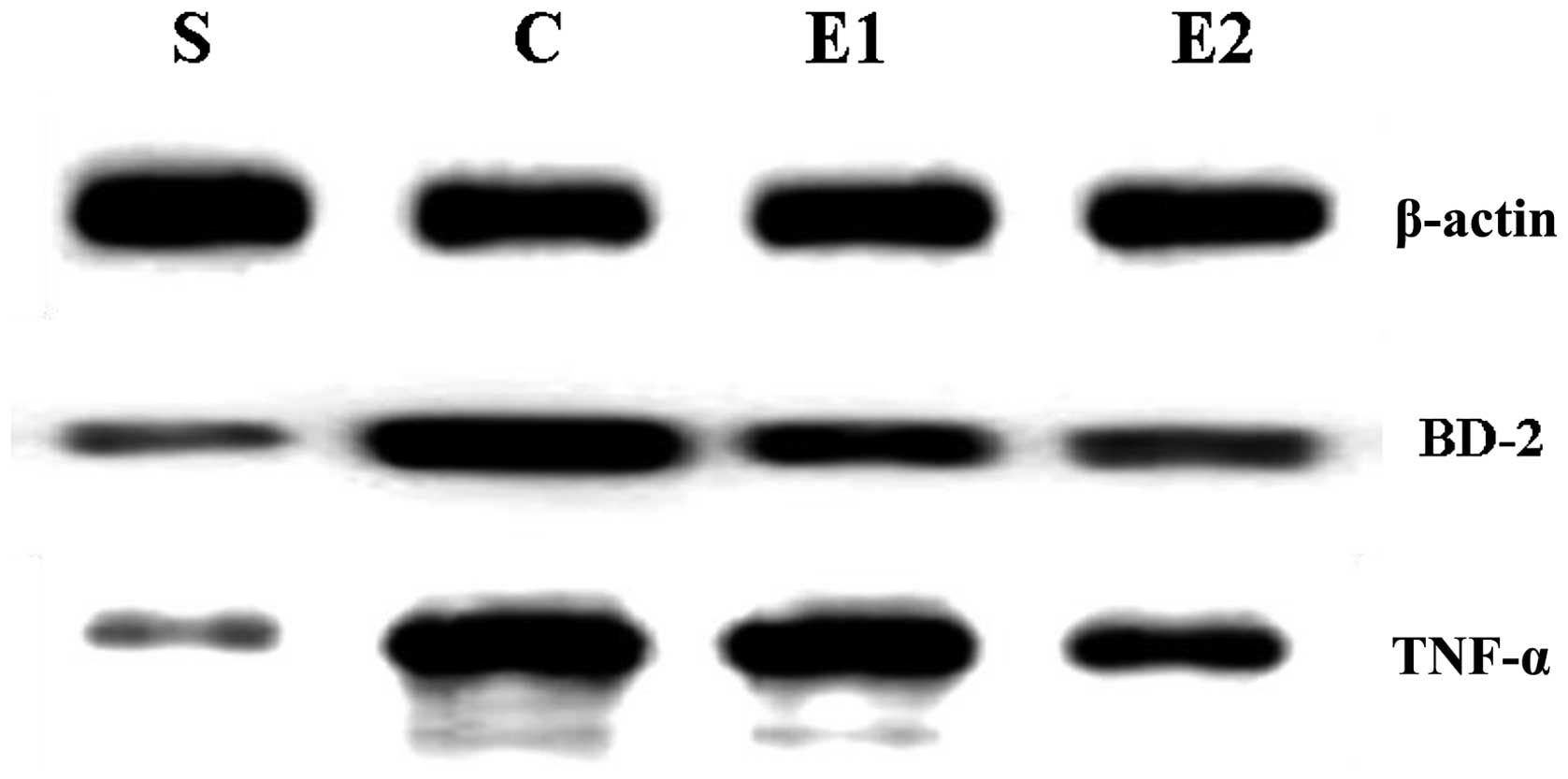
A negligible level of BD-2 mRNA and BD-2 and TNF-α
proteins were expressed in the S group; these indicators were
significantly increased in the C, E1 and E2
groups compared with the S group (P<0.01). The expression levels
of BD-2 mRNA and BD-2 and TNF-α proteins in the E1 and
E2 groups were decreased compared with those of the C
group and were significantly decreased in the E2 group
compared with those of the E1 group (P<0.01; Table I; Figs. 1 and 2).
