Introduction
Gastric cancer (GC) is one of the most common types
of cancer globally. It is considered to be the second most frequent
cause of cancer-related mortality worldwide (1–3). The
invasion and migration of GC are important factors leading to tumor
recurrence and affecting prognosis; however, the molecular
pathogenesis of GC metastasis is not well understood. For this
reason, understanding the mechanisms underlying GC as well as the
identification of novel molecular targets are of great
importance.
Phosphatase of regenerating liver-3 (PRL-3), a
metastasis-associated protein, belongs to the PRL family of protein
tyrosine phosphatases (PTP), which includes two other members,
PRL-1 and PRL-2 (4). The PTP
superfamily of phosphatases includes a large group of enzymes
important for the regulation of a wide variety of cell mechanisms,
including signal transduction, the cell cycle, differentiation,
cell transformation, adhesion and motility (5,6).
Evidence has accumulated for the association of PRL-3 with
oncogenic states, and several studies have linked its expression to
human cancer progression and metastasis, such as in the case of
malignant melanoma, as well as pancreatic, ovarian, breast and
nasopharyngeal cancer (7–11). In a previous study, we examined the
expression of PRL-3 in primary GC tissues and in peritoneal
metastases, and found that PRL-3 expression was significantly
higher in primary gastric carcinoma with peritoneal metastasis than
in peritoneal metastasis-negative gastric carcinoma (12). However, little is known regarding
the molecular mechanisms by which PRL-3 promotes motility, invasion
and metastasis.
The extracellular signal-regulated kinase 1/2 (ERK
1/2), part of the mitogen-activated protein kinase (MAPK) family,
is well-known for its role in numerous cell processes, such as cell
migration, invasion and proliferation (13,14).
One suggested mechanism whereby the ERK 1/2 pathway promotes
invasiveness in tumor cells is through the upregulation of matrix
metalloproteinases (MMPs), for extracellular matrix remodeling
(15), which are important in
tumor metastasis (16). A decrease
in cell adhesion and proteolytic degradation of collagen by MMPs
promotes the invasive migration of cells through the extracellular
matrix (17). PRL-3 has previously
been described as one of the PRLs that are also capable of
degrading the extracellular matrix; however, the molecular details
remain unclear (7). Previously, it
was demonstrated that an increased PRL-1 expression results in the
activation of ERK1/2, which stimulates MMP production, and
increases cell invasion and migration (18). It is known that PRL-3 has ≥75%
amino acid sequence similarity with PRL-1 and PRL-2 (19). Therefore, it is also necessary and
crucial to determine the relationship between PRL-3, ERK 1/2 and
MMP expression in human GC.
In the present study, we employed siRNA targeting
PRL-3 to explore the potential of new therapeutic targets in the
treatment of GC. Our results suggested that knockdown of PRL-3 was
able to inhibit GC invasion and migration, and significantly
decrease ERK 1/2 and MMP-7 expression, which provides novel
insights into tumorigenesis and may ultimately lead to more
effective therapies.
Materials and methods
Gastric tissues, cell lines and
cultures
Human gastric adenocarcinoma cancer cell lines,
SGC-7901, MKN-45, MKN-28 and BGC-823, were obtained from the Type
Culture Collection of the Chinese Academy of Sciences (Shanghai,
China). The human gastric mucosa cell lines, GES-1 and HFE-145,
were preserved in our central laboratory. Cells were cultured in
Dulbecco’s Modified Eagle’s medium (DMEM, Gibco-BRL, Carlsbad, CA,
USA) containing 10% fetal bovine serum (FBS), penicillin (100 U/ml)
and streptomycin (100 μg/ml). Cells were incubated at 37°C in a
humidified incubator with 5% CO2. All human gastric
tissue samples, including eight gastric tissues and 20 malignant
tissues, were obtained from the General Surgery Department of the
First Affiliated Hospital of Nanchang University (Nanchang, China).
All samples were obtained with informed consent and approved by the
hospital institutional review board. The tissues were stored in
liquid nitrogen following removal from patients.
