Introduction
Currently, mild hypothermia is widely used to treat
ischemic and hypoxic encephalopathy in a clinical setting. Several
studies (1–4) have shown that mild hypothermia
inhibits the generation and release of oxygen free radicals and
inflammatory reactions following cerebral ischemia and hypoxia, to
protect the central nervous system by reducing the oxygen
consumption of brain tissues. Previous studies (5,6) have
identified that mild hypothermia improves nerve function in
ischemic and hypoxic encephalopathy by reducing neuronal apoptosis.
Apoptosis is closely associated with the caspase protease family.
Therefore, the protective effect of mild therapeutic hypothermia on
neurons may be associated with the caspase family proteases. Mild
hypothermia inhibits caspase-3 activation induced by
ischemia-reperfusion injury (7)
and reduces H2O2-induced caspase-3 activity
in myocardial cell injury (8). It
also reduces caspase-3 activity in the serum of neonates with
ischemic and hypoxic encephalopathy (9). Thus, the protective effect of mild
hypothermia treatment on the brain may be achieved by reducing
caspase-3 activity following hypoxia and ischemia. However, its
protective effect and mechanisms have not yet been fully
elucidated. The start time, duration and specific temperature used
when applying mild systemic and local hypothermia to the head
remain debatable; a limited number of studies have challenged the
protective effect of mild hypothermia on the brain (10–14).
Therefore, further studies are required to verify the protective
effect of mild hypothermia and its mechanism of action.
Studies on the protective effect of mild hypothermia
on the brain are divided into in vivo and in vitro
trials. In vivo trials usually simulate cerebral ischemia
and hypoxia by blocking blood flow to the brain, whereas in
vitro trials typically use the current neuronal oxygen-glucose
deprivation (OGD) model. In vivo trials have many
experimental factors and are characterized by poor control and
comparability. Nerve cells cultured in vitro retain the
physiological characteristics associated with in vivo
neurons and are widely cultured and used to replace in vivo
nerve cells in experimental studies. A model of a nerve cell
cultured in vitro in hypoxic, sugar-deficient medium has
been accepted as a suitable model for simulating brain tissue
ischemic injury in vivo. This model is widely used to
investigate cerebral ischemic and hypoxic diseases (15,16).
Currently, the majority of studies on the protective
effect of mild hypothermia are conducted using OGD and mild
hypothermia. However, immediately applying mild hypothermia
treatment is difficult during cerebral ischemia and hypoxia.
Usually, mild hypothermia treatment is conducted a period of time
after cerebral ischemic and hypoxic injury occurrence. To confirm
the protective effect of mild hypothermia following cerebral
ischemic and hypoxic injury, previous experimental methods must be
modified, in which mild hypothermia treatment is performed after
OGD. The present study used the in vitro experimental
method, which applies mild hypothermia after inducing OGD to
investigate the protective effect of mild hypothermia on neurons
and the potential underlying mechanisms.
Materials and methods
Cell culture
Approximately 0.1 g/l polylysine was used to seal
the culture dish for 3 days. Suckling rats aged 1–3 days were
provided by the Experimental Animal Center of Sun Yat-sen
University (Guangzhou, China). After disinfecting the skin of each
rat, the scalp and skull were cut open and brain tissues were
immediately removed and placed into a culture dish containing
phosphate-buffered saline (PBS). Using an operating microscope,
hippocampal tissues were separated and placed in a centrifuge tube
containing Dulbecco’s modified Eagle’s medium (DMEM)/F12 medium
(Gibco, Carlsbad, CA, USA) followed by gently sucking in and out
using a suction tube. Subsequently, the mixture was centrifuged for
5 min at 352 × g and the supernatant was removed. DMEM/F12
containing 10% fetal bovine serum (FBS; Hyclone, Logan, UT, USA)
was added to the remaining precipitate to resuspend the cells.
Additionally, trypan blue was used to count the number of active
cells. The active cells were inoculated into a culture dish
(1×106 cells/ml) and incubated in an incubator
containing 5% CO2 at 37°C. After 24 h, the medium was
replaced with a medium containing 2% B27. On the third day,
cytosine arabinoside at a final concentration of 5 μmol/l was added
to the cells for 24 h. The culture liquid was replaced once every
three days. Neuronal growth and cell morphology were observed. On
the 8th day, identification of neuronal microtubule-associated
protein 2 (MAP-2) using fluorescence immunohistochemistry was
conducted.