In the C, E1 and E2 groups,
the expression levels of BD-2 and TNF-α proteins were positively
correlated with one another (r=0.886, r=0.876 and r=0.878,
respectively; P<0.05 for all the groups).
Discussion
The systemic immune response is often caused by
lung, liver, heart and kidney I/R injury. The injury of these
remote organs usually induces an acute immune response that is
associated with white blood cell isolation and the release of
enzymes from lung tissues. This increases vascular permeability,
which causes perivascular and interstitial edema, leading to
pulmonary hypertension and edema. Lung diseases caused by I/R
injuries in these remote organs often lead to clinical respiratory
distress syndromes (17).
In the present study, ligation of the LAD in rats
for 45 min followed by relaxation for 3 h was applied to establish
the myocardial I/R model. During the experiment, elevation of the
ST-segment in the lead-II occurred and the corresponding region on
the heart surface darkened. The ligature was untied; when the
electrocardiogram ST segment was depressed >1/3 and the heart
surface had become its original red color, this indicated that the
myocardial tissue had recovered from the reperfusion. At the end of
the procedure, the serum CK-MB levels of the C, E1 and
E2 groups were significantly increased. Since CK-MB is
the most widely used enzyme for clinical diagnostic application in
acute myocardial infarction (AMI), these increased levels indicated
that the myocardial I/R model was successfully constructed in the
present study.
The PPI indicates the permeability of lung tissue to
proteins and lung I/R injury mainly appears to increase vascular
permeability. The results of this study showed that the PPI values
for the C, E1 and E2 groups were
significantly higher compared with those of the S group, indicating
that the permeability of the lung tissue increased after AMI and
the presence of lung injury. These results are consistent with the
results of previous studies (6),
which demonstrates that construction of the acute myocardial
I/R-induced lung injury model was successful.
Human BD-2 is mainly expressed in the skin, trachea
and lung tissue. In foreskin-derived keratinocytes, BD-2 is rapidly
expressed with the stimulation of TNF-α (within 1 h) and this lasts
for >48 h (18). According to
in vivo data, BD-2 protein levels in serum and BAL fluid are
increased in cystic fibrosis and infectious lung disease patients
(19). Alterations in the lung
defense mechanisms of patients with chronic obstructive pulmonary
diseases may promote the aggregation of bacteria in the peripheral
trachea. Following examination, BD-2 expression levels in the
peripheral trachea of these patients were increased. Patients who
smoked exhibited decreased BD-2 secretion in the main tracheal
epithelium, indicating that smoking may affect their condition
through the alteration of lung tissue defense mechanisms (20). Following remote organ I/R injuries,
numerous inflammatory cytokines are released by the lung tissue.
For example, in intestinal I/R, TNF-α, IL-6, IL-10, monocyte
chemoattractant protein-1 (MCP-1) and interferon-γ (IFN-γ) have
been monitored in lung homogenates and BAL fluid samples (6). Ischemia of the mesenteric artery for
30 min caused gap inflammatory cell infiltration of the lung
tissues and perivascular hemorrhagic edema occurred following
reperfusion for 1 h (21). The
results of the present study showed that the BD-2 mRNA level in the
rat lung tissue was upregulated following myocardial I/R,
accompanied by an increased expression of BD-2 and TNF-α proteins,
and the expression levels of these proteins were positively
correlated. Therefore, considering the results of previous studies,
we concluded that the release of numerous inflammatory mediators
may induce the upregulation of BD-2 mRNA in lung tissue following
myocardial I/R.
