Introduction
Stem cells may be used in the treatment of various
diseases due to their differentiation activity and self-renewal
potential. When subcultured over several generations, stem cells
are unable to undergo differentiation or self-renewal, mainly due
to the surrounding microenvironment, which is known to largely
control the fate of stem cells. Recently, various biomaterials have
been developed to maintain embryonic stem cell self-renewal.
Mesenchymal stem cells (MSCs), one type of adult stem cell, are
widely distributed in several tissues, including bone marrow, cord
blood and adipose tissue. MSCs are able to differentiate into a
variety of cell types, including adipocytes, osteocytes,
chondrocytes, myocytes and hepatocytes of the mesodermal lineage
and nerve and epithelial cells of the ectodermal lineage (1–6).
Besides differentiation, MSCs also release numerous cytokines to
support tissue regeneration and repair, including insulin-like
growth factor-1 (IGF-1), vascular endothelial growth factor-α
(VEGF-α) and macrophage chemokines (7). Numerous studies have shown that MSCs
stimulate the regeneration and repair of damaged tissues,
particularly in acute ischemic diseases, including acute myocardial
or cerebral infarction and various types of arthritis (1,3,5,6,8).
Furthermore, previous studies have shown that MSCs have special
immunological characteristics; therefore, MSCs may be transplanted
without a rejection reaction (9,10).
In addition, MSCs are involved in immune regulation, thus they have
the potential to be used as a novel therapy to treat rejection
following organ transplantation and autoimmune diseases.
Consequently, MSCs may have a therapeutic value in the treatment of
numerous serious diseases and a broad scope in future clinical
applications.
The regeneration, repair and immune regulation
abilities of MSCs are largely due to their multi-lineage
differentiation potential and paracrine actions. Currently, it is
difficult to maintain the stability of MSCs under in vitro
culture conditions, since aging, spontaneous differentiation into
osteocytes and a decreased proliferation ability have been reported
in MSC cultures under normal conditions (11–13).
The spontaneous differentiation of MSCs decreases their ability to
differentiate into other important cells, such as nerve and cardiac
muscle cells. The transplantation of these differentiated MSCs
(which are not morphologically similar to osteocytes) to non-bone
tissues has been shown to result in ectopic calcification and the
impairment of tissue functions (14). The paracrine actions of MSCs
cultured in vitro have also been found to decrease and their
myocardial protection ability was affected (15). Finally, the aging and
differentiation of MSCs cultured in vitro had significant
effects on their therapeutic outcome and safety.
Chitosan is a chitin derivative that is widely found
in nature. It is composed of D-glucosamine and
N-acetyl-glucosamine, which are joined by β (1→4) glycosidic bonds.
Due to its biocompatibility, non-immunogenicity and non-toxicity,
chitosan is compatible and cross-linked with collagen. The
different types of chitosan and its deacylation level have been
found to affect cell adhesion and proliferation (16–19).
Melanocytes and keratocytes cultured on chitosan-coated surfaces
have been shown to form spheroids, which were maintained in
three-dimensional (3D) culture systems (16,20).
In the present study, we investigated the effect of cell culture
using chitosan membranes on the stemness of MSCs and provided
information with regard to the clinical application of MSC-based
therapy.
Materials and methods
Ethics
This study was conducted in accordance with the
declaration of Helsinki. This study was conducted with approval
from the Ethics Committee of the First Affiliated Hospital of
Xinxiang Medical University, Weihui, China.
MSC culture
The first generation of bone marrow-derived human
mesenchymal stem cells (hMSCs) were provided by the Shengzhen
Baiwang Biotechnology Corporation (Shengzhen, China). hMSCs (100
cells/cm2) were thawed and recovered for 24 h, and then
cultured in full medium (CCM) with 17% fetal bovine serum (FBS) for
7–8 days until 70% fusion. hMSCs were passaged under the same
conditions and were used in the experiment before the third
generation.
Preparation of chitosan membranes
Chitosan powder (molecular weight, 510 kDa; 77.8%
deacetylation; Shengzheng Baiwang Biotechnology Corporation) was
dissolved in 1% aqueous acetic acid solution to obtain a 1%
chitosan solution. Next, 300 μl of the chitosan solution was coated
on a glass coverslip and dried out at room temperature. Following
neutralization with 0.5 N NaOH for 30 min, the glass coverslip was
washed with PBS 5 times and exposed to ultraviolet radiation for 1
h. The prepared chitosan membranes were used in further
experiments.
