Introduction
Acute lung injury (ALI) and its more severe form,
acute respiratory distress syndrome (ARDS), characterized by
non-cardiogenic pulmonary edema that results from the disruption of
the alveolar-capillary barrier and pulmonary capillary
permeability, are severe diseases with high clinical morbidity and
mortality. Although previous studies have reported a reduction in
mortality, due to the implementation of lung-protective ventilation
strategies, the mortality rate remains high (~40%) (1,2). One
of the main pathogenetic factors is sepsis and certain studies have
demonstrated that sepsis-induced ALI/ARDS is closely associated
with levels of lipopolysaccharide (LPS) in plasma (3). Systemic inflammatory response
syndrome (SIRS) is induced by pro- and anti-inflammatory cytokine
imbalance and has a detrimental role in LPS-induced ALI/ARDS. LPS
causes the simultaneous upregulation or downregulation of the
expression of specific inflammatory factors, which leads to changes
in the DNA methylation of these factors. LPS induces the
hypermethylation of the TNF-α promoter in human THP-1 monocytes. A
previous study indicated that epigenetics are significant in the
inflammatory process, regardless of whether it occurs locally or
systemically (4). Other studies
have demonstrated that IL-8 activation in human intestinal
epithelial cells is accompanied by H3K4, H3K9 and H3K27 methylation
at the IL-8 gene promoter following LPS stimulation (5). In addition, LPS induces aberrant
hypermethylation of Hic-1 in mouse embryonic fibroblasts lacking
p53 in culture (6). These findings
led us to hypothesize that altered DNA methylation in lung tissues
may play a major role in LPS-induced ALI/ARDS.
Epigenetics, including DNA methylation, histone
modifications and non-coding RNAs, affect the expression of
individual genes, shape developmental patterns and contribute to
the maintenance of cellular memory required for developmental
stability and tissue-specific changes (7). DNA methylation, as a major form of
epigenetic modification, is an important mechanism for the
regulation of genome function. DNA methylation has a fundamental
role in the regulation of gene transcription without altering the
sequence of the DNA (8). CpG
islands, defined as short DNA regions of genome containing a high
frequency of CG dinucleotides, are often located in the promoter in
the 5′ flanking region of housekeeping genes and a number of
tissue-specific genes. Cytosines located at CpG dinucleotides
catalyze this chemical modification and are targeted primarily by
the DNA methyltransferase family (9). DNA methylation regulates gene
expression by inhibiting the binding of transcription factors to
cognate cis elements and by facilitating the binding of
methyl-CpG-binding proteins, which directly or indirectly affect
the histone code and lead to chromatin condensation to inhibit
transcription factor binding (10). In previous studies, the effect of
DNA methylation has been associated with cancer, cardiovascular
disease, mental illness and human autoimmune diseases. Within the
lungs, aberrant DNA methylation is associated with tumorigenesis
(11), airway inflammation
(12) and other diseases (13).
In the current study, genome-wide analysis of DNA
methylation in rat lung tissues with LPS-induced ALI/ARDS was
performed using methylated DNA immunoprecipitation (MeDIP) and the
Roche-NimbleGen Rat DNA methylation 385K CpG islands plus promoter
arrays. Based on results of the MeDIP and arrays, associated genes
and chromosomes were determined, the correlation between DNA
methylation and CpG density was determined and gene ontology (GO)
and pathway analysis was performed. These results are likely to
provide insight into the therapy and prognosis of LPS-induced
ALI/ARDS.
Materials and methods
Animals and reagents
Male Sprague-Dawley rats (6–8-weeks old) weighing
180–220 g, were obtained from the Vital River Laboratory Animal
Technology Co., Ltd. (Beijing, China). All animals were allowed
food and tap water ad libitum. All experimental procedures
were in accordance with the Declaration of Helsinki of the World
Medical Association. Protocols were also approved by the
Institutional Animal Care and Use Committee of Binzhou Medical
University. LPS (Escherichia coli LPS, 055:B5) was purchased
from Sigma-Aldrich (St. Louis, MO, USA).
LPS-induced ALI animal model
Rats were fasted overnight but allowed water ad
libitum prior to induction of ALI. Animals were anesthetized
using 40 mg/kg chloral hydrate. LPS [10 mg/kg in phosphate-buffered
saline (PBS)] was instilled intratracheally to induce ALI. The
control group underwent the same procedure with intratracheal
instillation of PBS (10 mg/kg).
Pulmonary histopathology
The lower lobe of the right lung tissue was
harvested 12 h following LPS or PBS administration and fixed in 4%
paraformaldehyde for 5 days at 4°C. The lobe was embedded in
paraffin and cut into 5-μm sections. Hematoxylin and eosin staining
was performed according to the standard method to assess the lung
injury.
Genomic DNA extraction and
fragmentation
Genomic DNA was extracted from four lung tissue
samples (control and LPS-induced ALI, n=2 each) using a DNeasy
Blood and Tissue kit (Qiagen, Hilden, Germany) according to the
manufacturer’s instructions. Briefly, the lung tissue was ground
using a homogenizer on ice, lysed with proteinase K and tissue
lysis buffer for 3 h and then precipitated and washed. The genomic
DNA quality and quantity was assessed using the Nanodrop
spectrophotometer (Thermo Fisher Scientific Inc., Wilmington, DE,
USA) and a A260/A280 ratio between 1.7 and
2.0 was considered a criterion for quality control. The genomic DNA
of each sample was sonicated between 200 and 1,000 bp with a
Bioruptor sonicator (Diagenode Inc., Denville, NJ, USA) on ‘LOW’
mode for 10 cycles of 30 sec ‘ON’ and 30 sec ‘OFF’.