BD-2 expression is regulated by downstream effectors
(including the transcription factor NF-κB or AP-1) and activated by
excessive levels of proinflammatory cytokines (for example, IL-1β,
TNF-α and EGF). A number of studies have shown that NF-κB
inhibitors significantly inhibit BD-2 mRNA upregulation; a possible
explanation for this is that the binding sites of numerous
transcription factors (including NF-κB) are the same as that of the
BD-2 gene promoter (22).
Following myocardial I/R injuries, numerous inflammatory cytokines
(including TNF-α) enter the lung tissue and further activate
transcription factors, including NF-κB. These bind with
cis-acting elements and then upregulate BD-2 gene expression
at the transcription level (22).
Edaravone is a novel free radical scavenger, which
has been shown to exert a protective effect in the I/R injuries of
several organs. For example, in rabbit cardiomyocyte
hypoxia-reoxygenation and heart I/R models, edaravone was shown to
reduce cell damage and the myocardial infarction area during the
reperfusion (oxygen) period (23,24).
A number of studies have reported that the use of edaravone reduced
a variety of lung injuries, including in the in vitro lung
I/R injury model (25), in acute
lung injury caused by bleomycin (26) and in canine lung transplantation
(27). This indicates that
edaravone has the potential to have a protective effect in the
donor lung ischemia protective solution and on lung receptors in
lung transplantation reperfusion. Edaravone (8 mg/kg) has been
shown to significantly reduce malondialdehyde levels in lung tissue
following acute pancreatitis, inhibit neutrophil infiltration and
lung tissue damage and reduce IL-6 and TNF-α levels (28).
In the present study, the CK-MB and PPI values of
the two edaravone-treated groups were lower compared with those of
the C group, indicating that damage to the heart and lungs was
reduced. The levels of inflammatory cytokine TNF-α in these two
groups were also decreased compared with the C group; similar to
TNF-α, BD-2 protein expression was reduced and BD-2 mRNA expression
was downregulated. This showed that edaravone protects I/R injury
by inhibiting the release of proinflammatory cytokines, thus
reducing the BD-2 gene and protein expression levels.
Edaravone exhibited dose-dependent protection in
organ tissue damage. Nakamura et al(29) showed that of the 0.5-, 3-, 6- and
10-mg/kg doses of edaravone applied in a rat acute cerebral
hemorrhage model, only 6 and 10 mg/kg had a protective effect on
brain tissue. Edaravone has been shown to reduce the Fas-induced
(Fas/CD95 is a cell surface protein belonging to the TNF receptor
family) mortality in fulminant hepatic failure in a dose-dependent
manner (30). Yuan et
al(31) confirmed that
edaravone used at an early stage with a high concentration in a rat
Parkinson’s disease model significantly reduced the degree of
damage on autonomous behavior. In the present study, the use of 10
mg/kg edaravone caused CK-MB and PPI values to decrease to even
lower levels compared with the decrease observed with 3 mg/kg
edaravone. The expression levels of TNF-α and BD-2 proteins and
BD-2 mRNA were also further reduced, indicating that edaravone
decreased myocardial I/R lung tissue BD-2 gene and protein
expression in a dose-dependent manner.
Due to time limitations, the present study did not
investigate the time-dependent activity of edaravone in lung
tissue. Different durations of treatment with various doses of
edaravone may affect BD-2 expression. This requires investigation
in further studies.
In conclusion, the BD-2 gene is upregulated in lung
tissue following myocardial I/R and there was a significant
inhibition of this upregulation of BD-2 after edaravone treatment;
this occurred in a dose-dependent manner. This may have been caused
by a reduction in TNF-α production, inhibiting the TNF-α-induced
BD-2 gene expression and resulting in the reduction of BD-2 mRNA
and protein expression. However, the exact underlying mechanism
remains to be fully elucidated.