Plasmids and transfection
The human PRL-3-specific siRNA was based on NCBI
reference sequences (GenBank: PRL-3, NM_032611). The siRNA
sequences used for PRL-3 gene silencing were designed based on
published data, as follows: Sense: 5′-GATCCGTGACCTATGACAAAACGCTTCAA
GAGAGCGTTTTGTCATAGGTCACTTTTTGGAAA-3′ and antisense:
5′-AGCTTTTCCAAAAAGTGACCTATGACAAA
ACGCTCTTTGAAGCGTTTTGTCATAGGTCACG-3′ (20). The negative control sequences were
as follows: Sense: 5′-GAT CCGGTTATGTACAGGAACGCATTCAAGAGATGCGTT
CCTGTACATAACCTTTTTGGAAA-3′ and antisense:
5′-GCCAATAGATGTCCTTGCGTAAGTTCTCTACGCAA
GACATGTATTGGAAAAACCTTTTCGA-3′. All siRNA transfections were
performed using Lipofectamine 2000 (Invitrogen Life Technologies,
Carlsbad, CA, USA) in Opti-MEM (Invitrogen Life Technologies)
according to the manufacturer’s instructions, with a final siRNA
concentration of 100 nM.
Quantitative real-time polymerase chain
reaction (qRT-PCR) analysis
Total RNA from tumor cells or tissues was isolated
with a total RNA extraction kit (Sangon Biotech, Shanghai, China)
according to the manufacturer’s instructions. The primer sequences
for qRT-PCR analysis were designed and synthesized as follows:
Forward: 5′-CACGCTCAGCACCTTCATTG-3′ and reverse:
5′-GGTGAGCTGCTTGCTGTTGA-3′ for PRL-3; forward:
5′-CGCTACACGCAGTTGCAGTACA-3′ and reverse: 5′-AAGCGCAGCAGGATCTGGA-3′
for ERK 1; forward: 5′-TGTTCCCAAATGCTGACTCCAA-3′ and reverse:
5′-TCGGGTCGTAATACTGCTCCAGATA-3′ for ERK 2; forward:
5′-TGGACGGATGGTAGCAGTCT-3′ and reverse:
5′-TCTCCATTTCCATAGGTTGGAT-3′ for MMP-7; forward:
5′-GGCGGCACCACCATGTACCCT-3′ and reverse:
5′-AGGGGCCGGACTCGTCATACT-3′ for β-actin. RNA was first
retrotranscribed using the TaqMan® Reverse Transcription
kit (Applied Biosystems, Carlsbad, CA, USA), and then RT-PCR was
carried out using the TaqMan SYBR-Green master mix (Applied
Biosystems). The relative expression of the mRNA was normalized to
β-actin mRNA. The threshold cycle value (Ct) was defined as the
fractional cycle number at which the fluorescence passed an
invariable threshold. The comparative Ct method was used to
calculate the relative abundance of mRNA compared with that of
β-actin expression (21).
Western blot analysis
The cells were harvested, washed twice with 1X PBS
and lysed in 100 μl radioimmunoprecipitation assay (RIPA) lysis
buffer. Protein concentrations were determined using a
bicinchoninic acid (BCA) kit (Tiangen, China). Proteins were
resolved by 10% sodium dodecyl sulphate-polyacrylamide gel
electrophoresis (SDS-PAGE) and transferred to polyvinylidene
difluoride (PVDF) membranes (Roche Diagnostics GmbH, Mannheim,
Germany), which were then blocked with 5% non-fat milk in
Tris-buffered saline for 3 h, and incubated overnight with primary
antibodies. The secondary antibodies used were horseradish
peroxidase-conjugated goat anti-rabbit or -mouse IgG from Tiangen
(Beijing, China). Membranes were incubated with the secondary
antibodies for 1 h at room temperature, and proteins were detected
using an ECL Western Blotting Analysis system (Amersham Biosciences
Corp., Piscataway, NJ, USA).
Cell migration and invasion analysis
Cell migration assays were performed using 8.0-μm
pore size Transwell inserts (Costar, Cambridge, MA), with certain
modifications. Cell invasion was investigated using Matrigel-coated
8.0-μm filter invasion chambers (BD Biosciences, San José, CA,
USA). Cells were incubated for 24 h (for migration assay) or 48 h
(for invasion assay) at 37°C in a humidified atmosphere of 5%
CO2. Cells on the upper surface of the membrane were
removed using cotton tips after the indicated incubation times. The
migrant cells attached to the lower surface were stained with
crystal violet (500 μl of 5 mg/ml crystal violet dissolved in 20%
methanol) and incubated for 30 min. Cells were then soaked in 33%
ice-cold acetic acid and oscillated for 10 min. The ice-cold acetic
acid was then assessed by measuring the absorbance at 570 nm using
a microplate reader (Tecan, Shanghai, China).