MeDIP and microarray analysis
Sonicated genomic DNA (1 μg) was used for
immunoprecipitation with a mouse monoclonal anti-5-methylcytosine
antibody (Diagenode Inc.). DNA was heat-denatured at 94°C for 10
min, rapidly cooled on ice and immunoprecipitated with 1 μl primary
antibody overnight at 4°C with rocking agitation in 400 μl
immunoprecipitation buffer (0.5% BSA in PBS). A total of 200 μl
anti-mouse IgG magnetic beads were added and the mixture was
incubated to recover the immunoprecipitated DNA fragments for an
additional 2 h at 4°C with agitation. Following
immunoprecipitation, five immunoprecipitation washes were performed
with ice-cold immunoprecipitation buffer. Washed beads were
resuspended in TE buffer with 0.25% sodium dodecyl sulfate (SDS)
and 0.25 mg/ml proteinase K for 2 h at 65°C and then allowed to
cool to room temperature. MeDIP DNA was purified using Qiagen
MinElute columns (Qiagen). MeDIP-enriched DNA was amplified using
the GenomePlex® Complete Whole Genome Amplification kit from
Sigma-Aldrich. Amplified DNA samples were purified using the
QIAquick PCR purification kit (Qiagen). Purified DNA was quantified
using the ND-1000 Nanodrop. For DNA labeling, the NimbleGen
Dual-Color DNA Labeling kit was used according to the
manufacturer’s instructions (NimbleGen Systems, Inc., Madison, WI,
USA). The DNA (1 μg) of each sample was incubated for 10 min at
98°C with 1 OD or 40 μl of Cy5-9mer (MeD1P sample) or Cy3-9mer
(input sample) primers. Next, 100 pmol deoxynucleoside
triphosphates and 100 units Klenow fragment (New England Biolabs,
Inc., Ipswich, MA, USA) were added. The mix was incubated at 37°C
for 2 h. The reaction was terminated by adding 10 μl 0.5 M EDTA and
the labeled DNA was purified by isopropanol/ethanol precipitation.
Microarrays were hybridized at 42°C for 16–20 h with Cy3/5-labeled
DNA in NimbleGen hybridization buffer/hybridization component A in
a hybridization chamber (Hybridization System, NimbleGen Systems).
Following hybridization, washing was performed using the NimbleGen
Wash Buffer kit. For array hybridization, Roche-NimbleGen’s Rat
Promoter plus CpG Island array was used. The array has a 385K
format array design containing gene promoters [−1,300 to +500 bp of
the start site of the transcript (TSS)]. A total of 15,809 CpG
islands were covered by ~385,000 probes. Array data were extracted
and analyzed using NimbleScan and SignalMap software. Only genes
with consistent differences between the two control and two LPS
groups were considered.
Quantitative reverse
transcription-polymerase chain reaction (qRT-PCR) confirmation of
gene methylation changes
A MeDIP assay, combined with qPCR, was used to
quantitatively evaluate the methylation status of candidate genes
in the lung tissues derived from control and ALI/ARDS groups. MeDIP
was performed as described. Purified DNA from the
immunoprecipitated DNA complexes and the input DNA was analyzed by
qRT-PCR on the ABI PRISM 7900 system (Applied Biosystems, Bedford,
MA, USA). Primers used were as follows: Mapk3, forward
5′-CCCTTCAGACTGCTTCCTCA-3′ and reverse 5′-CTT GGGCTGTCAGACTTGGT-3′;
Pak1, forward 5′-GAATTT GTGGTACAGCAGGACAT-3′ and reverse
5′-CCACTGAGG CTATCTTTGACG-3′; Rac2, forward 5′-TTACCCATCACC
CACCACC-3′ and reverse 5′-TTCCGTTTCCTCCTGCCTC-3′. Relative changes
in gene methylation were determined by measuring the amount of
detected genes in immunoprecipitated DNA following normalization
against input DNA.
Statistical analysis
Data are expressed as the mean ± SD. For the
chromosome distribution of genes and the number of genes in high
CpG density promoters (HCP), intermediate CpG density promoters
(ICP) and low CpG density promoters (LCP), positive/negative genes
were compared using the Chi-square test. P<0.05 was considered
to indicate a statistically significant difference (14).
Results
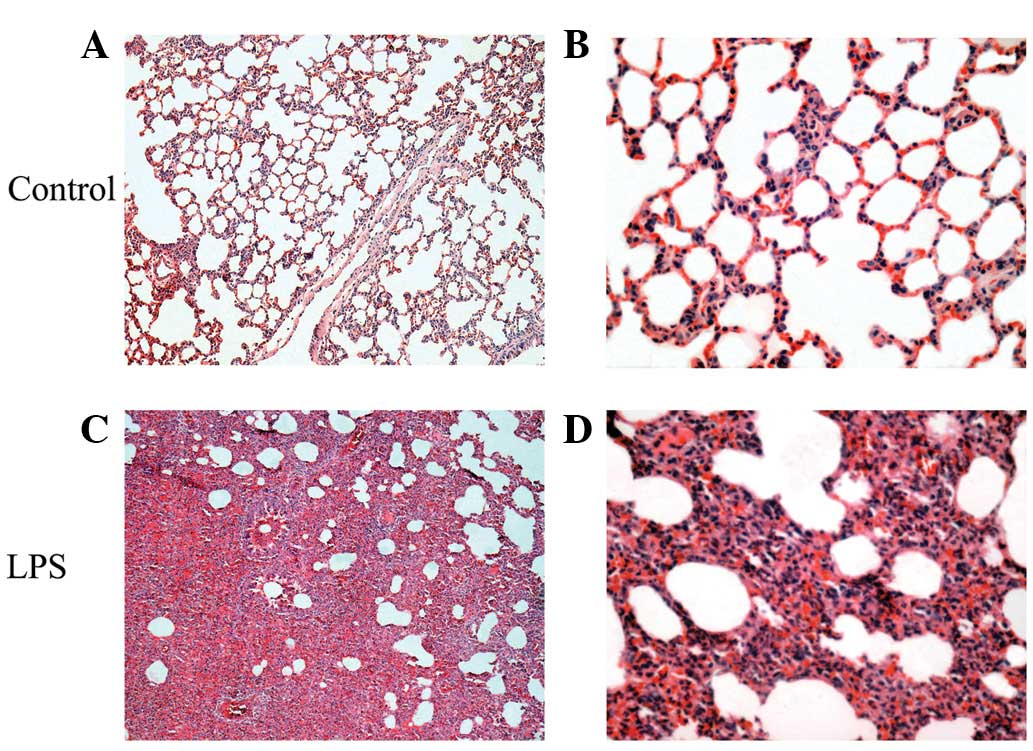
Histological changes in lung tissues
No histological alterations were found in the
control group (Fig. 1A and B). In
the LPS-induced ALI/ARDS group, microscopic changes were observed
12 h following LPS administration. The observed inflammatory
alterations were characterized by alveolar wall thickness, alveolar
and interstitial edema and hemorrhage, interstitial infiltration by
neutrophils and the complete consolidation of a section of the lung
tissue (Fig. 1C and D).