Acknowledgements
This study was supported by the Funding Project
‘Tackle Key Problems in Science and Technology’ of the Provincial
Health Bureau of Shanxi Province (No. 200948).
References
|
1
|
Murry CE, Jennings RB and Reimer KA:
Preconditioning with ischemia: a delay of lethal cell injury in
ischemic myocardium. Circulation. 74:1124–1136. 1986. View Article : Google Scholar
|
|
2
|
Mohan IK, Khan M, Wisel S, et al:
Cardioprotection by HO-4038, a novel verapamil derivative, targeted
against ischemia and reperfusion-mediated acute myocardial
infarction. Am J Physiol Heart Circ Physiol. 296:H140–H151. 2009.
View Article : Google Scholar
|
|
3
|
Kutala VK, Khan M, Mandal R, et al:
Attenuation of myocardial ischemia-reperfusion injury by
trimetazidine derivatives functionalized with antioxidant
properties. J Pharmacol Exp Ther. 317:921–928. 2006. View Article : Google Scholar
|
|
4
|
Tang YH, Xu JJ, Li JX and Cheng XS: Remote
postconditioning induced by brief pulmonary ischemia and
reperfusion attenuates myocardial reperfusion injury in rabbits.
Chin Med J (Engl). 124:1683–1688. 2011.
|
|
5
|
Kitashiro S, Sugiura T, Tamura T, et al:
Factors associated with increased extravascular lung water in
cardiac tamponade and myocardial ischemia. Crit Care Med.
27:2229–2233. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
He X, Han B, Mura M, et al: Anti-human
tissue factor antibody ameliorated intestinal ischemia
reperfusion-induced acute lung injury in human tissue factor
knock-in mice. PLoS One. 3:e15272008. View Article : Google Scholar
|
|
7
|
Faubel S: Pulmonary complications after
acute kidney injury. Adv Chronic Kidney Dis. 15:284–296. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Andrés-Hernando A, Altmann C, Ahuja N, et
al: Splenectomy exacerbates lung injury after ischemic acute kidney
injury in mice. Am J Physiol Renal Physiol. 301:F907–F916.
2011.PubMed/NCBI
|
|
9
|
Shu Q, Shi Z, Zhao Z, et al: Protection
against Pseudomonas aeruginosa pneumonia and sepsis-induced
lung injury by overexpression of beta-defensin-2 in rats. Shock.
26:365–371. 2006.
|
|
10
|
Hu Q, Zuo P, Shao B, et al: Administration
of nonviral gene vector encoding rat beta-defensin-2 ameliorates
chronic Pseudomonas aeruginosa lung infection in rats. J
Gene Med. 12:276–286. 2010.PubMed/NCBI
|
|
11
|
Wu QP, Yao SL and Fang XM: Study of rat
beta-defensin-2 gene and protein expression in
ventilator-associated pneumonia. Zhongguo Wei Zhong Bing Ji Jiu Yi
Xue. 17:353–356. 2005.(In Chinese).
|
|
12
|
Liu KX, Chen SQ, Zhang H, et al:
Intestinal ischemia/reperfusion upregulates beta-defensin-2
expression and causes acute lung injury in the rat. Injury.
40:950–955. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Yamazaki K, Miwa S, Toyokuni S, et al:
Effect of edaravone, a novel free radical scavenger, supplemented
to cardioplegia on myocardial function after cardioplegic arrest:
in vitro study of isolated rat heart. Heart Vessels. 24:228–235.
2009. View Article : Google Scholar
|
|
14
|
Liu N, Shang J, Tian F, et al: In vivo
optical imaging for evaluating the efficacy of edaravone after
transient cerebral ischemia in mice. Brain Res. 1397:66–75. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Shimoda M, Iwasaki Y, Okada T, et al:
Edaravone inhibits apoptosis caused by ischemia/reperfusion injury
in a porcine hepatectomy model. World J Gastroenterol.
