Introduction
Multiple myeloma (MM) is a B-cell malignancy
characterized by the clonal proliferation of neoplastic plasma
cells in the bone marrow (BM). MM is an incurable disease (1). Bortezomib (Velcade®) was
developed as an anticancer drug that acts by inhibiting the 26S
proteasome complex and exhibiting a strong cytotoxic effect against
MM cells (2–4). Nevertheless, numerous MM patients who
initially respond well, inevitably develop resistance to this
drug.
Previous studies have demonstrated that MM cells
become refractory to bortezomib possibly due to their increased
constitutive NF-κB activity, which is further increased when
cultured with MM patient-derived bone marrow stromal cells (BMSCs).
In addition, increased NF-κB activity in the MM RPMI8226 cell line
has been correlated with a decreased bortezomib sensitivity in
vitro(5,6).
Prostaglandin E2 (PGE2) has been shown to upregulate
the expression of Cox-2 or to contribute to its direct
stabilization (7–9). Upon binding with its cell surface
receptors, PGE2 has been shown to exert anti-apoptotic and
proliferative effects which may promote carcinogenesis and to a
certain extent, abrogate the anti-tumor effects of proteasome
inhibition (10). In addition,
NS-398 (a Cox-2 highly selective inhibitor) has been demonstrated
to inhibit cell growth and induce apoptosis by inhibiting the NF-κB
pathway (11). Thus, Cox-2
inhibition is a potentially effective way to enhance the efficacy
of bortezomib.
In the present study, the RPMI8226 cell line was
used as a model to determine the in vitro effects of NS-398
and bortezomib on MM RPMI8226 cells, with the aim of investigating
the possibile treatment of MM by combined use of NS-398 and
bortezomib.
Materials and methods
Cell culture and reagents
RPMI8226 cells (American Type Culture Collection,
Manassas, VA, USA) were cultured in RPMI-1640 medium supplemented
with 10% fetal calf serum (Gibco-BRL, Grand Island, NY, USA) and
maintained in a 5% CO2 incubator at 37°C. The highly
selective Cox-2 inhibitor, NS-398 (Sigma, St. Louis, MO, USA), and
the bortezomib (LC Laboratories, Woburn, MA, USA) were dissolved in
dimethyl sulfoxide (DMSO; Sigma) and stored at −20°C. The two drugs
were diluted in culture medium (10−4-10−2
mol/l) with <0.1% DMSO immediately prior to use. The study was
approved by the Ethics Committee of Fujian Medical University,
Fuzhou, China.
Cell growth assay
RPMI8226 cells were seeded in triplicate at a
density of 5×103 cells/well in 96-well plates and 180 μl
RPMI-1640 medium containing various concentrations of bortezomib
and/or NS-398. The cells were cultured for 24, 48 or 72 h.
Subsequently, 20 μl of MTT substrate (5 mg/ml) was added to each
well, and the plates were returned to the standard cell incubator
for an additional 4 h. The supernatants were then carefully removed
and 200 μl DMSO was added to each well. After the insoluble
crystals were completely dissolved, colorimetric analysis was
performed at a wavelength of 570 nm. The inhibition rate was
calculated as follows: 100% - (individual OD value / control OD
value)%. Each independent experiment was performed three times. The
formula presented by Jin (12) was
calculated as: Q = Ea + b / (Ea + Eb - Ea × Eb). Q was the
combination index; Ea + b represented the inhibition rate of the
combined drug (A+B); and Ea and Eb represented the inhibition rates
of A and B, respectively. When the Q value ranged betweeb 0.85 and
1.15, the combined drug effect was a simple ‘arithmetic’ sum. A Q
value of >1.15 indicated a synergic effect, while a Q value of
<0.85 indicated an antagonistic effect.