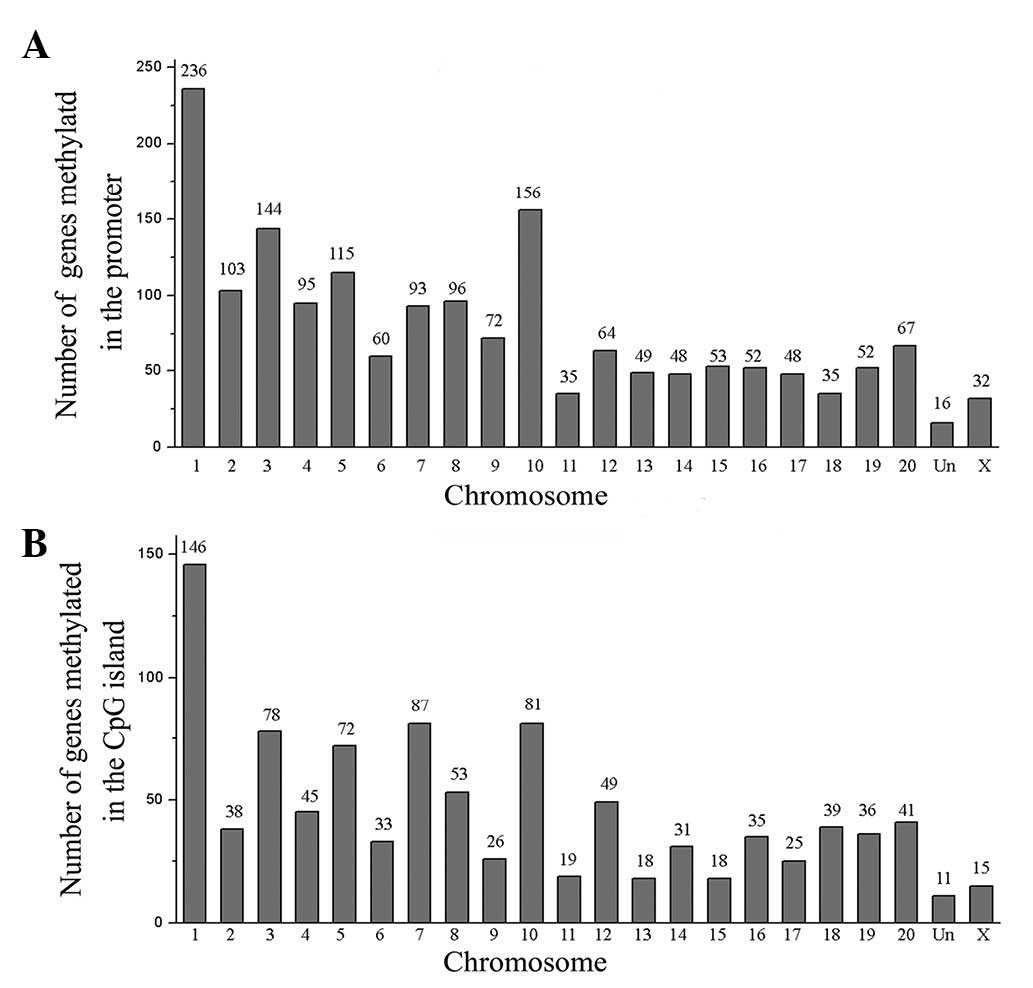
Chromosomal distribution
LPS-induced DNA methylation alterations were
initially observed in the chromosome. A total of 1,721 candidate
genes methylated in the promoter region were distributed across all
chromosomes (Fig. 2A). The results
indicate that the gene number of the chromosomes was statistically
significant: 236 genes on chromosome 1 (13.7%, P<0.01), 144
genes on chromosome 3 (8.4%, P<0.01), 115 genes on chromosome 5
(6.7%, P<0.01) and 156 genes on chromosome 10 (9.1%, P<0.01).
The 990 candidate genes methylated in the CpG island were also
distributed across all chromosomes (Fig. 2B). Results indicate that the gene
number of the chromosomes was statistically significant: 146 genes
on chromosome 1 (14.7%, P<0.01), 78 genes on chromosome 3 (7.9%,
P<0.01), 72 genes on chromosome 5 (7.3%, P<0.01), 81 genes on
chromosome 7 (8.2%, P<0.01) and 81 genes on chromosome 10 (8.2%,
P<0.01).
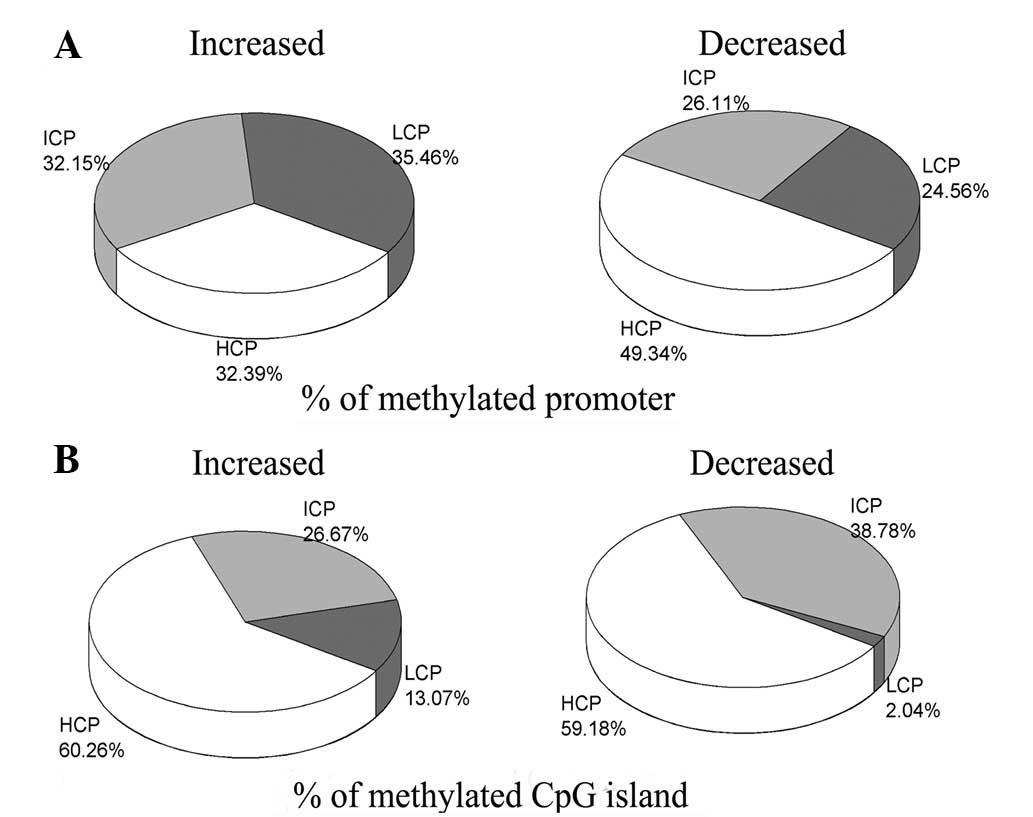
Levels of DNA methylation in the
promoters and CpG islands
The promoters were divided into three categories
based on CG content: HCP, ICP and LCP. The methylation level and
CpG density in the promoter were compared (Fig. 3A). In the group of genes in which
the degree of methylation was reduced by LPS-induced ALI/ARDs, the
number of methylated HCP genes was significantly higher (49.34%,
P<0.01) than the numbers of methylated ICP (26.11%) and LCP
(24.56%) genes. However, in the group of genes in which the degree
of methylation was increased by LPS-induced ALI/ARDs, the numbers
of methylated genes were similar in the HCP (32.39%), ICP (32.15%)
and LCP (35.46%) zones. In addition, differences in the methylation
levels of CpG islands were noted (Fig.
