Introduction
Steroid-induced avascular necrosis of the femoral
head (SANFH), frequently encountered in clinical practice, is a
progressive pathological process. It occurs due to obstruction of
the blood circulation in the femoral head induced by the improper
use of hormones. Additionally, SANFH is able to induce in
situ avascular necrosis and disability in a high proportion of
patients. When no treatment is provided to patients, the femoral
heads may deform or even collapse, impairing hip joint function and
causing permanent disability (1).
Currently, the incidence of SANFH is markedly increasing and has
the potential to become the leading cause of ANFH. Numerous
theories have been suggested in an attempt to explain the
development of SANFH, including fat embolism (2,3),
intravascular coagulation (3,4),
intrafemoral head pressure change (5) and osteoporosis (6). However, the pathological mechanism of
SANFH has not yet been fully elucidated (7–9).
According to previous studies (10–12),
the lipometabolism disorder and intravascular coagulation theories
may be used to explain the development of SANFH.
11β-hydroxysteroid dehydrogenases (11β-HSDs),
including 11β-HSD type 1 (11β-HSD1) and type 2 (11β-HSD2), are the
key metabolic enzymes for catalyzing the interconversion of active
glucocorticoids (cortisol and corticosterone) and inert 11-keto
forms (cortisone and 11-dehydrocorticosterone) (13). To date, numerous studies have
demonstrated that 11β-HSD1 and 11β-HSD2 affect fat cell and
endotheliocyte function (14–17).
During fat cell and endotheliocyte dysfunction, an abnormal blood
flow in terminal vessels is induced and causes complications,
including intravascular coagulation, microcirculation disturbance
and vascular embolization, which may lead to femoral head necrosis
(17). Therefore, the expression
of 11β-HSD1 and 11β-HSD2 may be important in SANFH development.
To the best of our knowledge, no previous studies
have investigated the association between 11β-HSD and SANFH.
Results of the present study revealed the correlation between
11β-HSD and SANFH, via the determination of 11β-HSD1 and 11β-HSD2
expression in a rabbit model; this may have a significant reference
value for the use of steroids in clinical practice.
Materials and methods
Animals
Healthy adult New Zealand rabbits (weight, 2.6–3.2
kg) were obtained from the Experimental Animal Center of Zhongnan
Hospital of Wuhan University (Wuhan, China). All the experimental
protocols used in this study were approved by the Animal Care and
Use Committee of our Hospital.
Preparation of the SANFH rabbit model and
sample collection
The SANFH rabbit model was prepared according to
previously described methods (1,18),
with minor modifications. Briefly, 48 healthy New Zealand rabbits
were randomly divided into 3 groups (n=16/group); the normal (no
treatment), control (vehicle only) and treatment groups (SANFH
model). Rabbits of the control and treatment groups were injected
with horse serum (10 ml/kg; HyClone Laboratories, Inc., Logan, UT,
USA) through an ear vein. After 2 weeks, 6 ml/kg horse serum was
similarly injected once a day for 2 days, followed by an injection
of 20 mg/kg methylprednisolone (Pharmacia and Upjohn Company,
Puurs, Belgium) into the abdomen of rabbits in the treatment group
twice a week for 2 weeks. Following the methylprednisolone
injection, 200,000 units of penicillin was injected into the
buttock of each rabbit. In the normal and control groups, an equal
amount of saline was injected into the buttock muscle. Three
animals from each group were examined using magnetic resonance
imaging (MRI) and histopathological analysis 2 weeks after the
hormone injection. Additionally, the blood and femur head samples
of 3 animals from each group were collected 0 (prior to hormone
injection), 2, 4 and 8 weeks after the hormone injection.
MRI
An orthogonal head coil was placed on the
anesthetized rabbit, with its center located on the hip joint, and
the fast spin echo (SE) was used. T2-weighted imaging
(T2WI; TR/TE, 2500/74 ms),
T1-weighted imaging (T1WI; TR/TE,
420/20 ms) and T2WI fat-suppression sequence
(FS-T2WI) were performed twice at the coronal
position.
Histopathological analysis
Following the sacrifice of each rabbit, both femoral
heads, including the metaphyses and thigh-bones, were removed. The
tissue sections were dissected and fixed in 10% formalin, embedded
in paraffin, cut into 5-μm-thick sections, de-paraffinized,
rehydrated using standard techniques and stained using hematoxylin
and eosin (H&E). The histopathological changes were evaluated
using a microscope (Olympus, Tokyo, Japan).