Flow cytometric analysis of apoptosis and
the cell cycle
RPMI8226 cells were seeded in quadruplicate at a
density of 5×106 cells/well in 6-well plates and 3 ml of
RPMI-1640 medium containing various concentrations of bortezomib
and/or NS-398. Subsequent to culturing for 48 h, the cells were
collected, washed twice with ice-cold phosphate-buffered saline
(PBS) and fixed with 70% ethanol at 4°C overnight. Following
washing with PBS, the cells were incubated in 0.5 ml PBS containing
50 μg/ml RNase A for ~30 min at 37°C. Propidium iodide (PI) was
then added (Keygen, Nanjing, China) to a final concentration of 50
μg/ml and incubated for 30 min on ice in the dark. The resultant
cell suspension was then subjected to flow cytometry
(Becton-Dickinson, Franklin Lakes, NJ, USA). The percentage of the
apoptotic cells (sub-G1) and the cells in the G0/G1, S and G2/M
phases was calculated using CellQuest software
(Becton-Dickinson).
Cox activity assay
The activity of Cox-2 was measured using the COX
Activity Assay kit (Cayman Chemical, Ann Arbor, MI, USA) according
to the manufacturer’s instructions. The cells were collected at
4°C. Cell pellets were then homogenized in the cold buffer provided
with the kit and centrifuged at 10,000 × g at 4°C for 10 min. Then,
10 μl of supernatant was added into each well of a 96-well plate
and mixed with 150 μl assay buffer and 10 μl heme. The supernatant
samples, which were boiled for 5 min, were matched with each well
as the controls. The 96-well plate was carefully agitated for 1 min
and incubated at 37°C for 5 min, then 20 μl colorimetric substrate
and arachidonic acid were added to each well. The plate was
carefully agitated again and incubated at 37°C for 5 min.
Colorimetric analysis was performed at a wavelength of 570 nm.
Western blot analysis
The cells were collected and lysed at 4°C for 30
min. Once the protein concentration had been determined using the
Lowry method, equal amounts of protein were separated on 6–15%
SDS-PAGE gels. The protein was transferred to polyvinylidene
fluoride (PVDF) membranes, which were blocked with 5% skimmed milk
in TBST (tris-buffered saline solution containing 0.1% Tween-20) at
4°C overnight, and then incubated with antibodies for NF-κB, Cox-2,
c-Myc, Bcl-2, survivin, cyclin D1 and GAPDH (Santa Cruz
Biotechnology Inc., Santa Cruz, CA, USA) for 2 h at room
temperature. Following washing with TBST, the membranes were
incubated with horseradish peroxidase (HRP)-labeled secondary
antibodies for 1 h at 37°C and then developed using the enhanced
chemiluminescence (ECL) reagent kit (Beyotime, Jiangsu, China).
Statistical analysis
Data were expressed as the mean ± standard deviation
(SD) and the Student’s t-test was used to determine the
significance of the differences between the drugs and controls.
P<0.05 was considered to indicate a statistically significant
difference.
