Introduction
Protein phosphatase 2, regulatory subunit A, α
(PPP2R1A) and β (PPP2R1B) are paralogous subunits of
the heterotrimeric protein phosphatase 2 (PP2A) holoenzyme, which
catalyzes the dephosphorylation of target substrate proteins
(www.ensembl.org) (1).
PPP2R1A and PPP2R1B belong to the
Huntington-elongation-A subunit-TOR (HEAT) repeat protein family,
and are required as scaffolds for the formation of the
heterotrimeric PP2A complex (2).
PPP2R1A and PPP2R1B each contain 15 tandemly repeated
HEAT motifs. It has been previously demonstrated that the HEAT
repeats 2–8 and 11–15 were involved in binding to the regulatory
and catalytic subunits of PP2A, respectively (3). In addition, mutations located in the
HEAT 2 and 11 motifs promoted tumorigenesis; the mutant
PPP2R1A proteins were defective in binding to the regulatory
and catalytic subunits of PP2A, resulting in a decrease in PP2A
activity and thus facilitating tumor progression (4).
A previous whole-exome sequencing attempt identified
recurrent PPP2R1A mutations in 3 out of 42 patients
(7.1%)with ovarian clear cell carcinoma (OCCC) (5). Subsequent studies confirmed this
finding and also revealed frequent PPP2R1A mutations in
patients with ovarian endometrioid carcinoma (6–8).
Additionally, a high frequency of PPP2R1A mutations was also
identified in several subtypes of endometrial carcinoma, with the
highest frequency demonstrated in the serous subtype (6–9).
Furthermore, previous studies revealed that PPP2R1A
mutations were present in patients with breast and lung
malignancies, but absent in patients with melanoma and Wilms’ tumor
(10,11), suggesting a general effect of
PPP2R1A mutations on human cancer. Until presently, the
majority of PPP2R1A mutations identified in human cancer
were restricted to codons 179–183 and 256–258, which correspond to
the HEAT 5 and HEAT 7 motifs, respectively (http://www.uniprot.org/) (5–9).
Therefore, the PPP2R1A codons 179–183 and 256–258 were
considered potential mutational hotspots in human malignancies,
particularly in ovarian and endometrial carcinoma.
PPP2R1B, the paralogous gene of
PPP2R1A, was demonstrated to be mutated in breast and
colorectal cancer, but not in ovarian cancer and certain other
types of cancer (10,12–21).
Although point mutations in the PPP2R1B gene have not been
detected in ovarian cancer, it should be noted that a relatively
small sample size was analyzed in these studies (16,17),
and loss of heterozygosity (LOH) of PPP2R1B has been
frequently observed in different subtypes of ovarian cancer
(16). Additionally, accumulating
evidence suggested that mutations in the homologous residues of
paralogous genes were frequently observed in human malignancies
(22–24), which suggested that PPP2R1B
mutations may also present in patients with ovarian cancer. We
hypothesized that PPP2R1A and PPP2R1B mutations are
also involved in the pathogenesis of non-primary ovarian cancer.
Therefore, to examine whether PPP2R1A and PPP2R1B
mutations may be involved in the development of primary and
secondary ovarian cancer, 251 Chinese patients with diverse
subtypes of ovarian cancer were recruited to test this
hypothesis.
Materials and methods
Patients and clinical data
A total of 251 formalin-fixed, paraffin-embedded
(FFPE) ovarian cancerous and paired normal tissues were recruited
from the Jiangxi Provincial Maternal and Child Health Hospital
(Jiangxi, China). The sections were reviewed in a blinded manner by
two pathologists, and only those with >70% cancerous cells were
included in this study. In total, there were 234 primary and 17
secondary ovarian cancer samples. The primary ovarian cancer
subtypes included ovarian serous cancer (n=76), OCCC (n=43),
ovarian endometrioid carcinoma (n=37), mucinous ovarian carcinoma
(n=15), ovarian germ cell tumor (n=33), ovarian sex cord-stromal
tumor (n=18), other rare subtypes (n=12) and secondary ovarian
cancer (n=17) (Table I); while all
secondary types of cancer were Krukenberg tumors. This study
conformed to the principles of the Declaration of Helsinki, and
written informed consent was obtained from each subject prior to
the study. The institutional review board of the Jiangxi Provincial
Maternal and Child Health Hospital approved this study.