18:3520–3526. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Salvi S: Protecting the myocardium from
ischemic injury: a critical role for alpha(1)-adrenoreceptors?
Chest. 119:1242–1249. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Waisman D, Brod V, Dickstein R, et al:
Effects of inhaled nitric oxide on lung injury after intestinal
ischemia-reperfusion in rats. Shock. 23:150–155. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Chung WO and Dale BA: Innate immune
response of oral and foreskin keratinocytes: utilization of
different signaling pathways by various bacterial species. Infect
Immun. 72:352–358. 2004. View Article : Google Scholar
|
|
19
|
van Wetering S, Sterk PJ, Rabe KF and
Hiemstra PS: Defensins: key players or bystanders in infection,
injury, and repair in the lung? J Allergy Clin Immunol.
104:1131–1138. 1999.PubMed/NCBI
|
|
20
|
Pace E, Ferraro M, Minervini MI, et al:
Beta defensin-2 is reduced in central but not in distal airways of
smoker COPD patients. PLoS One. 7:e336012012. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Onder A, Kapan M, Gümüş M, et al: The
protective effects of curcumin on intestine and remote organs
against mesenteric ischemia/reperfusion injury. Turk J
Gastroenterol. 23:141–147. 2012.PubMed/NCBI
|
|
22
|
Steubesand N, Kiehne K, Brunke G, et al:
The expression of the beta-defensins hBD-2 and hBD-3 is
differentially regulated by NF-kappaB and MAPK/AP-1 pathways in an
in vitro model of Candida esophagitis. BMC Immunol.
10:362009. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Yamawaki M, Sasaki N, Shimoyama M, et al:
Protective effect of edaravone against hypoxia-reoxygenation injury
in rabbit cardiomyocytes. Br J Pharmacol. 142:618–626. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Wu TW, Zeng LH, Wu J and Fung KP:
Myocardial protection of MCI-186 in rabbit ischemia-reperfusion.
Life Sci. 71:2249–2255. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Reyes YA, Shimoyama T, Akamatsu H and
Sunamori M: MCI-186 (edaravone), a free radical scavenger,
attenuates ischemia-reperfusion injury and activation of
phospholipase A(2) in an isolated rat lung model after 18 h of cold
preservation. Eur J Cardiothorac Surg. 29:304–311. 2006. View Article : Google Scholar
|
|
26
|
Asai T, Ohno Y, Minatoguchi S, et al: The
specific free radical scavenger edaravone suppresses
bleomycin-induced acute pulmonary injury in rabbits. Clin Exp
Pharmacol Physiol. 34:22–26. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Xu JZ, Shen BZ, Li Y, et al: Edaravone
attenuates ischemia-reperfusion injury by inhibiting oxidative
stress in a canine lung transplantation model. Chin Med J (Engl).
121:1583–1587. 2008.PubMed/NCBI
|
|
28
|
Yang T, Mao YF, Liu SQ, et al: Protective
effects of the free radical scavenger edaravone on acute
pancreatitis-associated lung injury. Eur J Pharmacol. 630:152–157.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Nakamura T, Kuroda Y, Yamashita S, et al:
Edaravone attenuates brain edama and neurologic deficits in a rat
model of acute intracerebral hemorrhage. Stroke. 39:463–469. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Miyasou T, Kwon AH, Tsuji K, et al:
Edaravone prevents Fas-induced fulminant hepatic failure in mice by
regulating mitochondrial Bcl-xL and Bax. Shock. 30:212–216.
2008.PubMed/NCBI
|
|
31
|
Yuan WJ, Yasuhara T, Shingo T, et al:
Neuroprotective effects of edaravone-administration on
6-OHDA-treated dopaminergic neurons. BMC Neurosci. 9:752008.
View Article : Google Scholar : PubMed/NCBI
|