Determination of total cholesterol and
triglyceride contents
The automatic biochemistry analyzer (Advia 1650;
Siemens Medical Solutions, Erlangen, Germany) was used to determine
the total cholesterol and triglyceride contents in the blood
samples of SANFH rabbits.
Western blotting
Total proteins of the femoral head tissue were
extracted and equal amounts of proteins (75 μg) were separated
using sodium dodecyl sulfate-polyacrylamide gel electrophoresis
(SDS-PAGE) and blotted on polyvinylidene difluoride (PVDF)
membranes. These were probed with anti-HSDB1 and anti-HSDB2 rabbit
polyclonal IgG (Wuhan Boster Bio-Engineering Co., Ltd., Wuhan,
China) and subsequently with goat anti-rabbit/HRP IgG (Wuhan Boster
Bio-Engineering Co., Ltd.), and detected using chemiluminescence.
To determine the protein loading, antibodies against β-actin were
used.
Statistical analysis
All the experiments were conducted in triplicate (at
least) and the data are presented as the mean ± standard deviation
(SD). The data were evaluated using one-way ANOVA, followed by
Dunnett’s multiple comparisons test between different groups. The
statistical significance of differences was analyzed using SPSS
software (SPSS for Windows 15.0; SPSS Inc., Chicago, IL, USA) and
P<0.05 was considered to indicate a statistically significant
difference.
Results
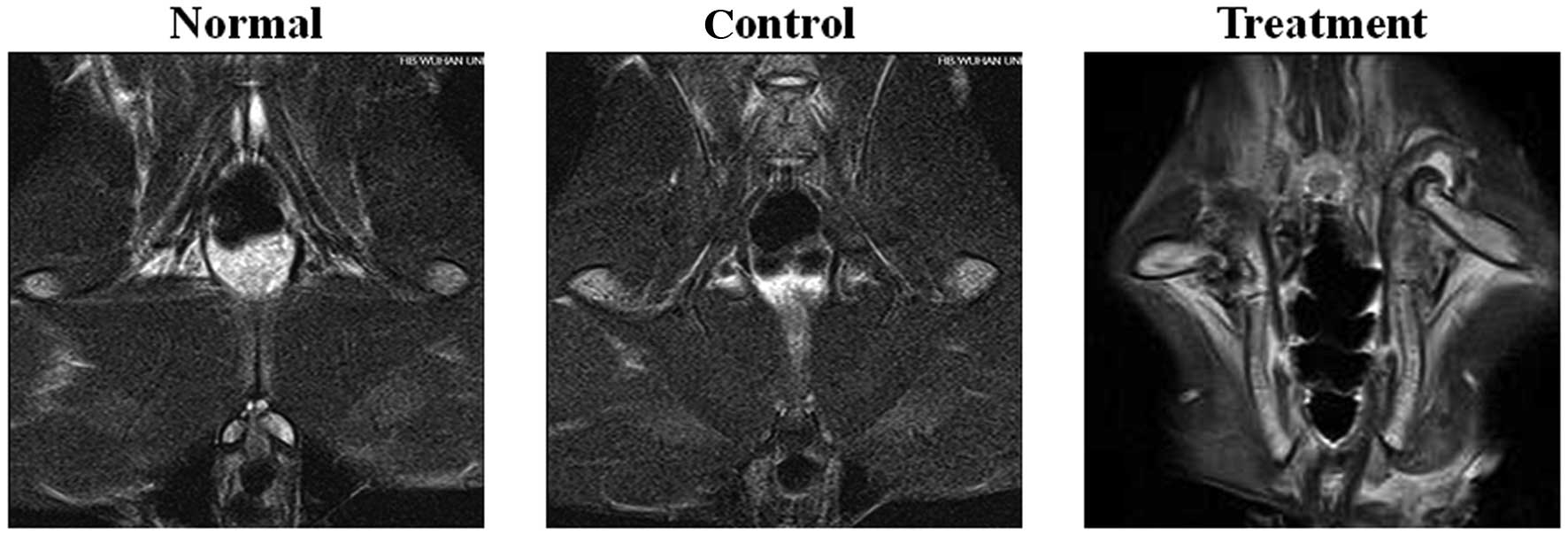
MRI examination
The femoral heads of rabbits in the normal and
control groups were symmetrical. In FS-T2WI,
low fat level signals were observed in the cortex of the femoral
head in the normal and control groups. By contrast, MRI in the
treatment group revealed a larger articular cavity of the femoral
head in rabbits of the treatment group. The high-level
FS-T2WI signal at the metaphyses indicated
that edema was present in the bone marrow in the treatment group
(Fig. 1).