 | Table IPatient characteristics and
PPP2R1A mutation distribution in Chinese patients with
primary and secondary ovarian cancer. |
Table I
Patient characteristics and
PPP2R1A mutation distribution in Chinese patients with
primary and secondary ovarian cancer.
| A. Patient
characteristics. |
|
|
Characteristics | Value |
|
| Age at diagnosis
(years) |
| Median | 47 |
| Minimum | 5 |
| Maximum | 75 |
| Affected ovary (no.
of patients) |
| Both | 106 |
| Left | 63 |
| Right | 82 |
|
| B. PPP2R1A
mutation distribution. |
|
| Cancer type | Frequency no.
(%) | Nucleotide
change | Amino acid
change |
|
| Primary |
| Epithelial |
| Serous | 0/76 (0.0) | - | - |
| Clear cell | 0/43 (0.0) | - | - |
| Endometrioid | 1/37 (2.7) | c.771G>T | p.W257C |
| Mucinous | 0/15 (0.0) | - | - |
|
Undifferentiated | 0/3 (0.0) | - | - |
| Unclassified | 0/4 (0.0) | - | - |
| Transitional
cell | 0/3 (0.0) | - | - |
| Mixed | 0/2 (0.0) | - | - |
|
Non-epithelial |
| Germ cell
tumor | | | |
| Yolk sac | 0/11 (0.0) | - | - |
| Dysgerminoma | 0/7 (0.0) | - | - |
| Teratoma | 0/9 (0.0) | - | - |
| Mixed | 0/6 (0.0) | - | - |
| Sex cord-stromal
tumor |
| Granulosa
cell | 0/16 (0.0) | - | - |
|
Sertoli-Leydig | 0/2 (0.0) | - | - |
| Secondary |
| Krukenberg
tumors | 0/17 (0.0) | - | - |
| Total | 251 (100) | c.771G>T | p.W257C |
PPP2R1A and PPP2R1B mutational
analyses
Genomic DNA was isolated from archival FFPE tissues
using FFPE DNA kits (Omega Bio-Tek Inc., Doraville, GA, USA). For
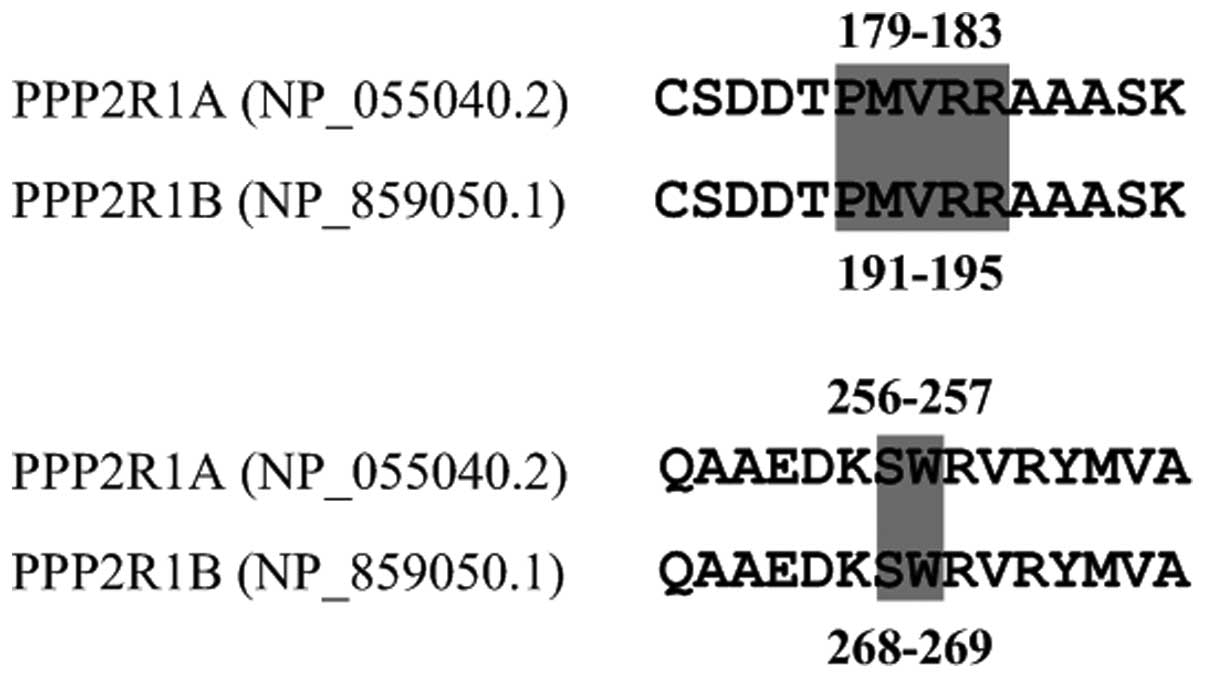
PPP2R1A, two short PCR fragments spanning codons 179–183 and
256–257 were amplified with the following primer pairs,
respectively: Forward 1: 5′-GTACTTCCGGAACCTGTGCT-3′ and reverse 1:
5′-AGCAAAACTCACCTGCTCGT-3′; forward 2: 5′-CTCTCCTCTCCCTAGGACTCG-3′
and reverse 2: 5′-TGTGAACTTGTCAGCCACCA-3′. For PPP2R1B, the
following primer pairs were used to amplify the PCR fragments
spanning codons 191–195 and 268–269, corresponding to the
homologous residues of codons 179–183 and 256–257 in PPP2R1A
(Fig. 1): Forward 1:
5′-ATTCCGTTCCTTGTGCTCAG-3′ and reverse 1:
5′-GGAGCATATCTGTGTCCCTTAAA-3′; forward 2:
5′-TAGGATTCAGTGCGCCTCCT-3′ and reverse 2:
5′-TGAAAATCTGTCAGCCACCA-3′. The PCR products were sequenced using
an ABI Prism 3730 DNA sequencer (Applied Biosystems, Foster City,
CA, USA). The identified mutation was confirmed as either somatic
or germline by sequencing the paired normal tissue.
Evolutionary conservation and protein
sequence homology analyses
Evolutionary conservation analysis of the
PPP2R1A mutation was performed using data obtained from
GenBank for 14 different species, including Homo sapiens
(PPP2R1A, GenBank accession no. NM_014225), Pan
troglodytes (XM_001174546), Pongo abelii (NM_001132813),
Macaca mulatta (NM_001257922.1), Nomascus leucogenys
(XM_003269709), Callithrix jacchus (XM_002762433), Rattus
norvegicus (NM_057140), Mus musculus (NM_016891),
Cricetulus griseus (XM_003509396), Canis lupus
familiaris (XM_845900), Bos taurus (NM_001037477),
Sus scrofa (NM_214024), Xenopus laevis (NM_001086666)
and Danio rerio (NM_213376).
Protein sequence homology analysis of human PPP2R1A
(GenBank accession no. NP_055040.2) and PPP2R1B (NP_859050.1) was
performed to determine the relationship between the two paralogous
genes, using DNASTAR software (DNASTAR, Inc., Madison, WI,
USA).
Results
Patient characteristics
A total of 251 patients with primary and secondary
ovarian cancer were recruited from the Department of Pathology,
Jiangxi Provincial Maternal and Child Health Hospital. The median
age of the patients was 47 years (range, 5–75), and 106 out of the
251 patients were affected in both ovaries, while the remaining 145
patients were affected in either the left or the right ovary
(Table IA).
PPP2R1A mutations in ovarian cancer
We screened the 234 primary and 17 secondary ovarian
cancer samples for the presence of PPP2R1A hotspot
mutations. The mutation type and frequency distribution are shown
in Table IB. A somatic,
heterozygous PPP2R1A mutation (c.771G>T, p.W257C) was
identified in 1 out of 37 primary ovarian cancer patients (2.7%)
with ovarian endometrioid carcinoma. The mutant sample was that of
a 46-year-old female, who was also diagnosed with ectopic
endometriosis in the benign ovary (Fig. 2). In addition, no PPP2R1A
mutations were detected in the remaining 250 patients with ovarian
cancer (Table IB).