Results
NS-398 and bortezomib act synergistically
to inhibit the growth of RPMI8226 MM cells
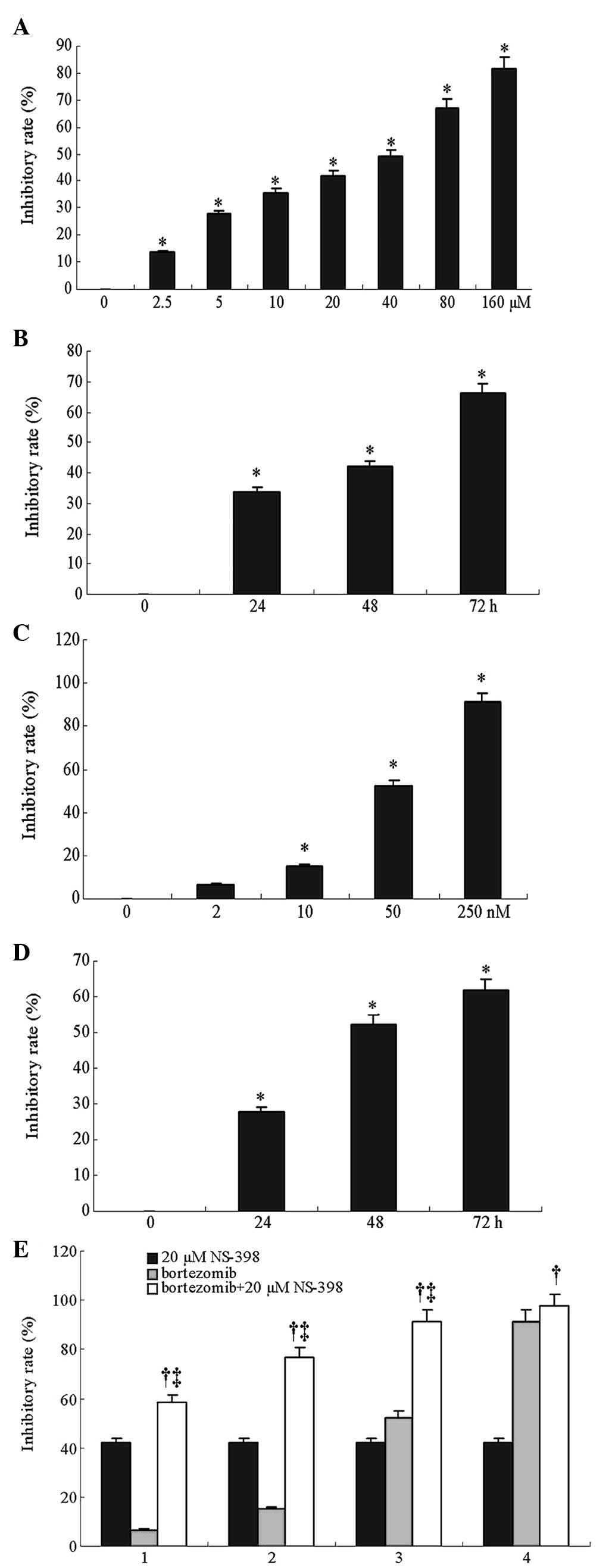
An MTT assay was performed to evaluate the effects
of NS-398 and bortezomib on the growth of the RPMI8226 cells. The
results showed that 20 μM NS-398 inhibited RPMI8226 cell growth
with inhibition rates of 33.7, 41.9 and 66.1% following 24-, 48-
and 72-h treatments, respectively. Moreover, exposure of the cells
to a 2.5–160 μM range of NS-398 for 48 h inhibited cell
proliferation in a dose-dependent manner. The half maximal
inhibitory concentration (IC50) of NS-398 for 48 h was
44.1 μM (Fig. 1A and B).
Similarly, bortezomib inhibited RPMI8226 cell growth in a dose- and
time-dependent manner. The IC50 of bortezomib for 48 h
was 48.4 nM (Fig. 1C and D). The
combination index was calculated to determine whether there was
synergy between NS-398 and bortezomib in the inhibition of MM cell
growth. The results showed that following treatment of the cells
with 2, 10, 50 and 250 nM of bortezomib for 48 h, the inhibitory
rate of cell growth was 6.7, 15.1, 52.3 and 91.2%, respectively,
while the inhibitory rate was 41.9% following treatment with 20 μM
NS-398. When 20 μM NS-398 was combined with these concentrations of
bortezomib, the inhibitory rate of cell growth was increased to
58.7, 76.9, 91.4 and 97.7%, respectively (P<0.05), and the
combined index was 1.28, 1.52, 1.26 and 1.03, respectively
(Fig. 1E). These results
demonstrate that NS-398 and bortezomib act synergistically by
inhibiting the growth of RPMI8226 MM cells. To better characterize
the synergistic action between the two drugs, 50 nM bortezomib and
20 μM NS-398 were used in the subsequent experiments.