3B). In the decreased group, the number of methylated HCP genes
was also significantly higher (59.18%, P<0.01) than that of ICP
(38.78%) and LCP (2.04%) genes. A similar distribution was observed
in the increased group: the methylation of the HCP zone (60.26%)
was identified to be significantly higher (P<0.01) than that of
the ICP (26.67%) and LCP (13.07%) zones.
GO annotation and pathway analysis
The GO project provides a controlled vocabulary to
describe gene and gene product attributes in any organism
(http://www.geneontology.org). The
ontology covers three domains: biological process, cellular
component and molecular function. In the present study, GO Ontology
was used to perform GO term analysis of the 1,721 genes in the
methylated in the promoter region and the 990 genes methylated in
the CpG island. Results indicate that the candidate genes are
associated with 755 biological processes, 79 cellular components
and 93 molecular functions. Genetic studies of ALI/ARDS have
largely focused on candidate genes involved in the response to
external stimulus, intracellular signal transduction and negative
regulation of cell proliferation (15). Therefore, all genes in the three GO
terms were selected (Table I). GO
analysis of all candidate genes revealed 146 genes involved in the
response to external stimulus, 205 genes in intracellular signal
transduction and 65 genes in negative regulation of cell
proliferation. Next, 14 methylated genes from the GO term results
which are etiologically involved in the LPS-induced ALI/ARDS were
selected (Table II). The Kyoto
Encyclopedia of Genes and Genomes (KEGG) pathway database
(http://www.genome.jp/kegg) was used to
perform pathway analysis of these candidate genes. The analysis
divided the candidate genes into 38 signaling pathways, and the 10
enrichment pathways involved in immune and inflammatory responses
were selected. The included pathways were: neuroactive
ligand-receptor interaction, neurotrophin signaling pathway, MAPK
signaling pathway, cholinergic synapse, mTOR signaling pathway, Fcγ
R-mediated phagocytosis, regulation of actin cytoskeleton, vascular
endothelial growth factor (VEGF) signaling pathway, B cell receptor
signaling pathway and T cell receptor signaling pathway (Table III).
 | Table IGO annotation of the candidate genes
identified by microarray. Methylated genes involved in the response
to external stimulus, intracellular signal transduction and
negative regulation of cell proliferation. |
Table I
GO annotation of the candidate genes
identified by microarray. Methylated genes involved in the response
to external stimulus, intracellular signal transduction and
negative regulation of cell proliferation.
| GO term | Focus genes | Gene name |
|---|
| Intracellular signal
transduction | 205 |
ADRA2B//PAK1//MAPK3//MAP2K2//TGFB1//MAPK12//RGD1562846//CDKN1A//
FOXM1//HTR6//PTGER3//GCGR//CNR1//GNAZ//MC3R//GHRH//AVPR1B//
GLP2R//PTHLH//GNAS//ADRB1//ADORA2A//GALR1//ADCY5//DRD3//NPR3//
INSL3//GRM7//GRIK3//FZD1//NMUR1//CASR//ATP2B4//LAT//RCAN2//ALMS1//
RCAN3//SIK1//MARK2//SOCS3//MAP4K2//SRPK2//STK4//RPS6KA5//MAST1//
CARD9//CSNK2B//SNIP1//AZI2//RIPK2//AGT//ERC1//TRIB1//ROR2//STRADB//
DAB2IP//WNT7B//GAB1//MAPK8IP1//GH1//F2R//MRAS//RAB4B//ARL3//
RAB6A//RHOQ//ARHGEF7//RALB//ARFRP1//RAB35//ARL9//RAB40B//
RHOBTB1//RASL12//RAB40C//DNAJA3//RGD1307615//REM1//DIRAS1//RAC2//
RAB20//RAB1B//GRB2//SYNGAP1//RASSF1//RSU1//CDC42EP1//ARHGDIA//
XPA//PDE4D//MIF//FGF1//PIK3R1//PLEKHA1//LIME1//RELN//TGFB2//PTPN6//
GPER//UBE3A//TBXA2R//PDE7A//IGFBP1//FOXO3//RPS6KB1//EIF4EBP1//
DISC1//HIF1A//AKT1S1//TMEM127//CYTH1//IQSEC3//CYTH4//GSTP1//CRHR2//
TAOK1//STMN3//ARHGEF15//ARHGEF3//FARP1//PLEKHG4//RGNEF//NGFR//
LPAR1//LPAR2//SOX11//PTK6//IL6//IL3//PHLDA3//PDPK1//NUP62//SLC20A1//
ATP2C1//TRADD//CXXC5//UBE2V1//LTBR//MAVS//NEK6//GOLT1B//MYLK2//
P2RX7//PTPRC//INSR//FGFR2//LPAR3//FGFR1//FLT4//IGFBP4//AKT2//RPS6KB2//
MAP3K10//FZD5//LOC682999//SNAI1//CDC34//MECOM//SERPINF2//CD27//
LEPROT//RASA2//TNK1//CMKLR1//ADA//VEGFB//F7//TCF7L2//CCL11//DUSP6//
NDRG2//FGF21//ARHGAP8//MYBBP1A//CASP3//RGS7//ECEL1//UNC13A//
PDZD2//GUCY2E//SMAD7//CSPG4//PSEN2//DUSP1//PBP2//PLCL2//SPSB3//
ADCY1//PDZD8//ARHGAP29//HMHA1//PLCZ1//ASB10//SOCS5 |