Model establishment and groups
An incubator containing three gases was preset to a
hypoxic status (37°C, 0.1% O2, 5% CO2, 94.5%
N2). On the 8th day of culture, the hippocampal neurons
and culture medium were removed and 2 ml of sugar-free Earle’s
liquid (6.80 g NaCl, 0.40 g KCl, 0.20 g CaCl2, 0.20 g
MgSO4·7H2O, 1.14 g
NaH2PO4·2H2O and 2.20 g
NaHCO3 were dissolved in triple-distilled water to
prepare a 1,000-ml solution. The pH value of the solution was
adjusted to 7.4 and a micropore filter used for filtration
sterilization) was added, followed by culture in a hypoxic
incubator for 2 h. The cells were then collected and the original
culture medium was added. The cells were placed into the common
incubator (37°C, 19% O2, 5% CO2) or the mild
hypothermic incubator (32°C, 19% O2, 5% CO2)
according to the different groups; the cells were observed and
detected after 24 h of reoxygenation. The experimental cells were
randomly divided into 4 groups (n=6); the normal control, simple
OGD and two mild hypothermic groups (for 6 h or 24 h following
OGD). With the exception of the normal control group, the duration
of OGD for the groups was 2 h. After OGD, all cells were treated
with reoxygenation for 24 h, followed by detection. The present
study was carried out in strict accordance with the recommendations
in the Guide for the Care and Use of Laboratory Animals of the
National Institutes of Health (17). The animal use protocol was reviewed
and approved by the Institutional Animal Care and Use Committee
(IACUC) of Sun Yat-sen memorial Hospital of Sun Yat-sen University
(Guangzhou, China).
Morphological observation
For the various groups of cells, the cell culture
medium was removed following reoxygenation for 24 h. Pre-cooled
prefixation liquid at 4°C (2% glutaric dialdehyde and 2.5%
paraformaldehyde) was added to perform fixation for 15 min. The
cells were removed with a cell scraper and centrifuged for 10 min
at a low temperature at 626–1,409 × g. This caused cells in the
centrifuge tube to precipitate into a mass. Finally, the cells were
stored at 4°C and sent to the Electron Microscopy Laboratory in the
North Campus of Sun Yat-sen University (Guangzhou, China) for
observation.
Lactic acid dehydrogenase (LDH) release
rate detection
Following cell injury, cytoplasmic LDH was partially
released into the culture medium. A higher LDH release rate
indicated more serious cell injury. LDH release rate (%) =
extracellular LDH activity/total cell LDH activity. LDH activity
was detected in a 7600-010 full automatic biochemical analyzer
(Hitachi, Tokyo, Japan). Firstly, 50 μl of the culture medium was
used to detect extracellular LDH activity. Then, Triton X-100
lysate was added for cell disruption. The supernatant was collected
for detection of LDH activity (18).
MTT assay
The neurons were cultured in a 96-well plate until
day 8. The OGD experimental methods and groups were the same as
those mentioned previously. Following reoxygenation for 24 h, 20 μl
of MTT solution at a concentration of 5 mg/ml (Amresco, Inc.,
Solon, OH, USA) was added to each well. The cells were incubated at
37°C for 4 h. Subsequently, the supernatant was removed and 150 μl
dimethyl sulfoxide (DMSO; Amresco, Inc.) was added to each well.
After the plate was agitated for 10 min, the absorbance of each
well was detected at 492 nm using a microplate reader. The mean of
the various wells in each group was considered to be the final
result. Cytoactivity (%) = optical density (OD) of the test
group/OD of the normal control group.
Flow cytometry
Following reoxygenation for 24 h, the groups of
cells were digested with trypsin, collected and centrifuged. The
supernatant was then removed and the cells were resuspended using
binding buffer solution. Annexin V-FITC (Bender MedSystems, Vienna,
Austria) and propidium iodide (PI; Sigma, St. Louis, MO, USA) were
added, followed by shaking. The mixture was allowed to react for 15
min at room temperature in the dark and a flow cytometer
(Becton-Dickinson, Franklin Lakes, NJ, USA) was used to determine
the apoptotic rate.