NS-398 promotes bortezomib-induced
apoptosis and the cell cycle arrest of RPMI8226 MM cells
To analyze the synergistic action between NS-398 and
bortezomib, apoptosis and the cell cycle of the RPMI8226 cells
treated by these two drugs were examined. As shown in Fig. 2, following treatment of the
RPMI8226 cells with 50 nM bortezomib and 20 μM NS-398, the rate of
apoptosis was 22.4 and 19.6%, respectively. The combination of the
bortezomib and NS-398 treatments led to an apoptotic rate of 61.2%,
which was higher than the sum of the rate when the cells were
treated with each drug alone. In addition, Table I shows the percentage of cells
arrested in the G0/G1 phase as 47.5±3.8 and 41.6±3.3% following the
bortezomib and NS-398 treatments, respectively. These results were
higher than that of the control (25.4±3.3%; P<0.05). The
combined drug treatment led to a higher percentage of cells being
arrested in the G0/G1 phase. Taken together, these data suggest
that NS-398 promotes bortezomib-induced apoptosis and the cell
cycle arrest of MM cells.
 | Table INS-398 and/or bortezomib-induced cell
cycle arrest in RPMI8226 cells. |
Table I
NS-398 and/or bortezomib-induced cell
cycle arrest in RPMI8226 cells.
| Treated types | G0/G1 (%) | S (%) | G2/M
(%) |
|---|
| Control | 25.4±3.4 | 51.3±3.5 | 23.3±1.5 |
| NS-398 | 41.6±3.3a | 38.6±2.7a | 19.8±1.4 |
| Bortezomib | 47.5±3.8a | 29.9±2.2a | 22.6±1.3 |
| Combination | 70.1±5.4b | 10.6±1.6b | 19.2±1.4 |
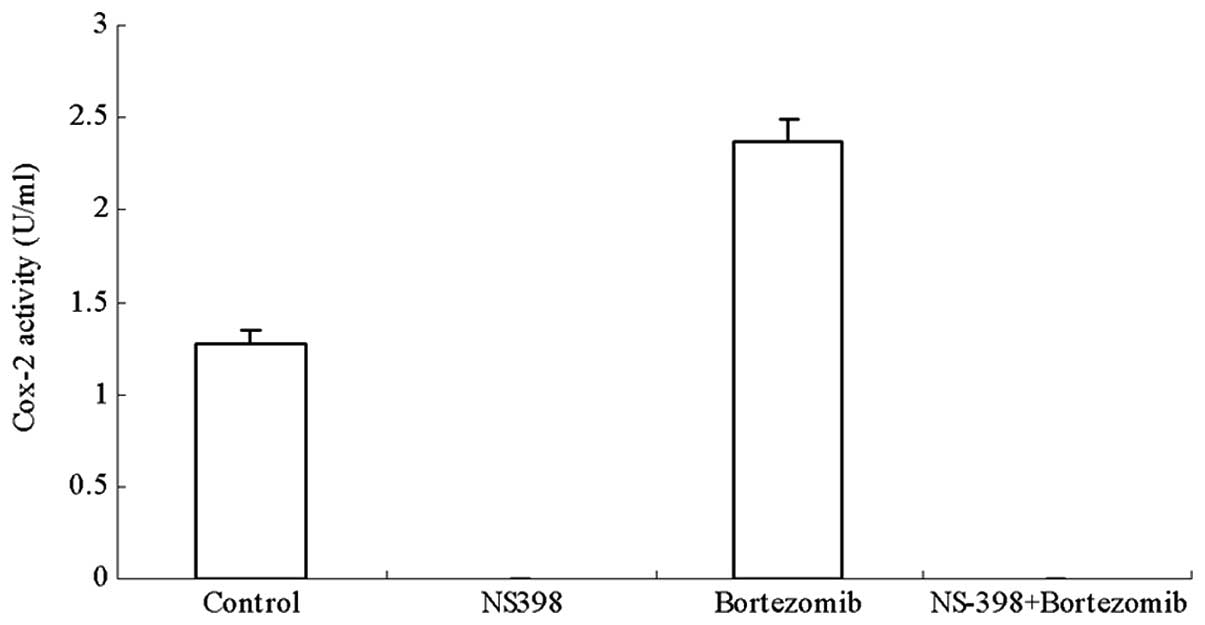
NS-398 inhibits bortezomib-induced Cox-2
activity in RPMI8226 MM cells
As shown in Fig. 3,
NS-398 completely suppressed the increased Cox-2 activity induced
by bortezomib in the RPMI8226 cells (Fig. 3). To investigate whether this was
due to the downregulation of Cox-2 expression caused by NS-398
treatment, a western blot analysis was performed to investigate the
protein level of Cox-2. It was observed that NS-398 had no obvious
effect on the high Cox-2 protein level in the RPMI8226 cells
treated with bortezomib (Fig. 4),
suggesting that NS-398 inhibits the bortezomib-induced Cox-2
activity in RPMI8226 cells without modulating the expression of
Cox-2.