Histopathological observations
As shown in Fig. 2,
changes in the periosteum, cartilage, trabeculae and hematopoietic
organization were observed. The periosteum of the femoral heads in
the control and normal groups were smooth, and cartilage cells were
arranged in an organized manner. The trabeculae were intact and
their arrangement was regular, compact and full. The bone cells in
the trabeculae were clearly visible with few empty bone lacunae.
There were abundant medullary hematopoietic and small fat cells
with a normal morphology. By contrast, the periosteum of the
femoral heads in the treatment group was incomplete with partially
shed cartilage cells. There were a few thin trabeculae with a
disordered texture and a number of trabeculae were broken into
fragments. A few spindle-shaped osteoblasts were distributed along
the trabeculae. The medullary hematopoietic areas were poorly
organized, with fewer cells, a sparse capillary network and
partially obstructed blood vessels (Fig. 2).
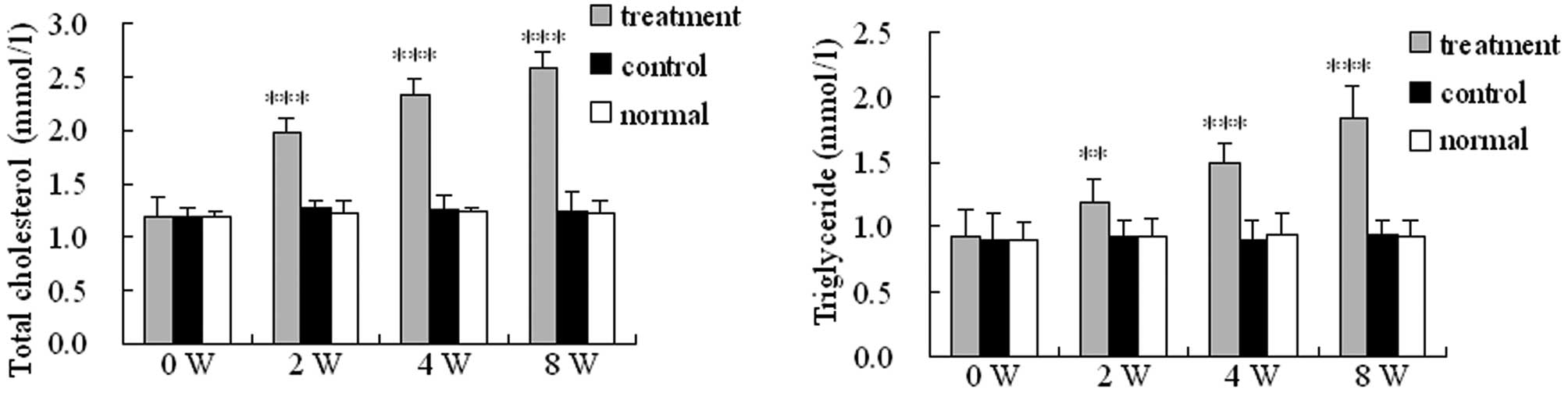
Levels of total cholesterol and
triglycerides in the blood of SANFH rabbits
The total cholesterol and triglyceride levels in the
blood of rabbits in the treatment group were significantly higher
compared with those of the control and normal groups (P<0.01;
Fig. 3). Furthermore, the total
cholesterol and triglyceride levels in the blood of rabbits in the
treatment group gradually increased following injection until the
end of the observation period.