Caspase-3 activity
Caspase-3 is an effector of apoptosis. Apoptosis is
more pronounced with increased caspase-3 activity. The total
protein in the neuronal cytoplasm was extracted and then the actual
caspase-3 concentration was detected. Subsequently, 200 μg of
caspase-3 was collected and treated using the caspase-3
colorimetric determination reagent kit (BioVision, Inc., Milpitas,
CA, USA), according to the manufacturer’s instructions. The
absorbance at 405 nm of each well was detected with a microplate
reader. Fold increase of caspase activity = OD of the test group/OD
of the normal control group.
Statistical analysis
The measurement data were expressed as the mean ±
standard deviation (SD). A t-test was used to compare the means
between samples. A test for the homogeneity of variance was
conducted. All the calculations were performed using SPSS 13.0
statistical software. P<0.05 was considered to indicate a
statistically significant difference.
Results
Hippocampal neuron culture and
identification results
Following MAP-2 immunofluorescence staining,
cortical neurons cultured for 8 days emitted fluorescence when
examined under a fluorescence microscope (green light excitation).
The endochylema and axons of the hippocampal neurons emitted a high
level of fluorescence, whereas the nuclei emitted no fluorescence
(Fig. 1A). Nonspecific nuclear
fluorescent staining with Hoechst 33258 revealed all the nuclei
(including glial nuclei; Fig. 1B).
After the two images were combined, the hippocampal neurons were
revealed to account for >90% of the cultured cells (Fig. 1C).
Protective effect of mild hypothermia on
neuronal morphology
After 24 h of culture under OGD/reoxygenation,
various groups of hippocampal neurons were observed. In the normal
control group, the organelles were integrated and regularly
arranged. Mitochondria and endoplasmic reticulum were smooth, no
edema, cavities or swelling were observed, with clear cell
structure (Fig. 2A). In the simple
OGD group, the organelles were not integrated and were partially
degraded. Their structures were unclear. The mitochondria appeared
swollen and more cavities were observed (Fig. 2B). In the group treated with mild
hypothermia for 6 h following OGD, the organelles were not
integrated and the cell structure was unclear. However, the
mitochondria was slightly swollen and cavities were visible
(Fig. 2C). In the group treated
with mild hypothermia for 24 h following OGD, the organelles were
more integrated and the arrangement was regular. Few cavities were
observed in the mitochondria and it was swollen. No edema was