NS-398 promotes bortezomib-induced
downregulation of NF-κB signaling in RPMI8226 MM cells
It is well-known that bortezomib inhibits NF-κB
activity in MM cells. Therefore, we examined the activity of NF-κB
and the expression of its downstream targets, including Bcl-2,
c-Myc, cyclin D1 and survivin in RPMI8226 MM cells. Western blot
analysis showed that the levels of NF-κB p65, a marker of the
activation of NF-κB signaling, as well as those of Bcl-2, c-Myc,
cyclin D1 and survivin, were significantly decreased upon combined
treatment with NS-398 and bortezomib, compared with the untreated
control or treatment with NS-398 or bortezomib alone. These results
demonstrate that NS-398 and bortezomib act synergistically to
inhibit NF-κB signaling in RPMI8226 MM cells.
Discussion
Cox-2 is important in the regulation of growth and
apoptosis in tumor cells (13,14).
Previous studies have indicated that Cox-2 is highly expressed in
MM, and its overexpression has often been used as an indicator of
poor prognosis (15). Cox-2
knockdown has been shown to induce the growth inhibition and
apoptosis of RPMI8226 MM cells (16). NS-398 has been demonstrated to
inhibit growth and induce the apoptosis of MM cells through
Cox-2-dependent and -independent pathways (17). A previous study showed that the
combination of bortezomib and aspirin, a classical Cox inhibitor,
acted synergistically to inhibit proliferation and induce the
apoptosis of human colorectal cells (10). However, to the best of our
knowledge, no studies have been reported on the synergistic effects
between NS398 and bortezomib in MM cells.
In the present study, NS-398 and bortezomib
inhibited RPMI8226 cell growth in a time- and dose-dependent
manner. When 20 μM NS-398 was combined with various concentrations
(2–250 nM) of bortezomib, the combined index ranged between 1.26
and 1.52, with the exception of 1.03, when a combination of 20 μM
of NS-398 and 250 nM bortezomib was used, suggesting that this
combination had synergetic growth inhibitory effects on the
RPMI8226 cells.
To elucidate the mechanisms by which NS-398 enhanced
the cytotoxicity of bortezomib in the MM RPMI8226 cells, we first
examined apoptosis induction. Our data showed that the apoptotic
rate of the combined group was significantly higher than that of
either NS-398 or bortezomib treatment alone. This is in agreement
with a previous study which indicated that NS-398 enhanced the
growth inhibition of MM cells by dexamethasone or thalidomide
treatment, and that the synergic effect was mainly mediated by the
increase of apoptosis (18). In
addition, we showed that NS-398 promotes bortezomib-induced cell
cycle arrest of MM cells at the G0/G1 phase.
The sensitization of RPMI8226 cells to bortezomib
was thought to be associated with the decreased activity of Cox-2.