Statistical analysis
Statistical analyses were carried out using SPSS
version 16.0 (SPSS, Inc., Chicago, IL, USA). The data are expressed
as the means ± standard deviation, and the significance of the data
was determined by one-way ANOVA analysis. P<0.05 was considered
to indicate a statistically significant difference.
Results
Gastric cell lines express variable
levels of PRL-3
We first examined the protein and mRNA expression
levels of PRL-3 in six gastric cell lines, using western blot
analysis and qRT-PCR. The six gastric cell lines included two
normal gastric cell lines, GES-1 and HFE-145, and four malignant
human GC cell lines with different degrees of cell differentiation,
BGC-823, MKN-28, SGC-7901 and MKN-45 (SGC-7901 and MKN-45 were
low-grade cell lines, and BGC-823 and MKN-28 were high-grade cell
lines). Among the six gastric cell lines that expressed PRL-3 at
various levels, SGC-7901 cells demonstrated the highest level of
PRL-3 protein (Fig. 1A) and mRNA
(Fig. 1B) expression, while GES-1
cells exhibited the lowest PRL-3 protein and mRNA expression;
SGC-7901 cell PRL-3 mRNA expression was 4.602-fold greater compared
with the GES-1 cell line (P<0.05). Therefore, we selected the
SGC-7901 cell line for subsequent RNA interference studies.
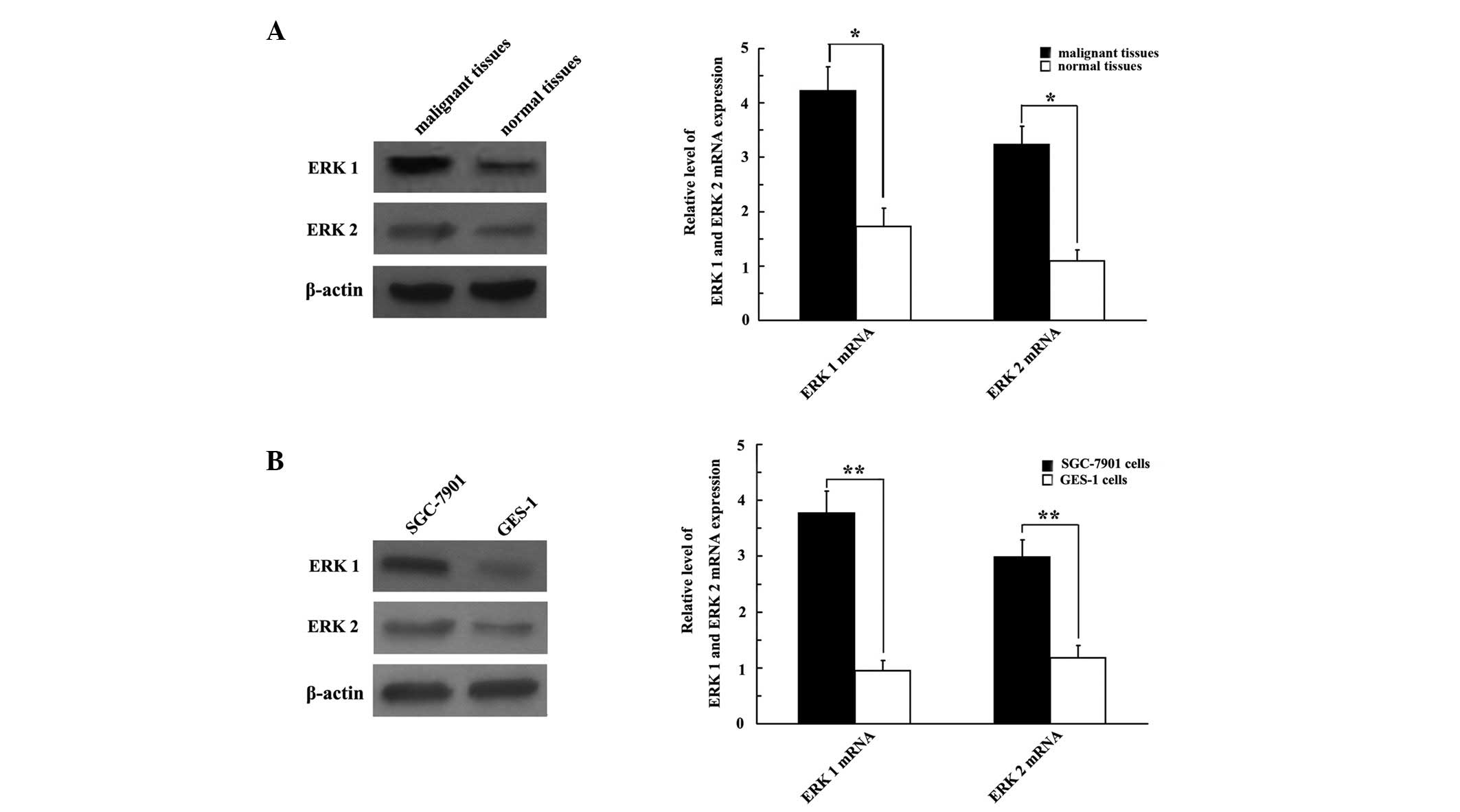
ERK 1/2 is highly expressed in GC
samples
We examined GC cell lines and tissues to elucidate
whether ERK 1/2 was expressed in GC. The results revealed that the
expression of ERK 1/2 protein was upregulated in both GC tissues
and cell lines (Fig. 2A and B,
respectively), compared with normal gastric tissues and cell lines.
The qRT-PCR analysis revealed that the SGC-7901 cell line exhibited
strong ERK 1/2 mRNA expression; ERK 1 and ERK 2 mRNA were 3.968-
and 2.525-fold greater, respectively, compared with the GES-1 cell