observed (Fig. 2D).
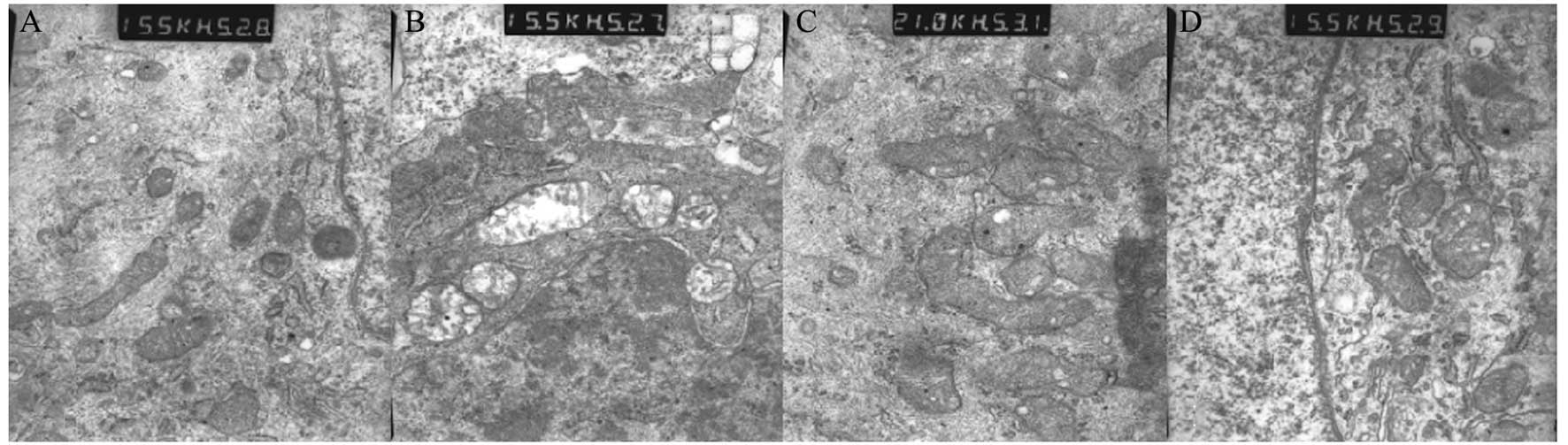 | Figure 2Protective effect of mild hypothermia
on neuron morphology. (A) In the normal control group, organelles
were integrated and regularly arranged. Mitochondria and
endoplasmic reticulum were smooth and no edema, cavities or
swelling were observed, with clear cell structure (x15,500). (B) In
the simple OGD group, the organelles were not integrated, partial
organelle degradation was evident and the cell structure was
unclear. The mitochondria appeared swollen and cavities were
visible (x15,500). (C) In the mild hypothermia for 6 h following
OGD group, the organelles were not integrated and the cell
structure was unclear. However, the mitochondria were slightly
swollen and cavities were visible (x21,000). (D) In the mild
hypothermia for 24 h following OGD group, the organelles were more
integrated and the arrangement was regular. Few cavities were
observed in the mitochondria and it was swollen. No edema was
observed (x15,500). OGD, oxygen-glucose deprivation. |
Mild hypothermia reduces neuronal LDH
release rate
Following OGD, the LDH release rate of nerve cells
was markedly increased; the LDH release rate of the simple OGD
group was significantly higher compared with that of the normal
control group (P<0.01). No significant difference in LDH release
rate was observed between the mild hypothermia for 6 h following
OGD group and the simple OGD group (P>0.05). Compared with the
simple OGD group, the LDH release rate of the mild hypothermia for
24 h following OGD group was significantly reduced and the
difference was statistically significant (P<0.01; Fig. 3).
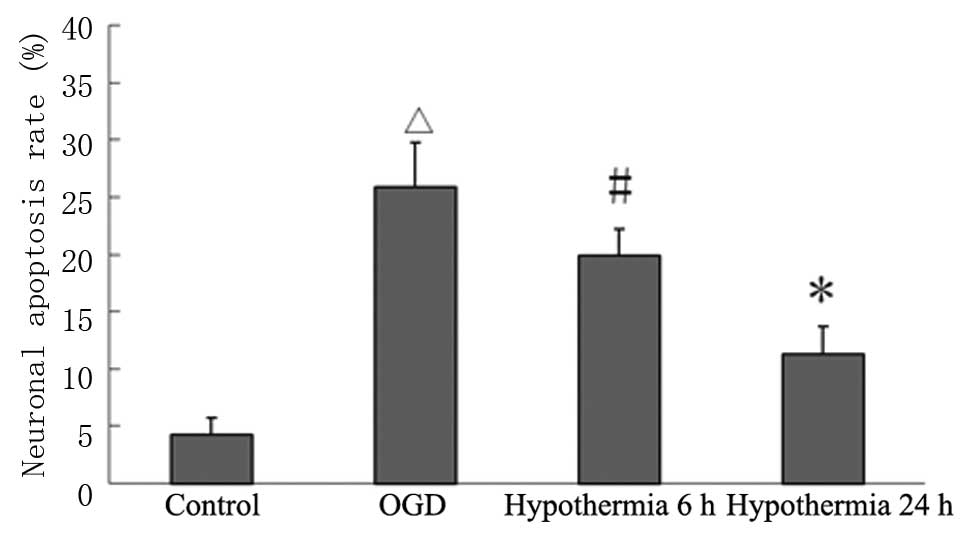
Mild hypothermia increases neuronal
activity and reduces the apoptotic rate
Following OGD, the cytoactivity of nerve cells was
significantly reduced, while the neuronal apoptotic rate
significantly increased; the cytoactivity of the simple OGD group
was significantly lower compared with that of the normal control
group, whereas the neuronal apoptotic rate was significantly higher
in the simple OGD group compared with the normal control group
(P<0.01). No significant differences in cytoactivity and
neuronal apoptotic rate were observed between the mild hypothermia
for 6 h group and the simple OGD group (P>0.05). Compared with
the simple OGD group, the cytoactivity of the mild hypothermia for
24 h group was significantly higher and the neuronal apoptotic rate
was significantly reduced. Both differences were statistically
significant (P<0.01; Figs. 4
and 5).