The evolutionary conservation analysis suggested
that the PPP2R1A p.W257C mutation was highly conserved among the 14
species ranging from Homo sapiens to Danio rerio
(Fig. 3).
PPP2R1B mutations in ovarian cancer
We sequenced the genomic region of PPP2R1B
spanning p.P191-R195 and p.S268-W269, the homologous residues
corresponding to the potential mutational hotspots in the
PPP2R1A gene. However, no mutations were detected among the
234 primary and 17 secondary patients with ovarian cancer.
Discussion
Recurrent PPP2R1A mutations have been
identified in OCCC patients in a whole-exome sequencing study
(5). This observation was
confirmed by four studies that followed, and frequent
PPP2R1A mutations were also identified in several other
subtypes of ovarian and endometrial carcinoma (6–9). In
addition, previous studies detected PPP2R1A and
PPP2R1B mutations in human malignancies with variable
frequencies (10–21,25).
In the present study, 234 primary and 17 secondary
Chinese patients with ovarian cancer were analyzed for the presence
of potential PPP2R1A and PPP2R1B hotspot mutations. A
previously reported PPP2R1A p.W257C (c.771G>T) somatic mutation
was detected in the sample from one patient with ovarian
endometrioid carcinoma out of our 251 samples. This particular
patient was also diagnosed with ectopic endometriosis in the benign
ovary. We failed to detect the presence of any mutations in the
remaining individuals. In addition, no PPP2R1B mutations
were detected in our samples.
The combined results of previous studies
demonstrated that PPP2R1A mutations frequently occurred in
breast, lung and endometrial carcinoma, as well as in OCCC and
ovarian endometrioid carcinoma (5–11).
Furthermore, the mutation frequencies of PPP2R1A in OCCC and
ovarian endometrioid carcinoma patients were 4.1–9.1% (mean, 7.4%)
and 10.0–12.2% (mean, 11.1%), respectively (5–8).
However, the mutation frequencies of PPP2R1A in our OCCC and
ovarian endometrioid carcinoma samples were 0% (0/43) and 2.7%
(1/37), respectively, which were lower than those observed in the
European and American counterparts (5–8). One
reason for these discrepancies may be population differences,
suggesting that PPP2R1A mutations may increase the
propensity for ovarian cancer when present in European and American
individuals compared with Chinese individuals. An alternative
explanation for these differences may be the relatively small
sample size analyzed in our study, therefore, larger sample sizes
would be required to test this theory. Moreover, we did not detect
any PPP2R1A mutations in OSC or ovarian mucinous carcinoma,
which was consistent with previous observations (6,8).
A previous study demonstrated that PPP2R1A p.E64D,
p.E64G and p.R418W mutations located in the HEAT 2 and 11 motifs,
respectively, contributed to carcinogenesis by impairing the
activity of PP2A (http://www.uniprot.org/) (4). Mutations of the PPP2R1A codon
257 have been frequently observed in ovarian cancer (6–8), and
are highly conserved across multiple species (Fig. 3). Additionally, the PPP2R1A p.W257C
mutation was located in the HEAT 7 repeat motif (http://www.uniprot.org/), which has been proposed to
be the domain interacting with the regulatory subunits of PP2A, and
thus crucial for PP2A activity (3). Overall, we speculated that the
PPP2R1A p.W257C mutation may contribute to the pathogenesis of
Chinese patients with ovarian endometrioid carcinoma. Nevertheless,
the pathogenicity of the PPP2R1A p.W257C mutation remains unclear,
therefore, functional assays are required to decipher the potential
role of this mutation in the development of ovarian cancer.
We also screened for potential mutations of
p.P191-R195 and p.S268-W269, the homologous residues corresponding
to potential mutational hotspots in the PPP2R1A gene.
However, no mutations were evident in our samples. This was
consistent with previous observations that PPP2R1B mutations
were absent in patients with ovarian cancer (16,17).