line (P<0.01; Fig. 2B).
Consistently, ERK 1/2 mRNA was overexpressed in malignant tissues
compared with normal gastric tissues (P<0.05; Fig. 2A).
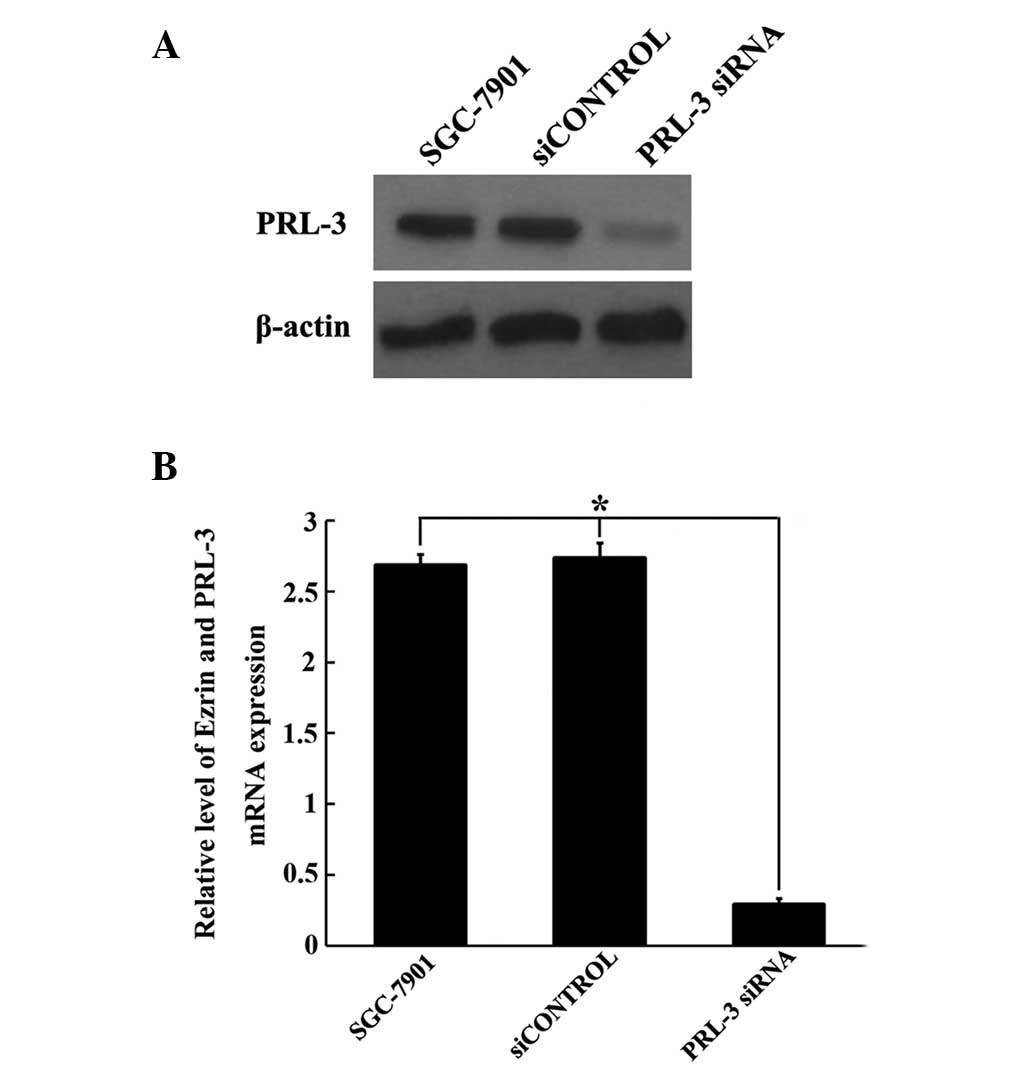
Identification of the efficiency of PRL-3
siRNA
As the PRL-3 levels were significantly higher in
tumor cells compared with normal cells, we aimed to determine
whether synthetic PRL-3 siRNA inhibited the expression of the PRL-3
gene in GC cells. To examine the efficiency of the specific PRL-3
siRNA, we used scrambled siRNA as the control. Following 48 h of
siRNA transfection, PRL-3 mRNA and protein expression levels were
measured by qRT-PCR and western blot analysis, respectively. As
shown in Fig. 3, PRL-3 siRNA was
capable of specifically and efficiently suppressing PRL-3
expression at the mRNA and protein level, compared with cells
treated with siCONTROL and untreated cells (P<0.05; Fig. 3). Considering the significant
silencing effect of PRL-3 siRNA, we used this siRNA for subsequent
experiments.
Downregulation of PRL-3 inhibits invasion
and migration of SGC-7901 human GC cells
To investigate the molecular mechanisms by which
PRL-3 promotes cell invasion and migration in human carcinoma
cells, following transfection with PRL-3 siRNA or the negative
vector (siCONTROL) for 48 h, SGC-7901 cells were subjected to
invasion or migration assays. As demonstrated in Fig. 4, cells transfected with PRL-3 siRNA
exhibited a 66.7% decrease in invasion and a 68.1% decrease in
migration, compared with control vector cells and untreated cells
(P<0.05; Fig. 4A and B,
respectively).
Inhibiting PRL-3 downregulation of ERK
1/2 and MMP-7 expression in SGC-7901 cells
ERK 1/2 is a member of the MAPK family and has been
demonstrated to be associated with cell motility and invasion
(13,14). MMP-7, the downstream target of ERK