Mild hypothermia reduces caspase-3
activity in neuronal cytoplasm
Following OGD, the caspase-3 activity of the nerve
cells significantly increased; the caspase-3 activity of the simple
OGD group was significantly higher compared with that of the normal
control group (P<0.01). Compared with the simple OGD group, the
caspase-3 activity of the mild hypothermia for 6 h and the mild
hypothermia for 24 h groups was significantly lower and these
differences were statistically significant (P<0.05; Fig. 6).
Discussion
Nerve cells cultured in vitro retain the
relevant physiological characteristics of in vivo neurons.
Currently, they are widely cultured to replace in vivo nerve
cells in experimental studies (19–22).
The in vitro OGD model controls the extracellular
environment more simply and accurately than the in vivo
model. Therefore, it is often used to investigate changes in
biochemistry and cell morphology induced by ischemia and hypoxia
and the relevant molecular biology mechanisms (23). Simulating ischemia and hypoxia
among in vivo neurons using the OGD model is currently an
important research topic and the model is widely used to
investigate ischemic and hypoxic encephalopathy (24–27).
Mild hypothermia remains vital to treatment
following cerebral ischemic and hypoxic injury. However, the
specific methods and details for the application of mild
hypothermia have not yet been unified and different viewpoints
exist with regard to its curative effect. In clinical research,
Yokoyama et al(28)
performed a multicenter investigation in Japan which showed that
the circulation of 452 patients following cardiopulmonary
resuscitation was restored after receiving mild hypothermia
treatment for 31.5±13.9 h. Core temperature was maintained at
33.9±0.4°C. Consequently, the survival rate of the patients after
30 days was 80.1%, with 55.3% of the patients retaining good nerve
function. This result is significantly higher compared with that of
patients who did not receive mild hypothermia treatment in previous
studies (29). Walters et
al(30) reviewed the
previously published literature and identified that after restoring
the spontaneous circulation of cardiac arrest patients, mild
hypothermia treatment improved nerve function and increased their
survival rate. However, large-scale, high-level, multicenter and
prospective studies in this area are limited. A number of studies
(31) have demonstrated that mild
hypothermia treatment improved the nerve function and long-term
prognosis of acute spinal cord injury. In a previous study on
animals (32), 33°C mild
hypothermia treatment significantly improved the survival rate of a
hemorrhagic shock rat model. Although the treatment disrupted
coagulation function to a certain extent, it did not affect the
prognosis. Noguchi et al(33) found that mild hypothermia inhibited
ischemia-reperfusion injury and arteriolar vasoconstriction in a
gerbil model of cerebral ischemia, thereby improving the survival
rate in cerebral ischemic injury. Dalen et al(34) demonstrated that mild hypothermia
significantly increased the neuronal survival rate following OGD
and reduced the generation of inflammatory mediators in an OGD
model of nerve cells cultured in vitro. Its protective
effect was unrelated to oxygen concentration following
reoxygenation. Liu et al(35) showed that mild hypothermia improved
cellular metabolism in the OGD model of rat brain sections cultured
in vitro, and that earlier hypothermia administration was
more effective. As previously mentioned, it is generally considered
that mild hypothermia has a protective effect on cerebral ischemic
and hypoxic injury. However, a consensus has not been reached and
further research is required.
In the present study, the cell injury-associated
indicators in the mild hypothermia for 24 h following OGD group
were significantly improved (P<0.01) and the cell morphology was
also significantly improved compared with the simple OGD group.
Furthermore, the LDH release rate was significantly reduced, the
MTT assay showed that cytoactivity was significantly increased and
the flow cytometry assay demonstrated that the neuronal apoptotic
rate was significantly reduced. However, the cell injury-associated
indicators in the mild hypothermia for 6 h following OGD group were
not significantly improved compared with the simple OGD group
(P>0.05). Mild hypothermia treatment for 24 h after OGD markedly
relieves neuronal hypoxic and sugar-deficient injury caused by OGD,
whereas mild hypothermia treatment for 6 h did not have a
significant protective effect. These results suggest that mild
hypothermia treatment after OGD requires application for a long
duration, which is in agreement with previously reported research
results (34,36,37).
Therefore, for ischemic and hypoxic cerebral injury, mild
hypothermia treatment for 24 h is more effective. These results
provide an effective theoretical basis for clinical mild
hypothermia treatment in ischemic and hypoxic encephalopathy.
However, a longer treatment period may not necessarily be better in
terms of curative effect due to the potential side-effects of mild
hypothermia. Therefore, further studies should be performed to
determine the optimal treatment duration.