Our data indicated that NS-398 was able to completely inhibit Cox-2
activity, i.e., not only the endogenous activity in RPMI8226 cells,
but also the enhanced activity induced by the bortezomib treatment.
Notably, we found that NS-398 had no effects on Cox-2 expression,
which was in agreement with a previous study (10). Thus, our results indicate that the
NS-398-mediated inhibition of Cox-2 activity may increase the
cytotoxicity of bortezomib in the MM cells.
Cox-2 inhibitors are known to act synergistically
with chemotherapeutic agents in numerous cancer cells by
downregulating the NF-κB target genes, such as Bcl-2, c-Myc and
survivin (19–22). In the present study, we found that
several downstream targeted genes of NF-κB signaling, including
Bcl-2, c-Myc, cyclin D1 and survivin, were highly expressed in the
RPMI8226 cells, indicating that the activation of NF-κB signaling
contributes to the development of myeloma. Following the combined
treatment with NS-398 and bortezomib, the expression of these NF-κB
target genes was significantly decreased. These data indicate that
the inhibition of the NF-κB pathway is essential to the synergistic
effect of NS-398 and bortezomib on the MM cells.
Bortezomib is used in MM patients based on the
inhibition of NF-κB activity by the prevention of the proteasomal
degradation of IκBα. However, a previous study indicated that
bortezomib-induced cytotoxicity in MM cells is not associated with
the inhibition of NF-κB activity (23). This raised a question with regard
to whether the NF-κB pathway remains a major therapeutic target of
MM. Thus, we investigated the activity of NF-κB in the RPMI8226 MM
cells. Western blot analysis indicated that the level of NF-κB p65
in cells treated with a combination of bortezomib and NS-398 was
significantly lower than in cells treated with bortezomib or NS-398
alone. Taken together, these results show that NF-κB signaling is
involved in the synergistic action between NS-398 and bortezomib
against MM cells.
In summary, it was found that NS-398 and bortezomib
acted synergistically to inhibit cell growth, arrest the cell cycle
at the G1 phase and induce the apoptosis of MM cells. NS-398
enhanced the efficacy of bortezomib against MM cells in
vitro and this was associated with the inhibition of NF-κB
signaling. These findings suggest that the combined use of NS-398
and bortezomib may constitute a promising novel treatment protocol
for MM patients.
Acknowledgements
This study was supported by the National Natural
Science Foundation of China (grant no. 81172259).
References
|
1
|
Merchionne F, Perosa F and Dammacco F: New
therapies in multiple myeloma. Clin Exp Med. 7:83–97. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Richardson PG, Barlogie B, Berenson J, et
al: A phase 2 study of bortezomib in relapsed, refractory myeloma.
N Engl J Med. 348:2609–2617. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Hideshima T, Mitsiades C, Akiyama M, et
al: Molecular mechanisms mediating antimyeloma activity of
proteasome inhibitor PS-341. Blood. 101:1530–1534. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Nencioni A, Grünebach F, Patrone F, et al:
Proteasome inhibitors: antitumor effects and beyond. Leukemia.
21:30–36. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Markovina S, Callander NS, O’Connor SL, et
al: Bortezomib-resistant nuclear factor-kappaB activity in multiple
myeloma cells. Mol Cancer Res. 6:1356–1364. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Markovina S, Callander NS, O’Connor SL, et
al: Bone marrow stromal cells from multiple myeloma patients
uniquely induce bortezomib resistant NF-kappaB activity in myeloma
cells. Mol Cancer. 9:1762010. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Levine L: Proteasome inhibitors: their
effects on arachidonic acid release from cells in culture and
arachidonic acid metabolism in rat liver cells. BMC Pharmacol.
4:152004. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Rockwell P, Yuan H, Magnusson R and
Figueiredo-Pereira ME: Proteasome inhibition in neuronal cells
induces a proinflammatory response manifested by upregulation of
cyclooxygenase-2, its accumulation as ubiquitin conjugates, and
production of the prostaglandin PGE(2). Arch Biochem Biophys.