Generation and dissociation of
spheroids
hMSCs were cultured at a low density (1,000
cells/well) in 6-well plates containing chitosan membranes. Cell
growth was observed daily using an inverted microscope. To obtain
spheroid-derived cells, spheroids were incubated with trypsin/EDTA
for 5–30 min (depending on the size of the spheroid). During
dissociation, the cells were pipetted every 2–3 min.
Proliferation and survival ratio of MSCs
cultured using chitosan membranes
The cell numbers of MSCs cultured with or without
chitosan membranes were analyzed using the fluorescent dye Hoechst
33528 on days 1, 3 and 10 of culture. Spheroids and cells were
digested in papain solution (Sigma, San Francisco, CA, USA) and
then incubated with 0.1 mg/ml Hoechst 33528 dye (Sigma). The
concentration was measured by a fluorescence spectrophotometer
(F2500; Hitachi, Tokyo, Japan) at room temperature using an
excitation and emission wavelength of 365 and 458 nm, respectively.
The cell number was calculated based on a standard curve and the
cell activity was measured using a propidium iodide (PI) staining
assay (Sigma Resources and Technologies, Inc., Santa Clara, CA,
USA). PI solution (2 mg/ml) was added to the cell suspension and
the percentage of unstained cells was defined as cell activity.
RNA extraction and quantitative real-time
PCR (qRT-PCR) analysis
Total RNA was extracted from the cells using TRIzol
reagent (Invitrogen, Carlsbad, CA, USA) according to the
manufacturer's instructions. The isolated RNA was reverse
transcribed and amplified using the RevertAid First Strand cDNA
Synthesis Kit (MBI Fermentas, St. Leon-Rot, Germany). cDNA was then
used as a template for qRT-PCR to quantitatively analyze the gene
expression levels with the DyNAmo Flash SYBR Green qPCR kit
(Finnzymes, Espoo, Finland). β-actin was amplified and used as an
internal standard to normalize the expression of tested genes. The
primers of the genes investigated are listed in Table I.
 | Table IPrimers used for quantitative
real-time PCR (qRT-PCR). |
Table I
Primers used for quantitative
real-time PCR (qRT-PCR).
| Gene | Primer sequence | Annealing temperature
(°C) |
|---|
| β-actin | Sense:
AACTACCTTCAACTCCATC
Antisense: TGATCTTGATCTTCATTGTG | 55 |
| Oct4 | Sense:
ACATCAAAGCTCTGCAGAAA
Antisense: CTGAATACCTTCCCAAATAGAAC | 60 |
| Sox2 | Sense:
TGCGAGCGCTGCACAT
Antisense: GCAGCGTGTACTTATCCTTCTTCA | 58 |
| Nanog | Sense:
AATACCTCAGCCTCCAGCAGAT
Antisense: TGCGTCACACCATTGCTATTCTT | 60 |
MSC differentiation into nerve cells
MSCs were primarily cultured for 3 days to induce
differentiation into nerve cells. Following the addition of 10
ng/ml epidermal growth factor (EGF; Gibco-BRL, Rockville, MD, USA),
20 ng/ml hepatocyte growth factor (HGF; R&D Systems,
Minneapolis, MN, USA) and 20 ng/ml VEGF (R&D Systems) were
added to the culture medium, and the cells were cultured for an
additional 10 days.
Induction of chondrogenic
differentiation
To induce chondrogenic differentiation, basic medium
was replaced by chondrogenic differentiation medium following a
primary culture for 3 days. Chondrogenic differentiation medium
contained 10 ng/ml transforming growth factor β3 (TGFβ3)
(CytoLab/PeproTech, Rehovot, Israel), 0.1 mM dexamethasone, 50
mg/ml L-ascorbic acid 2-phosphate, 40 mg/ml L-proline, 1% insulin
transferrin selenium (ITS-premix 100; Sigma), 1% penicillin and 1%
streptomycin. The medium was replaced twice a week. For
morphological observation, the cells were incubated in precooled
acetone and washed with PBS. The cells were then stained with
Mayer's hematoxylin solution and washed once with toluidine blue
and water. Safranin O was then applied for 5 min to stain the
cells. Subsequently, 95% alcohol, absolute alcohol and xylene were
sequentially used to treat the cells once every 2 min. Finally,
differentiation was observed under a microscope.
Statistical analysis
The data were analyzed using SPSS 12.0.1 software
and are presented as the mean ± standard error (SE). P<0.05 was
considered to indicate a statistically significant difference.
Results
Morphology of MSCs cultured using
chitosan membranes
MSCs cultured in a 2D culture system had a typical
long polygonal morphology. However, spheroid formation was observed
within 1–2 days when the cells were transferred to chitosan
membranes. Cell size was reduced by 75% compared with cells
cultured in a 2D system following trypsin digestion, similar to
spheroids generated using the pendant drop method (Fig. 1).