Mild hypothermia improves nerve function in ischemic
and hypoxic encephalopathy by reducing neuronal apoptosis. Cell
apoptosis is closely associated with the caspase protease family.
As a cysteine protease family, caspases are critical in apoptosis
and are involved in the common pathway of apoptosis. Its members
are effector molecules of caspase-3, which implement apoptosis. The
period prior to caspase-3 activation is known as the reversible
stage of apoptosis and the later period is known as the
irreversible stage of apoptosis. Therefore, effectively inhibiting
the occurrence and development of apoptosis by reducing caspase-3
activity is possible. Since caspase-3 is closely associated with
apoptosis, numerous studies have utilized it as an indicator for
evaluating the curative effects of drugs or methods on cerebral
ischemic and hypoxic injury (38–41).
Mild hypothermia may achieve its protective effect on the brain by
reducing caspase-3 activity following hypoxia and ischemia.
In the present study, the caspase-3 activity of
nerve cells following OGD significantly increased compared with
normal control cells (P<0.01) and was positively associated with
an increase in the neuronal apoptotic rate. This suggests that
nerve cells may induce programed neuronal cell death following OGD
by activating caspase-3, leading to a clear increase in apoptosis.
Compared with the simple OGD group, the caspase-3 activity in the
mild hypothermia for 24 h group was significantly decreased
(P<0.01) and the neuronal apoptotic rate was also significantly
reduced. This indicates that mild hypothermia may inhibit neuronal
apoptosis by reducing caspase-3 activity. Compared with the simple
OGD group, the neuronal apoptotic rate in the mild hypothermia for
6 h group was decreased, but the difference was not significant.
However, caspase-3 activity was significantly reduced (P<0.05).
The decrease in caspase-3 activity did not cause a significant
reduction in the neuronal apoptotic rate, which is not consistent
with the expected results of this study. These results suggest that
the apoptotic mechanism is complex and that caspase-3 is only one
of several factors that may affect it. This study showed that a
decrease in caspase-3 activity may be one of the molecular
mechanisms for the protective effect of mild hypothermia on the
brain. These mechanisms are highly complex and remain to be fully
elucidated; thus, further studies are required.
This study was an in vitro trial; however,
nerve cells cultured in vitro are not identical to in
vivo nerve cells. In vivo neurons are more complex and
are widely connected to each other. They also have a lower
tolerance for ischemia and hypoxia; thus, they are more easily
damaged. Consequently, in vitro experiments are not
equivalent to in vivo experiments, and in vitro
experiments are unable to replace multicenter clinical studies. The
present study provides only a direction and basis for further
clinical studies and a model for investigating the relevant
mechanisms.
In this study, the duration of mild hypothermia
treatment was 6 or 24 h, without examining other time-points.
Additionally, mild hypothermia was not applied for >24 h. For
the treatment temperature, only 32°C was selected and the effects
of other temperatures and comparisons between high-temperature
groups were not investigated. Therefore, the present study is not
adequately comprehensive. Direction for future studies may include
improvement of the present study to obtain a more comprehensive and
reliable conclusion.
References
|
1
|
Bayir H, Clark RS and Kochanek PM:
Promising strategies to minimize secondary brain injury after head
trauma. Crit Care Med. 31(Suppl 1): S112–S117. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Kinoshita K, Chatzipanteli K, Vitarbo E,
Truettner JS, Alonso OF and Dietrich WD: Interleukin-1beta
messenger ribonucleic acid and protein levels after
fluid-percussion brain injury in rats: importance of injury
severity and brain temperature. Neurosurgery. 51:195–203. 2002.
View Article : Google Scholar
|
|
3
|
Marion DW: Moderate hypothermia in severe
head injuries: the present and the future. Curr Opin Crit Care.