| Negative regulation
of cell proliferation | 65 |
SULF1//KRIT1//TGFB1//ASCL2//CASP3//DLG1//SCGB1A1//PTPN6//GSTP1//LTA//
DAB2IP//APOD//PIK3R1//TRIB1//SF1//NDRG2//ANG1//GAL//LST1//SOX11//
FGFR2//GPC3//TGFB2//PTCH1//RUNX3//LRP6//KRT4//PAK1//TSPO//GATA2//
WT1//AGT//CEBPA//IL6//JUN//NOS1//FOXA3//BDKRB2//PHB//ADORA2A//F2R//
PPARG//BMP2//WISP2//PTGES//NUP62//GABBR1//INSL3//BECN1//CDKN1A//
ALDH1A2//PTPRU//TFF1//SOX7//ENPP7//ROR2//CDH5//TMEM127//STK4//
FZD5//PTPRF//DNAJA3//IRF6//LEFTY1//KLF13 |
| Response to external
stimulus | 146 |
BECN1//WIPI2//MAP1LC3A//ATG9A//CLN3//TRPM4//CCL11//PPARG//IL6//
ALOX5AP//MIF//F7//CNR1//LTA//CMKLR1//CXCL2//S100A8//CCR10//RAC2//
JUN//JUND//RELN//MGP//ACCN1//KCNA5//BMP2//HIF1A//P2RX7//CTSB//
RPS6KB1//PTCH1//ACCN3//NGFR//NKX2-1//PLA2G10//APBB1//SEMA6C//
SEMA3A//MYH10//TGFB2//EFNA2//NFASC//RUNX3//GDF7//EPHB3//PGRMC1//
KLF7//NRCAM//CEBPA//TBXA2R//PIK3R1//COX4I1//DDIT3//PLEC//CHAT//
SLC6A19//OPN4//TULP1//PDE6C//HOXA2//FOXA3//CARTPT//GAS2L1//PDE6B//
GRK1//TSPO//RARRES2//FOXG1//AGT//MAPK3//BAD//CYBA//BNIP3//LTBR//
HABP4//F2R//CR1L//ZIC2//ACE//GSTP1//LIPC//NOS1//INSR//SDS//TH//AKT2//
THRA//SLC1A2//CDKN1A//PRDM4//G6PD//GH1//CLPS//GHRH//GHSR//SOCS3//
PNLIPRP2//SLC22A3//IGF2R//PTGES//SCAMP3//RPL36AL//DUSP1//DKK1//
CLK2//LEFTY1//PITX2//ALDH1A2//ADA//ORM1//HMGCS1//ALPL//TGFB1//
TRIM25//EGR3//MYBBP1A//PFKFB1//AK3//ACADS//SSTR3//AANAT//DSCAM//
FOXE1//SCGB1A1//SETD6//ADORA2A//CNR2//HSPD1//DRD3//ATP2B2//CDH23//
FGF7//VEGFB//ZFP354A//LRP6//SERPINF2//LTC4S//PTK7//WNT7B//RXRB//
PTK6//FZD1//SKP2//LPAR1//MECOM//RPS6KA5 |
 | Table IIMethylated gene association studies
in acute lung injury and acute respiratory distress syndrome
(ALI/ARDS). |
Table II
Methylated gene association studies
in acute lung injury and acute respiratory distress syndrome
(ALI/ARDS).
| Gene symbol | Protein name | Description |
|---|
| Ace |
Angiotensin-converting enzyme | Catalyzes the
conversion of angiotensin I to angiotensin; plays a role in
regulation of blood pressure. |
| Akt2 | RAC-β
serine/threonine protein kinase | Involved in
phosphatidylinositol 3-kinase-mediated signaling. |
| Casp3 | Caspase-3 | Apoptotic
cysteine-aspartic acid protease that may play a role in neuronal
cell death regulation and other apoptotic processes. |
| Cebpb |
CCAAT/enhancer-binding protein β | Transcription
factor that binds to CCAAT motif on DNA and may facilitate IL-6
induced transcriptional activation. |
| Cxcl2 | C-X-C motif
chemokine 2 | Chemokine involved
in the pulmonary inflammatory response. |
| IL6 | Interleukin-6 | Cytokine involved
in development and possibly in neurodegenerative processes. |
| Mapk3 | Mitogen-activated
protein kinase 3 | Kinase involved in
intracellular signalling; component of MAPK signaling pathway. |
| Mif | Macrophage
migration inhibitory factor | Inhibits random
migration of macrophages and is involved in the pathogenesis of
several inflammatory diseases. |
| Mylk2 | Myosin light chain
kinase 2, skeletal/cardiac | Kinase;
phosphorylates a serine in the N-terminus of a myosin light
chain. |
| Pak1 |
Serine/threonine-protein kinase PAK 1 | Serine/threonine
protein kinase; binds and complexes specifically with activated
(GTP-bound) p21, leading to inhibition of p21 GTPase activity. |
| Rac2 | Ras-related C3
botulinum toxin substrate 2 | Exhibits GTPAse
activity, protein binding (homolog); involved in actin cytoskeleton
organization and biogenesis, bone resorption; associated with
neutrophil immunodeficiency syndrome. |
| Tgfb1 | Transforming growth
factor | Binds the TGF β
receptor; plays a role in regulation of cell growth and β-1
proliferation; induces synthesis of extracellular matrix proteins
and may play a role in fibrosis. |
| Tgfb2 | Transforming growth
factor β-2 | Binds the
transforming growth factor-β receptor; plays a role in regulation
of cell growth and proliferation; may be involved in
mesenchymal-epithelial cell interactions during development. |
| Vegfb | Vascular
endothelial growth factor B | Mouse homolog is a
growth factor; involved in the promotion of angiogenesis. |
 | Table IIIPathway analysis of the candidate
genes identified by microarray. |
Table III
Pathway analysis of the candidate
genes identified by microarray.