1/2, contributes to cell motility by remodeling the extracellular
matrix. In the present study, we used western blot analysis and
qRT-PCR to examine the changes in ERK 1/2 and MMP-7 protein and
mRNA expression post-transfection with PRL-3 siRNA. The western
blot analysis revealed that 72 h post-transfection, the PRL-3 siRNA
resulted in significant decreases in ERK 1/2, phosphorylated ERK
1/2 (pERK 1/2) and MMP-7 protein levels, compared with untreated
controls or cells transfected with siCONTROL (Fig. 5A). The qRT-PCR results also
demonstrated that ERK 1/2 and MMP-7 mRNA levels were similarly
affected 72 h post-transfection (P<0.05, P<0.01 and
P<0.05, respectively; Fig.
5B-D).
Discussion
The majority of cancer-related mortalities are due
to tumor metastases rather than primary tumors (22,23).
Failure of treatment for GC is mainly caused by metastasis and
invasion of the tumor cells to the neighboring organs. However, the
specific molecular changes in GC cells that promote the metastatic
process are largely unclear. Understanding the metastasic
mechanisms is important for aiding and improving the success of
treatment for this cancer.
Inhibiting specific gene expression by RNAi has
become an important method of cancer treatment (24,25).
Recently, PRL-3 was demonstrated to be overexpressed in GC cells,
and has been proposed to be a novel marker of poor outcome in GC
(26,27). To explore whether PRL-3 may become
a potential molecular target for gene therapy of GC, we employed
RNAi technology to downregulate PRL-3 expression in a human GC cell
line, SGC-7901. Compared with the control group, the mRNA and
protein expression levels were significantly decreased in the cells
transfected with PRL-3 siRNA. These results indicated that PRL-3
siRNA was able to effectively and specifically silence the
expression of PRL-3 in SGC-7901 cells.