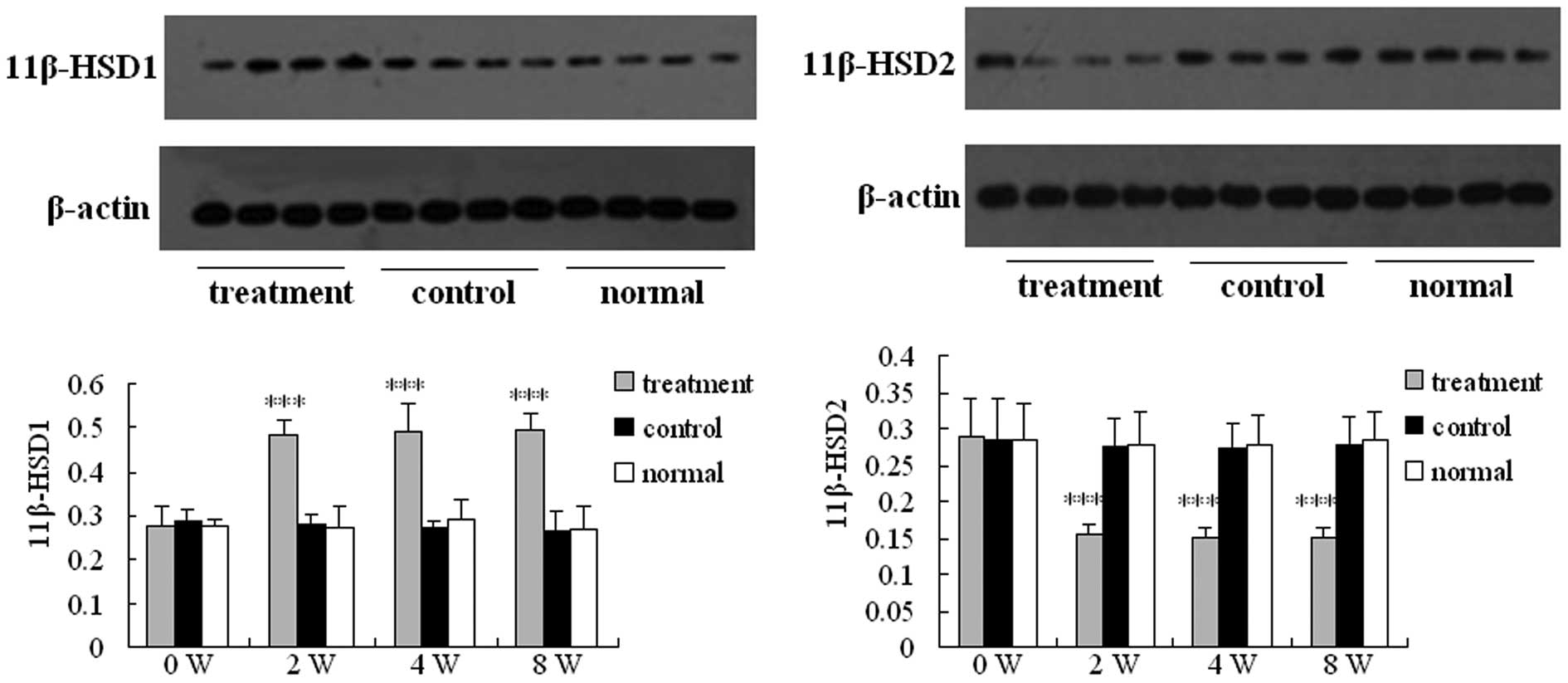
Western blotting
The expression levels of 11β-HSD1 and 11β-HSD2
proteins were determined using western blotting (Fig. 4). The expression levels of 11β-HSD1
and 11β-HSD2 proteins in the control and normal groups maintained a
stable level. By contrast, the expression levels of 11β-HSD1
protein in the treatment group increased 2 weeks after the
injection, whereas the expression levels of 11β-HSD2 protein in the
treatment group decreased 2 weeks after the injection.
Additionally, the expression levels of 11β-HSD1 protein in the
treatment group were significantly higher compared with those of
the control and normal groups at each time-point after the
injection (P<0.001). However, the expression levels of 11β-HSD2
protein in the treatment group were significantly lower compared
with those of the control and normal groups at each time point
after the injection (P<0.001). The relative protein level was
normalized to the intensity of β-actin, as determined by western
blotting.
Discussion
Steroids have been used in clinical practice to
inhibit inflammation, allergy and immune responses in numerous
diseases. In 2003, steroids were widely used to treat severe acute
respiratory syndrome (19).
However, serious adverse reactions may be induced by steroid use,
with SANFH being one of the most common. According to the results
of previous studies, an increasing number of ANFH cases caused by
steriod use have been reported and SANFH often occurs at an age of
30–50 years. Without treatment, hip joint dysfunction occurs in
SANFH patients and this disease is considered to be an irreversible
process (20,21). Numerous therapeutic methods have
previously been used to treat SANFH, including hip arthroplasty,
prosthesis and reconstructive surgery of the femoral head, in
addition to treatments without surgery; however, these treatments
were not particularly effective (22–24).