| Signaling
pathway | Focus genes | Gene name |
|---|
| Neuroactive ligand
receptor interaction | 55 |
ADORA2A//ADRA1D//ADRA2B//ADRB1//APLNR//AVPR1B//BDKRB2//
CHRM1//CHRM5//CHRNA4//CNR1//CNR2//CRHR2//DRD3//F2R//GABBR1//
GABRA3//GABRG3//GABRR3//GALR1//GCGR//GH1//GHSR//GLP2R//
GPR35//GRIA2//GRIK3//GRIK4//GRM7//HRH3//HTR1D//HTR6//LPAR1//
LPAR2//LPAR3//MC3R//MC5R//NMUR1//NTSR1//P2RX7//P2RY14//PPYR1//
PTGER3//PTGIR//SCTR//SSTR3//TAAR1//TAAR3//TAAR4//TAAR6//TAAR9//
TBXA2R//THRA//TSPO//UTS2R |
| Neurotrophin
signaling pathway | 25 |
AKT2//AKT3//ARHGDIA//BAD//CALML3//CAMK2G//FOXO3//GAB1//
GRB2//IRAK2//JUN//MAP2K2//MAPK12//MAPK3//NFKBIE//NGFR//NTF4//
PIK3R1//PIK3R2//PRDM4//PSEN2//RIPK2//RPS6KA5//YWHAG//YWHAH |
| MAPK signaling
pathway | 42 |
AKT2//AKT3//CACNB2//CACNG1//CACNG5//CACNG6//CACNG8//CASP3//
CHP2//DAXX//DDIT3//DUSP1//DUSP14//DUSP6//FGF1//FGF21//FGF7//
FGFR1//FGFR2//GRB2//JUN//JUND//MAP2K2//MAP4K2//MAPK12//MAPK3//
MAPK8IP1//MECOM//MRAS//NTF4//PAK1//PLA2G10//PLA2G2C//
PLA2G2F//PPP3R2//RAC2//RASA2//RPS6KA5//STK4//TAOK1//TGFB1//
TGFB2 |
| Cholinergic
synapse | 21 |
ADCY1//ADCY5//AKT2//AKT3//CAMK2G//CHAT//CHRM1//CHRM5//
CHRNA4//CREB3L3//GNB3//GNG7//GNG8//KCNJ2//KCNJ3//KCNJ4//
KCNJ6//MAPK3//PIK3R1//PIK3R2//SLC18A3 |
| mTOR signaling
pathway | 12 |
AKT2//AKT3//EIF4E//EIF4EBP1//HIF1A//MAPK3//PDPK1//PIK3R1//PIK3R2//
RPS6KB1//RPS6KB2//VEGFB |
| Fcγ R-mediated
phagocytosis | 19 |
AKT2//AKT3//AMPH//ARPC1B//ARPC2//ARPC4//DNM3//FCGR2A//LAT//
LIMK1//MAPK3//PAK1//PIK3R1//PIK3R2//PIP5K1B//PTPRC//RAC2//
RPS6KB1//RPS6KB2 |
| Regulation of actin
cytoskeleton | 33 |
ACTB//APC2//ARHGEF7//ARPC1B//ARPC2//ARPC4//BDKRB2//CHRM1//
CHRM5//F2R//FGD1//FGF1//FGF21//FGF7//FGFR1//FGFR2//ITGB4//LIMK1//
MAP2K2//MAPK3//MRAS//MYH10//MYL7//MYLK2//NCKAP1//PAK1//
PIK3R1//PIK3R2//PIP4K2B//PIP5K1B//PPP1CA//PPP1R12A//RAC2 |
| VEGF signaling
pathway | 14 |
AKT2//AKT3//BAD//CHP2//MAP2K2//MAPK12//MAPK3//PIK3R1//PIK3R2//
PLA2G10//PLA2G2C//PLA2G2F//PPP3R2//RAC2 |
| B cell receptor
signaling pathway | 15 |
AKT2//AKT3//CHP2//DAPP1//GRB2//JUN//MAP2K2//MAPK3//NFKBIE//
PIK3AP1//PIK3R1//PIK3R2//PPP3R2//PTPN6//RAC2 |
| T cell receptor
signaling pathway | 18 |
AKT2//AKT3//CDK4//CHP2//DLG1//GRB2//JUN//LAT//MAP2K2//MAPK12//
MAPK3//NFKBIE//PAK1//PIK3R1//PIK3R2//PPP3R2//PTPN6//PTPRC |
qRT-PCR validation of differential genes
in the microarrays
A subset of 3 genes, Mapk3, Pak1 and Rac2, that
reveal differential methylation between the control and ALI/ARDS
groups were validated using qRT-PCR to confirm the microarray
results independently. Mapk3 and Pak1 showed DNA methylation in the
control group. However, in the ALI/ARDS group, Rac2 was methylated.
A close correlation was observed between the microarray and qRT-PCR
data (Table IV), indicating the
accuracy of our microarray data and the significant induction in
the expression of candidate genes following LPS.
 | Table IVGene methylation changes determined
by quantitative reverse transcription-polymerase chain
reaction. |
Table IV
Gene methylation changes determined
by quantitative reverse transcription-polymerase chain
reaction.
| Gene | Sample | Input (Ct) | IP (Ct) | % |
|---|
| Mapk3 | Control | 23.757 | 27.986 | 1.066 |
| LPS | 24.141 | NA | NA |
| Pak1 | Control | 23.693 | 27.854 | 1.117 |
| LPS | 23.628 | 39.023 | 4.64E-04 |
| Rac2 | Control | 20.783 | 36.298 | 4.27E-04 |
| LPS | 20.717 | 25.101 | 0.958 |
Discussion
The present study reports, to the best of our
knowledge, the first genome-wide DNA methylation analysis of rat
lung tissues with LPS-induced ALI/ARDS. A genome-wide DNA
methylation analysis of lung tissues with ALI/ARDS was performed in
rats. In addition, the promoter regions of 1,721 genes and the CpG
islands of 990 genes were found to exhibit aberrant levels of DNA
methylation compared with normal lung tissues. Next, the DNA
methylation status of three candidate genes, Mapk3, Pak1 and Rac2,
was validated using qRT-PCR. The chromosomal locations of these
genes were identified and chromosomes 1, 3, 5, 7 and 10 were
identified to be the most common locations of these genes. Specific
genes on these chromosomes, including Mapk3 (16) and Lat (17) on chromosome 1, Mylk2 and Cebpb
(17) on chromosome 3, Rac2
(18) on chromosome 7 and Ace
(19) on chromosome 10, have been
reported to be critical factors in the development of ALI/ARDS.
Therefore, aberrant DNA methylation on chromosomes 1, 3, 5, 7 and
10 may be associated with the pathogenesis of ALI/ARDS.