8:111–114. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Okauchi M, Kawai N, Nakamura T, Kawanishi
M and Nagao S: Effects of mild hypothermia and alkalizing agents on
brain injuries in rats with acute subdural hematomas. J
Neurotrauma. 19:741–751. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Li H and Wang D: Mild hypothermia improves
ischemic brain function via attenuating neuronal apoptosis. Brain
Res. 1368:59–64. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Lin CH, Wu WS, Lin MT, Liu WP, Hsu RB and
Chang CP: Attenuating ischemia-induced H9c2 myoblasts apoptosis by
therapeutic hypothermia. Am J Med Sci. 339:258–265. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Kunimatsu T, Kobayashi K, Yamashita A,
Yamamoto T and Lee MC: Cerebral reactive oxygen species assessed by
electron spin resonance spectroscopy in the initial stage of
ischemia-reperfusion are not associated with hypothermic
neuroprotection. J Clin Neurosci. 18:545–548. 2011. View Article : Google Scholar
|
|
8
|
Diestel A, Drescher C, Miera O, Berger F
and Schmitt KR: Hypothermia protects H9c2 cardiomyocytes from
H2O2 induced apoptosis. Cryobiology.
62:53–61. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Liu CQ, Xia YF, Yuan YX, Li L and Qiu XL:
Effects of selective head cooling with mild hypothermia on serum
levels of caspase-3 and IL-18 in neonates with hypoxic-ischemic
encephalopathy. Zhongguo Dang Dai Er Ke Za Zhi. 12:690–692.
2010.(In Chinese).
|
|
10
|
Brodhun M, Fritz H, Walter B, et al:
Immunomorphological sequelae of severe brain injury induced by
fluid-percussion in juvenile pigs - effects of mild hypothermia.
Acta Neuropathol. 101:424–434. 2001.PubMed/NCBI
|
|
11
|
Fritz HG and Bauer R: Secondary injuries
in brain trauma: effects of hypothermia. J Neurosurg Anesthesiol.
16:43–52. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Inamasu J, Nakamura Y and Ichikizaki K:
Induced hypothemia in experimental traumatic spinal cord injury: an
update. J Neurol Sci. 209:55–60. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
McIntyre LA, Fergusson DA, Hébert PC,
Moher D and Hutchison JS: Prolonged therapeutic hypothemia after
traumatic brain injury in adults: a systematic review. JAMA.
289:2992–2999. 2003. View Article : Google Scholar
|
|
14
|
Robertson CL, Clark RS, Dixon CE, et al:
No long-term benefit from hypothermia after severe traumatic brain
injury with secondary insult in rats. Crit Care Med. 28:3218–3223.
2000. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Scorziello A, Pellegrini C, Forte L, et
al: Differential vulnerability of cortical and cerebellar neurons
in primary culture to oxygen glucose deprivation followed by
reoxygenation. J Neurosci Res. 63:20–26. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Wang S, Xing Z, Vosler PS, et al: Cellular
NAD replenishment confers marked neuroprotection against ischemic
cell death: role of enhanced DNA repair. Stroke. 39:2587–2595.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
National Research Council. Guide for the
Care and Use of Laboratory Animals. 8th Edition. The National
Academies press; Washington, DC: pp. 199–200. 2011
|
|
18
|
Abe K and Matsuki N: Measurement of
cellular 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium
bromide (MTT) reduction activity and lactate dehydrogenase release
using MTT. Neurosci Res. 38:325–329. 2000. View Article : Google Scholar
|
|
19
|
Bai Y, Meng Z, Cui M, et al: An
Ang1-Tie2-PI3K axis in neural progenitor cells initiates survival
responses against oxygen and glucose deprivation. Neuroscience.
160:371–381. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Labrande C, Velly L, Canolle B, et al:
Neuroprotective effects of tacrolimus (FK506) in a model of
ischemic cortical cell cultures: role of glutamate uptake and FK506
binding protein 12 kDa. Neuroscience. 137:231–239. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Lei Z, Ruan Y, Yang AN and Xu ZC: NMDA
receptor mediated dendritic plasticity in cortical cultures after
oxygen-glucose deprivation. Neurosci Lett. 407:224–229. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Wang ZF and Tang XC: Huperzine A protects
C6 rat glioma cells against oxygen-glucose deprivation-induced
injury. FEBS Lett. 581:596–602. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Montero M, Poulsen FR, Noraberg J, et al:
Comparison of neuroprotective effects of erythropoietin (EPO) and
carbamylerythropoietin (CEPO) against ischemia-like oxygen-glucose
deprivation (OGD) and NMDA excitotoxicity in mouse hippocampal
slice cultures. Exp Neurol. 204:106–117. 2007. View Article : Google Scholar
|
|
24
|
Baltan S, Murphy SP, Danilov CA, Bachleda
A and Morrison RS: Histone deacetylase inhibitors preserve white
matter structure and function during ischemia by conserving ATP and
reducing excitotoxicity. J Neurosci. 31:3990–3999. 2011. View Article : Google Scholar
|
|
25
|
Gwak MS, Cao L, Li L and Zuo Z: Isoflurane
preconditioning reduces oxygen-glucose deprivation-induced neuronal
injury via B-cell lymphoma 2 protein. Environ Toxicol Pharmacol.