Furthermore, the pathogenesis of SANFH remains unclear. Thus,
investigation into the pathogenesis of SANFH and novel therapeutic
strategies is urgently required.
In the present study, a rabbit model of SANFH was
established to explore potential molecular mechanisms underlying
SANFH development. MRI and histopathological analyses were used to
evaluate the SANFH rabbit model and these determined that the model
had been successfully established and was able to be used for
further investigation in this study.
Metabolic disorder has been hypothesized to be the
mechanism that underlies SANFH development. Following high hormone
intake, the serum concentration levels of lipids, including total
cholesterol and triglycerides, are increased, leading to
hyperlipidemia. This may cause fat embolism to be induced in the
peripheral vessels, leading to intravascular coagulation (3,7,25).
Subsequently, fibrin platelets are formed and thrombopoiesis
occurs, which induces osteonecrosis via microcirculation
disturbance in the femoral head (26–28).
Results of the present study demonstrated that the total
cholesterol and triglyceride levels in the blood of rabbits treated
with methylprednisolone were significantly increased compared with
those of the control and normal groups; these results provide
evidence in support of the metabolic disorder theory.
According to the results of previous studies,
glucocorticoids are able to affect fat metabolism and
endotheliocyte function, and may be associated with the necrosis of
the femoral head (17).
Additionally, glucocorticoids cause serious intramedullary fatty
infiltration and induce a diminished blood flow. Complications,
including intravascular coagulation, microcirculation disturbance
and vascular embolization, are induced by fat cell and
endotheliocyte dysfunction, which subsequently lead to ANFH.
11β-HSD1 and 11β-HSD2 are the two key enzymes that catalyze the
conversion of inert 11-keto forms (cortisone) to active cortisol,
or vice versa, thus regulating the access of glucocorticoids to
steroid receptors (13). Results
of the present study showed that the expression levels of 11β-HSD1
protein were increased, while the expression levels of 11β-HSD2
protein were decreased following injection. Therefore, these
results indicate that 11β-HSDs are important in the development of
SANFH and that 11β-HSDs are potentially important targets for
preventing the development of ANFH in steroid-treated patients.
References
|
1
|
Wen Q, Ma L, Chen YP, Yang L, Luo W and
Wang XN: A rabbit model of hormone-induced early avascular necrosis
of the femoral head. Biomed Environ Sci. 21:398–403. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Jones JP Jr: Intravascular coagulation and
osteonecrosis. Clin Orthop Relat Res. 277:41–53. 1992.PubMed/NCBI
|
|
3
|
Kawai K, Tamaki A and Hirohata K:
Steroid-induced accumulation of lipid in the osteocytes of the
rabbit femoral head. A histochemical and electron microscopic
study. J Bone Joint Surg Am. 67:755–763. 1985.PubMed/NCBI
|
|
4
|
Nishimura T, Matsumoto T, Nishino M and
Tomita K: Histopathologic study of veins in steroid treated
rabbits. Clin Orthop Relat Res. 334:37–42. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Wang GJ, Lennox DW, Reger SI, et al:
Cortisone-induced intrafemoral head pressure change and its
response to a drilling decompression method. Clin Orthop Relat Res.
159:274–278. 1981.PubMed/NCBI
|
|
6
|
Arlet J: Nontraumatic avascular necrosis
of the femoral head. Past, present, and future. Clin Orthop Relat
Res. 277:12–21. 1992.PubMed/NCBI
|
|
7
|
Jones JP Jr: Fat embolism and
osteonecrosis. Orthop Clin North Am. 16:595–633. 1985.PubMed/NCBI
|
|
8
|
Mont MA, Jones LC, Einhorn TA, Hungerford
DS and Reddi AH: Osteonecrosis of the femoral head. Potential
treatment with growth and differentiation factors. Clin Orthop
Relat Res. (Suppl 355): S314–S335. 1998.PubMed/NCBI
|
|
9
|
Drescher W, Bünger MH, Weigert K, et al:
Methylprednisolone enhances contraction of porcine femoral head
epiphyseal arteries. Clin Orthop Relat Res. 423:112–117. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Drescher W, Schneider T, Becker C, Hobolth
J, Rüther W, Hansen ES and Bünger C: Selective reduction of bone
blood flow by short-term treatment with high-dose
methylprednisolone: An experimental study in pigs. J Bone Joint
Surg Br. 83:274–277. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Feng Y, Yang SH, Xiao BJ, Xu WH, Ye SN,
Xia T, Zheng D, Liu XZ and Liao YF: Decreased in the number and
function of circulation endothelial progenitor cells in patients
with avascular necrosis of the femoral head. Bone. 46:32–40. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Fujioka M, Kubo T, Nakamura F, Shibatani
M, Ueshima K, Hamaguchi H, Inoue S, Sugano N, Sakai T, Torii Y,
Hasegawa Y and Hirasawa Y: Initial changes of non-traumatic
osteonecrosis of femoral head in fat suppression images: bone
marrow edema was not found before the appearance of band patterns.