In the current study, the differences in DNA
methylation patterns for 3 classes of CpG island, HCP, ICP and LCP,
were observed. Among the CpG island distribution categories, a
number of genes in HCP may be associated with housekeeping genes
and regulate developmental genes, whereas genes in LCP are largely
associated with tissue-specific genes (20), which indicates that, based on CpG
density, analyzing methylation changes may provide additional
insight. DNA methylation levels differed significantly among the 3
categories. The 1,721 genes methylated in the promoter region
include 452 genes with a decreased degree of methylation and 1,269
genes with an increased degree of methylation. The incidence of
methylated HCP genes in the decreased group was higher (n=223,
P<0.01). However, in the increased group, the incidence of
methylated genes in the three categories was not found to be
significant. A similar distribution was observed in the genes
methylated in the CpG island: methylated HCP genes in the decreased
and increased groups were markedly higher (P<0.01). Results
indicate that a higher number of housekeeping and developmental
genes are regulated than tissue-specific genes in the
pathophysiology of ALI/ARDS. Overall, the observations of the
current study demonstrated that DNA methylation is associated with
CpG density, DNA methylation has a higher incidence in HCP genes
compared with ICP and LCP genes and housekeeping and developmental
genes may play crucial roles in the pathophysiology of LPS-induced
ALI/ARDS.
From our methylated genes, which were association
studies with positive findings in ALI/ARDS, we identified 14
methylated genes. Among these genes, a substantial number have been
demonstrated to play a functional role in LPS-induced ALI/ARDS. Of
the 14 methylated genes, angiotensin-converting enzyme (ACE), is
the key enzyme that converts AT-I to AT-II and its functions are
involved in the positive regulation of apoptotic process,
angiotensin signaling process, the renin-angiotensin cascade
pathway and angiotensin II signaling pathway. ACE I/D polymorphism
affects the prognosis of ALI/ARDS (21). ALI/ARDS is characterized by
alveolar injury and increased pulmonary vascular permeability. Mura
et al(22) reported a
potential role for VEGF in promoting the repair of the
alveolar-capillary membrane during recovery from ALI/ARDS. Vegfb is
associated with the VEGF signaling pathway and is involved in the
promotion of angiogenesis. The methylation of Vegfb may affect
repair of the alveolar-capillary membrane and angiogenesis.
One of the principal mechanisms of LPS-induced
ALI/ARDS relates to the effects of the inflammatory response, which
leads to SIRS, including activation of leukocytes-alveolar
macrophages and sequestered neutrophils in the lungs. A previous
genetic study on ALI/ARDS reported that genes associated with the
inflammatory response are important in the development of ALI/ARDS.
The present study found that following genes associated with the
inflammatory response exhibited aberrant DNA methylation profiles:
i) Cebpb, CCAAT/enhancer-binding protein β, is a critical regulator
of the inflammatory responses and injury in the lungs (23); ii) Cxcl2 is a potent neutrophil
chemokine involved in the pulmonary inflammatory response, which is
linked to ventilator-induced ALI and hyperoxia-induced ALI.
Inhibition of its receptor leads to a marked decrease in neutrophil
sequestration and lung injury (24); iii) IL6 is a potent proinflammatory
cytokine and key factor in the development of ALI/ARDS (25); iv) Mylk2 encodes proteins involved
in multiple components of the inflammatory response, including
apoptosis, vascular permeability and leukocyte diapedesis. Myosin
light-chain kinase, a central cytoskeletal regulator encoded by
Mylk, has a key pathophysiological role in ALI (26); and v) Mif, macrophage migration
inhibitory factor, is involved in the pathogenesis of several
inflammatory diseases. Mif-induced neutrophils accumulate in the
alveolar space, indicating that Mif may be a useful target in the
reduction of neutrophil lung inflammation and ALI (27). These methylated genes are involved
in important mechanisms that underlie ALI/ARDS. However, further
studies are required to identify the correlation between the
aberrant methylation of these genes and the pathogenesis of
LPS-induced ALI/ARDS.
According to KEGG pathway analysis, 10 enrichment
pathways were selected. Of the top 10 enrichment pathways, MAPK is
an important signal transmitter from the cell surface to the
internal nucleus and is mainly involved in cell differentiation and
proliferation, apoptosis and regulation of immune and inflammatory
responses. MAPK initiates a cascade of inflammatory cytokines,
leading to an uncontrolled inflammatory response. LPS induces an
inflammatory reaction through the activation of the MAPK signaling
pathway. Thus, the MAPK signaling pathway may have an essential
role in the development of pulmonary inflammation and LPS-induced
ALI/ARDS. A total of 42 methylated genes are associated with the
MAPK signaling pathway and 7 have been associated with ALI/ARDS in
previous studies, including Akt2 (28), Casp3 (29), Mapk3 (16), Pak1 (30), Rac2 (18), Tgfb1 and Tgfb2 (31). These genes have a functional role
in the MAPK signaling pathway and aberrant methylation of these
genes may affect its activation and inflammatory response in
LPS-induced ALI/ARDS.
In summary, the Roche-NimbleGen Rat DNA methylation
385K CpG islands plus promoter array is a useful tool for studying
the genome-wide DNA methylation of lung tissues with LPS-induced
ALI/ARDS. Aberrant DNA methylation in ALI/ARDS was determined and
altered patterns of lung DNA methylation during the pathophysiology
of LPS-induced ALI/ARDS were observed. The identification of a lung
gene-specific methylation profile may provide valuable insight into
pathways that are likely to be epigenetically regulated. Further
analysis of DNA methylation is important for the understanding of
ALI/ARDS and may be of value for indicating prognostic biomarkers
and predictors of response to therapy and may constitute future
therapeutic targets.
Acknowledgements
This study was supported by grants from the Natural
Science Foundation of Shandong Province, China (Y2008C163). The
authors thank Kangchen Biotech Company for assistance with the
analyses.
Abbreviations:
|
LPS
|
lipopolysaccharide
|
|
ALI
|
acute lung injury
|
|
ARDS
|
acute respiratory distress
syndrome
|
|
PBS
|
phosphate-buffered saline
|
|
HCP
|
high density CpG promoters
|
|
LCP
|
low density CpG promoters
|
|
ICP
|
intermediate density CpG promoters
|
|
MeDIP
|
methylated DNA immunoprecipitation
|
|
GO
|
gene ontology
|
|
KEGG
|
Kyoto Encyclopedia of Genes and
Genomes
|
References
|
1
|
Rubenfeld GD, Caldwell E, Peabody E, et
al: Incidence and outcomes of acute lung injury. N Engl J Med.
353:1685–1693. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Zambon M and Vincent JL: Mortality rates
for patients with acute lung injury/ARDS have decreased over time.