It is known that PRL-3 is associated with the
progression and eventual metastasis of several types of human
cancer (28). Thus, we examined
the effect of PRL-3 suppression on the invasion of SGC-7901 cells
by mobility assays. The results demonstrated that the migratory
ability was significantly reduced through Matrigel-coated chamber
membranes compared with the control group. Therefore, there is a
strong correlation between PRL-3 expression levels and the invasion
or migration ability of human GC cells. These results are
consistent with previous studies demonstrating that inhibition of
PRL-3 signaling reduced the migration and invasion ability of tumor
cells (29,30).
Although the mechanism whereby PRL-3 affects cell
invasion remains undefined, several studies have clarified the
PRL-3 target molecules, a number of which are known to be involved
in cell motility (31). ERK 1/2
are crucial for various cell activities including proliferation,
migration and invasion (32,33).
MMPs are downstream target proteins of ERK 1/2. Among >20 MMPs,
MMP-7 appears to be one of the most important MMPs in human GC, as
it is highly overexpressed in GC (34,35).
Luo et al demonstrated that PRL-1 promoted motility and
invasion in HEK293 cells by increasing MMP-2 and MMP-9 expression
via the ERK 1/2 pathway (18). As
the amino acid sequences of PRL-1 and PRL-3 are >75% identical,
and the two sequences contain the C-terminal prenylation motif CAAX
to be prenylated (36,37), it is reasonable that PRL-3 may
possess similar abilities to PRL-1. Therefore, we hypothesized that
PRL-3 is capable of influencing the protein synthesis or activities
of ERK 1/2-MMP-7, leading to the facilitation of cell motility and
invasion.
To test this hypothesis, we examined the effect of
PRL-3 on the cell activities of ERK1/2, and found that the
expression of ERK 1/2 decreased significantly following
transfection with PRL-3 siRNA. Additionally, to investigate whether
PRL-3 siRNA had any effect on MMP-7, we used qRT-PCR and western
blot analysis to detect the expression levels of MMP-7. The results
demonstrated that the level of MMP-7 expression also decreased
significantly. To the best of our knowledge, this study provides
the first evidence that MMP-7 expression may be downregulated via
the inhibition of PRL-3 in GC. However, whether PRL-3 activates
MMP-7 through the ERK 1/2 pathway requires further
investigation.
The present study has demonstrated that PRL-3 and
ERK 1/2 proteins were highly expressed in GC cells and tissues, and
that downregulation of PRL-3 expression by siRNA inhibited the
invasion and migration of SGC-7901 GC cells. Furthermore, knockdown
of PRL-3 suppresses the expression levels of ERK 1/2 and MMP-7.
These findings suggest that further investigation of the regulation
of the PRL-3-ERK1/2-MMP-7 pathway may have therapeutic implications
for the prognosis and treatment of GC metastasis.
Acknowledgements
This study was supported by the Scientific and
Technological Project of Jiangxi Province (no. 2009BSB11216).
References
|
1
|
Compare D, Rocco A and Nardone G: Risk
factors in gastric cancer. Eur Rev Med Pharmacol Sci. 14:302–308.
2010.
|
|
2
|
Parkin DM, Bray F, Ferlay J and Pisani P:
Global cancer statistics, 2002. CA Cancer J Clin. 55:74–108. 2005.
View Article : Google Scholar
|
|
3
|
Petrocca F, Visone R, Onelli MR, Shah MH,
Nicoloso MS, de Martino I, Iliopoulos D, Pilozzi E, Liu CG, Negrini
M, et al: E2F1-regulated microRNAs impair TGFbeta-dependent
cell-cycle arrest and apoptosis in gastric cancer. Cancer Cell.
13:272–286. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Bessette DC, Qiu D and Pallen CJ: PRL
PTPs: mediators and markers of cancer progression. Cancer
Metastasis Rev. 27:231–252. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Andersen JN, Jansen PG, Echwald SM,
Mortensen OH, Fukada T, Del Vecchio R, Tonks NK and Møller NP: A
genomic perspective on protein tyrosine phosphatases: gene
structure, pseudogenes, and genetic disease linkage. Faseb J.