374:325–333. 2000. View Article : Google Scholar
|
|
9
|
Mbonye UR, Yuan C, Harris CE, et al: Two
distinct pathways for cyclooxygenase-2 protein degradation. J Biol
Chem. 283:8611–8623. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Voutsadakis IA, Patrikidou A, Tsapakidis
K, et al: Additive inhibition of colorectal cancer cell lines by
aspirin and bortezomib. Int J Colorectal Dis. 25:795–804. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Liu JF, Zhu GJ, Jamieson GG, et al: NS-398
induces apoptosis in human esophageal cancer cells through
inhibition of NF-kappaB downstream regulation of cyclooxygenase-2.
Cancer Invest. 27:17–23. 2009. View Article : Google Scholar
|
|
12
|
Jin ZJ: Addition in drug combination.
Zhongguo Yao Li Xue Bao. 1:70–76. 1980.(In Chinese).
|
|
13
|
Bae SH, Jung ES, Park YM, et al:
Expression of cyclooxygenase-2 (COX-2) in hepatocellular carcinoma
and growth inhibition of hepatoma cell lines by a COX-2 inhibitor,
NS-398. Clin Cancer Res. 7:1410–1418. 2001.PubMed/NCBI
|
|
14
|
Elder DJ, Halton DE, Crew TE and Paraskeva
C: Apoptosis induction and cyclooxygenase-2 regulation in human
colorectal adenoma and carcinoma cell lines by the
cyclooxygenase-2-selective non-steroidal anti-inflammatory drug
NS-398. Int J Cancer. 86:553–560. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Ladetto M, Vallet S, Trojan A, et al:
Cyclooxygenase-2 (COX-2) is frequently expressed in multiple
myeloma and is an independent predictor of poor outcome. Blood.
105:4784–4791. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Li QB, Chen ZC, You Y and Zou P: Small
interfering RNA of cyclooxygenase-2 induces growth inhibition and
apoptosis independently of Bcl-2 in human myeloma RPMI8226 cells.
Acta Pharmacol Sin. 28:1031–1036. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Ding J, Tsuboi K, Hoshikawa H, et al:
Cyclooxygenase isozymes are expressed in human myeloma cells but
not involved in anti-proliferative effect of cyclooxygenase
inhibitors. Mol Carcinog. 45:250–259. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Zhang M, Abe Y, Matsushima T, et al:
Selective cyclooxygenase 2 inhibitor NS-398 induces apoptosis in
myeloma cells via a Bcl-2 independent pathway. Leuk Lymphoma.
46:425–433. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Honjo S, Kase S, Osaki M, et al: COX-2
correlates with F-box protein, Skp2 expression and prognosis in
human gastric carcinoma. Int J Oncol. 26:353–360. 2005.PubMed/NCBI
|
|
20
|
Mizutani Y, Nakanishi H, Li YN, et al:
Enhanced sensitivity of bladder cancer cells to cisplatin mediated
cytotoxicity and apoptosis in vitro and in vivo by the selective
cyclooxygenase-2 inhibitor JTE-522. J Urol. 172:1474–1479. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Lin J, Hsiao PW, Chiu TH and Chao JI:
Combination of cyclooxygenase-2 inhibitors and oxaliplatin
increases the growth inhibition and death in human colon cancer
cells. Biochem Pharmacol. 70:658–667. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Kishimoto Y, Yashima K, Morisawa T, et al:
Effects of cyclooxygenase-2 inhibitor NS-398 on APC and c-myc
expression in rat colon carcinogenesis induced by azoxymethane. J
Gastroenterol. 37:186–193. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Hideshima T, Ikeda H, Chauhan D, et al:
Bortezomib induces canonical nuclear factor-κB activation in
multiple myeloma cells. Blood. 114:1046–1052. 2009.
|