Combined with the results of previous studies (16,17),
the absence of PPP2R1B mutations in the ovarian cancer
samples analyzed in our study suggested that PPP2R1B
mutations may not be actively involved in the development of
primary and secondary ovarian cancer. However, this conclusion
should be treated with caution, as the most frequent genetic
aberrations of PPP2R1B observed in human cancers were
heterozygous or homozygous LOH (10,19).
Furthermore, we did not exclude the possibility that there may be
mutations in other regions of this gene contributing to the
development of ovarian cancer.
In conclusion, we analyzed 251 Chinese patients with
primary and secondary ovarian cancer for the presence of
PPP2R1A and PPP2R1B mutations. The mutation
frequencies of PPP2R1A were 0.0 and 2.7% in OCCC and ovarian
endometrioid carcinoma patients, respectively, which were lower
than those of their corresponding European and American
counterparts. In addition, we did not detect any PPP2R1A
mutations in OSC and ovarian mucinous carcinoma, which was
consistent with previous observations. Moreover, no PPP2R1B
mutations were identified in our samples, and this observation was
consistent with previous studies on ovarian cancer. Our results
suggested that mutations of PPP2R1A, but not PPP2R1B,
may be involved in the pathogenesis of Chinese patients with
ovarian cancer.
Acknowledgements
The authors would like to thank the sample donors
who participated in this study. This study was supported by the
National Natural Science Foundation of China (grant nos. 81060052
and 81260384) and the Natural Science Foundation of Jiangxi
Province (grant no. 20114BAB215033).
References
|
1
|
Slupe AM, Merrill RA and Strack S:
Determinants for substrate specificity of protein phosphatase 2A.
Enzyme Res. 2011:3987512011. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Ruediger R, Roeckel D, Fait J, Bergqvist
A, Magnusson G and Walter G: Identification of binding sites on the
regulatory A subunit of protein phosphatase 2A for the catalytic C
subunit and for tumor antigens of simian virus 40 and polyomavirus.
Mol Cell Biol. 12:4872–4882. 1992.
|
|
3
|
Xu Y, Xing Y, Chen Y, Chao Y, Lin Z, Fan
E, Yu JW, Strack S, Jeffrey PD and Shi Y: Structure of the protein
phosphatase 2A holoenzyme. Cell. 127:1239–1251. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Chen W, Arroyo JD, Timmons JC, Possemato R
and Hahn WC: Cancer-associated PP2A Aalpha subunits induce
functional haploinsufficiency and tumorigenicity. Cancer Res.
65:8183–8192. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Jones S, Wang TL, Shih IM, Mao TL,
Nakayama K, Roden R, Glas R, Slamon D, Diaz LA Jr, Vogelstein B, et
al: Frequent mutations of chromatin remodeling gene ARID1A in
ovarian clear cell carcinoma. Science. 330:228–231. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
McConechy MK, Anglesio MS, Kalloger SE,
Yang W, Senz J, Chow C, Heravi-Moussavi A, Morin GB, Mes-Masson AM,
et al; Australian Ovarian Cancer Study Group. Subtype-specific
mutation of PPP2R1A in endometrial and ovarian carcinomas. J
Pathol. 223:567–573. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Nagendra DC, Burke J III, Maxwell GL and
Risinger JI: PPP2R1A mutations are common in the serous type of
endometrial cancer. Mol Carcinog. 51:826–831. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Shih IM, Panuganti PK, Kuo KT, Mao TL,
Kuhn E, Jones S, Velculescu VE, Kurman RJ and Wang TL: Somatic
mutations of PPP2R1A in ovarian and uterine carcinomas. Am J
Pathol. 178:1442–1447. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
McConechy MK, Ding J, Cheang MC, Wiegand
KC, Senz J, Tone AA, Yang W, Prentice LM, Tse K, Zeng T, et al: Use
of mutation profiles to refine the classification of endometrial
carcinomas. J Pathol. 228:20–30. 2012.PubMed/NCBI
|
|
10
|
Calin GA, di Iasio MG, Caprini E,
Vorechovsky I, Natali PG, Sozzi G, Croce CM, Barbanti-Brodano G,
Russo G and Negrini M: Low frequency of alterations of the alpha
(PPP2R1A) and beta (PPP2R1B) isoforms of the subunit A of the
serine-threonine phosphatase 2A in human neoplasms. Oncogene.