18:8–30. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Alonso A, Sasin J, Bottini N, Friedberg I,
Friedberg I, Osterman A, Godzik A, Hunter T, Dixon J and Mustelin
T: Protein tyrosine phosphatases in the human genome. Cell.
117:699–711. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Wu X, Zeng H, Zhang X, Zhao Y, Sha H, Ge
X, Zhang M, Gao X and Xu Q: Phosphatase of regenerating liver-3
promotes motility and metastasis of mouse melanoma cells. Am J
Pathol. 164:2039–2054. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Stephens B, Han H, Hostetter G, Demeure MJ
and Von Hoff DD: Small interfering RNA-mediated knockdown of PRL
phosphatases results in altered Akt phosphorylation and reduced
clonogenicity of pancreatic cancer cells. Mol Cancer Ther.
7:202–210. 2008. View Article : Google Scholar
|
|
9
|
Zeng Q, Dong JM, Guo K, Li J, Tan HX, Koh
V, Pallen CJ, Manser E and Hong W: PRL-3 and PRL-1 promote cell
migration, invasion, and metastasis. Cancer Res. 63:2716–2722.
2003.PubMed/NCBI
|
|
10
|
Radke I, Götte M, Kersting C, Mattsson B,
Kiesel L and Wülfing P: Expression and prognostic impact of the
protein tyrosine phosphatases PRL-1, PRL-2, and PRL-3 in breast
cancer. Br J Cancer. 95:347–354. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Zhou J, Wang S, Lu J, Li J and Ding Y:
Over-expression of phosphatase of regenerating liver-3 correlates
with tumor progression and poor prognosis in nasopharyngeal
carcinoma. Int J Cancer. 124:1879–1886. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Li ZR, Wang Z, Zhu BH, He YL, Peng JS, Cai
SR, Ma JP and Zhan WH: Association of tyrosine PRL-3 phosphatase
protein expression with peritoneal metastasis of gastric carcinoma
and prognosis. Surg Today. 37:646–651. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Sharma GD, He J and Bazan HE: p38 and ERK
1/2 coordinate cellular migration and proliferation in epithelial
wound healing: evidence of cross-talk activation between MAP kinase
cascades. J Biol Chem. 278:21989–21997. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Mu H, Wang X, Wang H, Lin P, Yao Q and
Chen C: Lactosylceramide promotes cell migration and proliferation
through activation of ERK1/2 in human aortic smooth muscle cells.
Am J Physiol Heart Circ Physiol. 297:H400–H408. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
McCawley LJ, Li S, Wattenberg EV and
Hudson LG: Sustained activation of the mitogen-activated protein
kinase pathway. A mechanism underlying receptor tyrosine kinase
specificity for matrix metalloproteinase-9 induction and cell
migration. J Biol Chem. 274:4347–4353. 1999. View Article : Google Scholar
|
|
16
|
Deryugina EI and Quigley JP: Matrix
metalloproteinases and tumor metastasis. Cancer Metastasis Rev.
25:9–34. 2006. View Article : Google Scholar
|
|
17
|
Björklund M and Koivunen E:
Gelatinase-mediated migration and invasion of cancer cells. Biochim
Biophys Acta. 1755:37–69. 2005.PubMed/NCBI
|
|
18
|
Luo Y, Liang F and Zhang ZY: PRL1 promotes
cell migration and invasion by increasing MMP2 and MMP9 expression
through Src and ERK1/2 pathways. Biochemistry. 48:1838–1846. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Zeng Q, Si X, Horstmann H, Xu Y, Hong W
and Pallen CJ: Prenylation-dependent association of
protein-tyrosine phosphatases PRL-1, -2, and -3 with the plasma
membrane and the early endosome. J Biol Chem. 275:21444–21452.