31:262–265. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Savoia C, Sisalli MJ, Di Renzo G,
Annunziato L and Scorziello A: Rosuvastatin-induced neuroprotection
in cortical neurons exposed to OGD/reoxygenation is due to nitric
oxide inhibition and ERK1/2 pathway activation. Int J Physiol
Pathophysiol Pharmacol. 3:57–64. 2011.PubMed/NCBI
|
|
27
|
Ziu M, Fletcher L, Rana S, Jimenez DF and
Digicaylioglu M: Temporal differences in microRNA expression
patterns in astrocytes and neurons after ischemic injury. PLoS One.
6:e147242011. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Yokoyama H, Nagao K, Hase M, et al;
J-PULSE-Hypo Investigators. Impact of therapeutic hypothermia in
the treatment of patients with out-of-hospital cardiac arrest from
the J-PULSE-HYPO study registry. Circ J. 75:1063–1070. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Cheah SO, Ong ME and Chuah MB: An eight
year review of exercise-related cardiac arrests. Ann Acad Med
Singapore. 39:542–546. 2010.PubMed/NCBI
|
|
30
|
Walters JH, Morley PT and Nolan JP: The
role of hypothermia in post-cardiac arrest patients with return of
spontaneous circulation: a systematic review. Resuscitation.
82:508–516. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Dietrich WD, Levi AD, Wang M and Green BA:
Hypothermic treatment for acute spinal cord injury.
Neurotherapeutics. 8:229–239. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Iwamoto S, Takasu A and Sakamoto T:
Therapeutic mild hypothermia: effects on coagulopathy and survival
in a rat hemorrhagic shock model. J Trauma. 68:669–675. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Noguchi K, Matsumoto N, Shiozaki T, et al:
Effects of timing and duration of hypothermia on survival in an
experimental gerbil model of global ischaemia. Resuscitation.
82:481–486. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Dalen ML, Frøyland E, Saugstad OD, Mollnes
TE and Rootwelt T: Post-hypoxic hypothermia is protective in human
NT2-N neurons regardless of oxygen concentration during
reoxygenation. Brain Res. 1259:80–89. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Liu J, Litt L, Segal MR, Kelly MJ,
Yoshihara HA and James TL: Outcome-related metabolomic patterns
from 1H/31P NMR after mild hypothermia treatments of oxygen-glucose
deprivation in a neonatal brain slice model of asphyxia. J Cereb
Blood Flow Metab. 31:547–559. 2011. View Article : Google Scholar
|
|
36
|
Jiang JY, Xu W, Li WP, et al: Effect of
long-term mild hypothermia or short-term mild hypothermia on
outcome of patients with severe traumatic brain injury. J Cereb
Blood Flow Metab. 26:771–776. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Shibuta S, Varathan S, Kamibayashi T and
Mashimo T: Small temperature variations alter edaravone-induced
neuroprotection of cortical cultures exposed to prolonged hypoxic
episodes. Br J Anaesth. 104:52–58. 2010. View Article : Google Scholar
|
|
38
|
Kunimatsu T, Yamashita A, Kitahama H,
Misaki T and Yamamoto T: Measurement of cerebral reactive hyperemia
at the initial post-ischemia reperfusion stage under normothermia
and moderate hypothermia in rats. J Oral Sci. 51:615–621. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Kuo JR, Lo CJ, Chang CP, Lin HJ, Lin MT
and Chio CC: Brain cooling-stimulated angiogenesis and neurogenesis
attenuated traumatic brain injury in rats. J Trauma. 69:1467–1472.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Shuja F, Tabbara M, Li Y, et al: Profound
hypothermia decreases cardiac apoptosis through Akt survival
pathway. J Am Coll Surg. 209:89–99. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Wang Z, Shi Q, Li S, Du J, Liu J and Dai
K: Hyperthermia induces platelet apoptosis and glycoprotein Ibalpha
ectodomain shedding. Platelets. 21:229–237. 2010. View Article : Google Scholar : PubMed/NCBI
|