19:1191–1195. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Ruteshouser EC, Ashworth LK and Huff V:
Absence of PPP2R1A mutations in Wilms tumor. Oncogene.
20:2050–2054. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Wang SS, Esplin ED, Li JL, Huang L, Gazdar
A, Minna J and Evans GA: Alterations of the PPP2R1B gene in human
lung and colon cancer. Science. 282:284–287. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Takagi Y, Futamura M, Yamaguchi K, Aoki S,
Takahashi T and Saji S: Alterations of the PPP2R1B gene located at
11q23 in human colorectal cancers. Gut. 47:268–271. 2000.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Tamaki M, Goi T, Hirono Y, Katayama K and
Yamaguchi A: PPP2R1B gene alterations inhibit interaction of
PP2A-Abeta and PP2A-C proteins in colorectal cancers. Oncol Rep.
11:655–659. 2004.PubMed/NCBI
|
|
15
|
Suraweera N, Robinson J, Volikos E,
Guenther T, Talbot I, Tomlinson I and Silver A: Mutations within
Wnt pathway genes in sporadic colorectal cancers and cell lines.
Int J Cancer. 119:1837–1842. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Wu R, Connolly DC, Ren X, Fearon ER and
Cho KR: Somatic mutations of the PPP2R1B candidate tumor suppressor
gene at chromosome 11q23 are infrequent in ovarian carcinomas.
Neoplasia. 1:311–314. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Campbell IG and Manolitsas T: Absence of
PPP2R1B gene alterations in primary ovarian cancers. Oncogene.
18:6367–6369. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Marsh A, Healey S, Lewis A, Spurdle AB,
Kedda MA, Khanna KK, kConFab, Mann GJ, Pupo GM, Lakhani SR and
Chenevix-Trench G: Mutation analysis of five candidate genes in
familial breast cancer. Breast Cancer Res Treat. 105:377–389. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Chou HC, Chen CH, Lee HS, Lee CZ, Huang
GT, Yang PM, Lee PH and Sheu JC: Alterations of tumour suppressor
gene PPP2R1B in hepatocellular carcinoma. Cancer Lett. 253:138–143.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Hemmer S, Wasenius VM, Haglund C, Zhu Y,
Knuutila S, Franssila K and Joensuu H: Alterations in the
suppressor gene PPP2R1B in parathyroid hyperplasias and adenomas.
Cancer Genet Cytogenet. 134:13–17. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Yeh LS, Hsieh YY, Chang JG, Chang WW,
Chang CC and Tsai FJ: Mutation analysis of the tumor suppressor
gene PPP2R1B in human cervical cancer. Int J Gynecol Cancer.
17:868–871. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Su F, Viros A, Milagre C, Trunzer K,
Bollag G, Spleiss O, Reis-Filho JS, Kong X, Koya RC, Flaherty KT,
et al: RAS mutations in cutaneous squamous-cell carcinomas in
patients treated with BRAF inhibitors. N Engl J Med. 366:207–215.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Van Raamsdonk CD, Griewank KG, Crosby MB,
Garrido MC, Vemula S, Wiesner T, Obenauf AC, Wackernagel W, Green
G, Bouvier N, et al: Mutations in GNA11 in uveal melanoma. N Engl J
Med. 363:2191–2199. 2010.PubMed/NCBI
|
|
24
|
Zou Y, Zeng Y, Zhang DF, Zou SH, Cheng YF
and Yao YG: IDH1 and IDH2 mutations are frequent in Chinese
patients with acute myeloid leukemia but rare in other types of
hematological disorders. Biochem Biophys Res Commun. 402:378–383.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Zhu Y, Loukola A, Monni O, Kuokkanen K,
Franssila K, Elonen E, Vilpo J, Joensuu H, Kere J, Aaltonen L and
Knuutila S: PPP2R1B gene in chronic lymphocytic leukemias and
mantle cell lymphomas. Leuk Lymphoma. 41:177–183. 2001. View Article : Google Scholar : PubMed/NCBI
|