2000. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Kato H, Semba S, Miskad UA, Seo Y, Kasuga
M and Yokozaki H: High expression of PRL-3 promotes cancer cell
motility and liver metastasis in human colorectal cancer: a
predictive molecular marker of metachronous liver and lung
metastases. Clin Cancer Res. 10:7318–7328. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Lawrie CH, Gal S, Dunlop HM, Pushkaran B,
Liggins AP, Pulford K, Banham AH, Pezzella F, Boultwood J,
Wainscoat JS, et al: Detection of elevated levels of
tumour-associated microRNAs in serum of patients with diffuse large
B-cell lymphoma. Br J Haematol. 141:672–675. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Chambers AF, Groom AC and MacDonald IC:
Dissemination and growth of cancer cells in metastatic sites. Nat
Rev Cancer. 2:563–572. 2002. View
Article : Google Scholar : PubMed/NCBI
|
|
23
|
Weiss L: Metastasis of cancer: a
conceptual history from antiquity to the 1990s. Cancer Metastasis
Rev. 19:193–383. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Sun P, Yu H, Zhang WQ, Hu M and Lv R:
Lentivirus-mediated siRNA targeting VEGF inhibits gastric cancer
growth in vivo. Oncol Rep. 28:1687–1692. 2012.PubMed/NCBI
|
|
25
|
Qin XJ, Dai DJ, Gao ZG, Huan JL and Zhu L:
Effect of lentivirus-mediated shRNA targeting VEGFR-3 on
proliferation, apoptosis and invasion of gastric cancer cells. Int
J Mol Med. 28:761–768. 2011.PubMed/NCBI
|
|
26
|
Dai N, Lu AP, Shou CC and Li JY:
Expression of phosphatase regenerating liver 3 is an independent
prognostic indicator for gastric cancer. World J Gastroenterol.
15:1499–1505. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Wang Z, Cai SR, He YL, Zhan WH, Chen CQ,
Cui J, Wu WH, Wu H, Song W, Zhang CH, et al: High expression of
PRL-3 can promote growth of gastric cancer and exhibits a poor
prognostic impact on patients. Ann Surg Oncol. 16:208–219. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Miskad UA, Semba S, Kato H, Matsukawa Y,
Kodama Y, Mizuuchi E, Maeda N, Yanagihara K and Yokozaki H: High
PRL-3 expression in human gastric cancer is a marker of metastasis
and grades of malignancies: an in situ hybridization study.
Virchows Arch. 450:303–310. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Ming J, Liu N, Jiang GC, Zhang QF, Qiu XS
and Wang EH: Downregulating PRL-3 inhibit migration and invasion of
lung cancer cell via RhoA and mDia1. Tumori. 98:370–376.
2012.PubMed/NCBI
|
|
30
|
Mizuuchi E, Semba S, Kodama Y and Yokozaki
H: Down-modulation of keratin 8 phosphorylation levels by PRL-3
contributes to colorectal carcinoma progression. Int J Cancer.
124:1802–1810. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Peng L, Jin G, Wang L, Guo J, Meng L and
Shou C: Identification of integrin alpha1 as an interacting protein
of protein tyrosine phosphatase PRL-3. Biochem Biophys Res Commun.
342:179–183. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Kwak DH, Lee JH, Kim T, Ahn HS, Cho WK, Ha
H, Hwang YH and Ma JY: Aristolochia manshuriensis Kom inhibits
adipocyte differentiation by regulation of ERK 1/2 and Akt pathway.
PLoS One. 7:e495302012. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Ma L, Lan F, Zheng Z, Xie F, Wang L, Liu
W, Han J, Zheng F, Xie Y and Huang Q: Epidermal growth factor (EGF)
and interleukin (IL)-1β synergistically promote ERK1/2-mediated
invasive breast ductal cancer cell migration and invasion. Mol
Cancer. 11:792012.
|
|
34
|
Yang Y, Shi H, Li X and Yi Y: Effects of
shRNA targeting maspin on invasion of gastric carcinoma SGC7901
cell line. Oncol Rep. 25:259–265. 2011.PubMed/NCBI
|
|
35
|
Koskensalo S, Mrena J, Wiksten JP,
Nordling S, Kokkola A, Hagström J and Haglund C: MMP-7
overexpression is an independent prognostic marker in gastric
cancer. Tumour Biol. 31:149–155. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Zeng Q, Hong W and Tan YH: Mouse PRL-2 and
PRL-3, two potentially prenylated protein tyrosine phosphatases
homologous to PRL-1. Biochem Biophys Res Commun. 244:421–427. 1998.
View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Cates CA, Michael RL, Stayrook KR, Harvey
KA, Burke YD, Randall SK, Crowell PL and Crowell DN: Prenylation of
oncogenic human PTP (CAAX) protein tyrosine phosphatases. Cancer
Lett. 110:49–55. 1996. View Article : Google Scholar : PubMed/NCBI
|