Magn Reson Imaging. 19:985–991. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Seckl JR and Walker BR: Minireview:
11beta-hydroxysteroid dehydrogenase type 1- a tissue-specific
amplifier of glucocorticoid action. Endocrinology. 142:1371–1376.
2001.PubMed/NCBI
|
|
14
|
Nakano D and Nishiyama A: Programmed
11β-hydroxysteroid dehydrogenase type 2 reduction: a possible cause
of adult-onset disease? J Hypertens. 29:201–203. 2011.
|
|
15
|
Kaur K, Hardy R, Ahasan MM, et al:
Synergistic induction of local glucocorticoid generation by
inflammatory cytokines and glucocorticoids: implications for
inflammation associated bone loss. Ann Rheum Dis. 69:1185–1190.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Kitajima M, Shigematsu M, Ogawa K, et al:
Effects of glucocorticoid on adipocyte size in human bone marrow.
Med Mol Morphol. 40:150–156. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Kerachian MA, Séguin C and Harvey EJ:
Glucocorticoids in osteonecrosis of the femoral head: a new
understanding of the mechanisms of action. J Steroid Biochem Mol
Biol. 114:121–128. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Matsui M, Saito S, Ohzono K, et al:
Experimental steroid-induced osteonecrosis in adult rabbits with
hypersensivity vasculitis. Clin Orthop Relat Res. 277:61–72.
1992.PubMed/NCBI
|
|
19
|
Shi B, Li G, Wang P, Yin W, Sun G, Wu Q
and Yu G: Effect of antler extract on corticosteroid-induced
avascular necrosis of the femoral head in rats. J Ethnopharmacol.
127:124–129. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Jones LC and Hungerford DS: Osteonecrosis:
etiology, diagnosis, and treatment. Curr Opin Rheumatol.
16:443–449. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Hungerford DS: Treatment of osteonecrosis
of the femoral head: everything’s new. J Arthroplasty. 22(Suppl 1):
91–94. 2007.
|
|
22
|
Beris AE, Payatakes AH, Kostopoulos VK, et
al: Non-union of femoral neck fractures with osteonecrosis of the
femoral head: treatment with combined free vascularized fibular
grafting and subtrochanteric valgus osteotomy. Orthop Clin North
Am. 35:335–343. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Nelson CL, Garrison RL, Walz BH and
McLaren SG: Resurfacing of only the femoral head - treatment for
young patients with osteonecrosis of the femoral head with
collapse, delamination and significant head involvement. J Ark Med
Soc. 100:162–163. 2003.PubMed/NCBI
|
|
24
|
Asano T, Takahashi KA, Fujioka M, et al:
ABCB1 C3435T and G2677T/A polymorphism decreased the risk for
steroid-induced osteonecrosis of the femoral head after kidney
transplantation. Pharmacogenetics. 13:675–682. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Jones JP Jr and Sakovich L: Fat embolism
of bone. A roentgenographic and histological investigation, with
use of intra-arterial lipiodol, in rabbits. J Bone Joint Surg Am.
48:149–164. 1966.PubMed/NCBI
|
|
26
|
Jones JP: Epidemiologlcal risk factors for
non-traumatic osteonecrosis. Orthopade. 29:370–379. 2000.(In
German).
|
|
27
|
Ichiseki T, Matsumoto T, Nishino M,
Kaneuji A and Katsuda S: Oxidative stress and vascular permeability
in steroid-induced osteonecrosis model. J Orthop Sci. 9:509–5l.
5:2004.PubMed/NCBI
|
|
28
|
Starklint H, Lausten GS and Arnoldi CC:
Microvascular obstruction in avascular necrosis.
Immunohistochemistry of l4 femoral heads. Acta Orthop Scand.
66:9–12. 1995. View Article : Google Scholar : PubMed/NCBI
|