Chest. 133:1120–1127. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Wang HM, Bodenstein M and Markstaller K:
Overview of the patolgy of three widely used animal models of acute
lung injury. Eur Surg Res. 40:305–316. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
El Gazzar M, Yoza BK, Hu JY, Cousart SL
and McCall CE: Epigenetic silencing of tumor necrosis factor alpha
during endotoxin tolerance. J Biol Chem. 282:26857–26864. 2007.
|
|
5
|
Angrisano T, Pero R, Peluso S, et al:
LPS-induced IL-8 activation in human intestinal epithelial cells is
accompanied by specific histone H3 acetylation and methylation
changes. BMC Microbiol. 10:1722010. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Tatemichi M, Hata H, Tazawa H and Nakadate
T: Lipopolysaccharide induces aberrant hypermethylation of Hic-1 in
mouse embryonic fibroblasts lacking p53. Anticancer Res.
28:2101–2108. 2008.PubMed/NCBI
|
|
7
|
Kiefer JC: Epigenetics in development. Dev
Dyn. 236:1144–1156. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Holliday R and Pugh JE: DNA modification
mechanisms and gene activity during development. Science.
187:226–232. 1975. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Leonhardt H and Bestor TH: Structure,
function and regulation of mammalian DNA methyltransferase. EXS.
64:109–119. 1993.PubMed/NCBI
|
|
10
|
Klose RJ and Bird AP: Genomic DNA
methylation: the mark and its mediators. Trends Biochem Sci.
31:89–97. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Heller G, Zielinski CC and
Zöchbauer-Müller S: Lung cancer: from single-gene methylation to
methylome profiling. Cancer Metastasis Rev. 29:95–107. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Adcock IM, Tsaprouni L, Bhavsar P and Ito
K: Epigenetic regulation of airway inflammation. Curr Opin Immunol.
19:694–700. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Boellmann F, Zhang L, Clewell HJ, Schroth
GP, Kenyon EM, Andersen ME and Thomas RS: Genome-wide analysis of
DNA methylation and gene expression changes in the mouse lung
following subchronic arsenate exposure. Toxicol Sci. 117:404–417.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Jia RZ, Zhang X, HU P, Liu XM, Hua XD,
Wang X and Ding HJ: Screening for differential methylation status
in human placenta in preeclampsia using a CpG island plus promoter
microarray. Int J Mol Med. 30:133–141. 2012.PubMed/NCBI
|
|
15
|
Flores C, Pino-Yanes MM and Villar J: A
quality assessment of genetic association studies supporting
susceptibility and outcome in acute lung injury. Crit Care.
12:R1302008. View
Article : Google Scholar : PubMed/NCBI
|
|
16
|
Di Paola R, Cisafulli C, Mazzon E,
Genovese T, Paterniti I, Bramanti P and Cuzzocrea S: Effect of
PD98059, a selective MAPK3/MAPK1 inhibitor, on acute lung injury in
mice. Int J Immunopathol Pharmacol. 22:937–950. 2009.PubMed/NCBI
|
|
17
|
Grigoryev DN, Finigan JH, Hassoun P and
Garcia JG: Science review: searching for gene candidates in acute
lung injury. Crit Care. 8:440–447. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Yao HY, Chen L and Xu C: Inhibition of Rac
activity alleviates lipopolysaccharide-induced acute pulmonary
injury in mice. Biochim Biophys Acta. 1810:666–674. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Flores C, Pino-Yanes MM, Casula M, Casula
M and Villar J: Genetics of acute lung injury: past, present and
future. Minerva Anestesiol. 76:860–864. 2010.PubMed/NCBI
|
|
20
|
Saxonov S, Berg P and Brutlag DL: A
genome-wide analysis of CpG dinucleotides in the human genome
distinguishes two distinct classes of promoters. Proc Natl Acad Sci
USA. 103:1412–1417. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Adamzik M, Frey U, Sixt S, Knemeyer L,
Beiderlinden M, Peters J and Siffert W: ACE I/D but not AGT (-6)A/G
polymorphism is a risk factor for mortality in ARDS. Eur Respir J.
29:482–488. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Mura M, dos Santos CC, Stewart D and Liu
M: Vascular endothelial growth factor and related molecules in
acute lung injury. J Appl Physiol. 97:1605–1617. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Yan C, Wu M, Cao J, Tang H, Zhu M, Johnson
PF and Gao H: Critical role for CCAAT/enhancer-binding protein β in
immune complex-induced acute lung injury. J Immunol. 189:1480–1490.
2012.
|
|
24
|
Belperio JA, Keane MP, Burdick MD, et al:
Critical role for CXCR2 and CXCR2 ligands during the pathogenesis
of ventilator-induced lung injury. J Clin Invest. 110:1703–1716.
2002. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Flores C, Ma SF, Maresso K, Wade MS,
Villar J and Garcia JG: IL-6 gene-wide haplotype is association
with susceptibility to acute lung injury. Transl Res. 152:11–17.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Han YJ, Ma SF, Wade MS, Flores C and
Garcia JG: An intronic MYLK variant associated with inflammatory
lung disease regulates promoter activity of the smooth muscle
myosin light chain kinase isoform. J Mol (Berl). 90:299–308. 2012.
View Article : Google Scholar
|
|
27
|
Takahashi K, Koga K, Linge HM, et al:
Macrophage CD74 contributes to MIF-induced pulmonary inflammation.
Respir Res. 10:332009. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Ikegami M, Falcone A and Whitsett JA:
STAT-3 regulates surfactant phospholipid homeostasis in normal lung
and during endotoxin-mediated lung injury. J Appl Physiol.
104:1753–1760. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Perl M, Chung CS, Perl U, Thakkar R,
Lomas-Neira J and Ayala A: Therapeutic accessibility of
caspase-mediated cell death as a key pathomechanism in indirect
acute lung injury. Crit Care Med. 38:1179–1186. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Birukova AA, Xing J, Fu P, et al: Atrial
natriuretic peptide attenuates LPS-induced vascular leak: role of
PAK1. Am J Physiol Lung Cell Mol Physiol. 299:L652–L663. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Leite-Junior JH, Garcia CS,
Souza-Fernandes AB, et al: Methylprednisolone improves lung
mechanics and reduces the inflammatory response in pulmonary but
not in extrapulmonary mild acute lung injury in mice. Crit Care
Med. 36:2621–2628. 2008. View Article : Google Scholar : PubMed/NCBI
|