Spheroid cell viability
Since MSC spheroids have limited access to
nutritional agents, cell viability was investigated. The 10,000 or
25,000 MSC spheroid was cultured for 3 days and 90% of harvested
cells survived. The number of apoptotic cells slightly increased
with the duration of culture (Fig.
2). Growth curves for cultured MSCs were plotted for the
different culture materials used. MSC proliferation was found to be
maintained after spheroid formation.
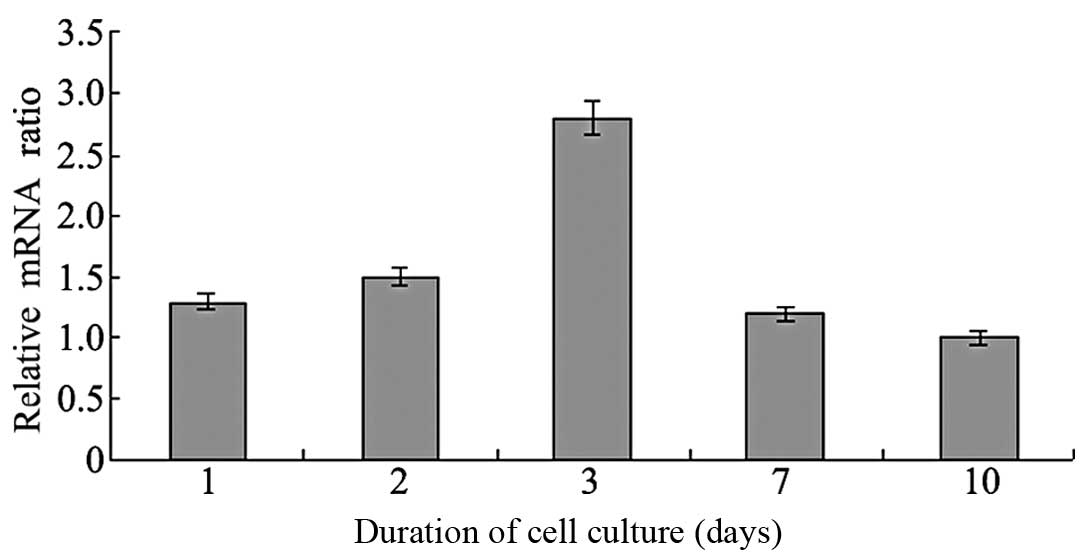
Effects of spheroid formation on the
expression of stemness marker genes
To determine the regulatory roles of stemness marker
genes in MSC culture, the expression levels of Oct4, Sox2 and Nanog
mRNA with different culture materials were analyzed by RT-PCR
(Figs. 3 and 4). A ratio >1 was considered to
indicate upregulated gene expression, while <1 showed
downregulation. The expression levels of these genes in MSCs
cultured using chitosan membranes were upregulated within 1–3 days,
and downregulated after 7 days. This indicated that spheroid
formation by MSCs cultured using chitosan membranes may be able to
maintain the expression of MSC stemness marker genes.
Effect of culture using chitosan
membranes on MSC differentiation
Cells cultured in medium supplemented with growth
factors were differentiated and transformed into cells with a long
and thin morphology. The change in cell morphology was similar to
that of nerve cells; for example, the cytoplasmic area around the
nucleolus was smaller (Fig. 5).
MSCs cultured using chitosan membranes had an increased
differentiation efficiency (P<0.001). These results indicated
that MSCs were able to differentiate into nerve cells in the
presence of these growth factors, and that the MSCs cultured on
chitosan membranes had an increased potential for this
differentiation. When TGFβ3 was added to the medium of MSCs
cultured using chitosan membranes, cells differentiated into
cartilage after 2 weeks, and chitosan membranes were found to aid
this type of differentiation (Fig.
6). These results indicated that spheroids formed by MSCs
cultured using chitosan membranes were similar to MSC spheroids
generated by the pendant drop method. This method maintained the
stemness ability of MSCs and may aid the induction of
differentiation under specific conditions.
Discussion
Stem cells are known for their self-renewal ability
and may be induced to differentiate into specific cell types when
cultured under particular conditions. They are also considered to
be important candidates for tissue engineering. However,
pluripotent stem cells lose their stemness ability when cultured
in vitro. To maintain the self-renewal ability of
undifferentiated embryonic stem cells (ESCs), the feeder layer,
such as mouse embryonic fibroblasts (MEFs) or conditional medium,
is required to provide a stable environment (21,22).
Numerous natural or synthetic polymeric materials have been
developed to replace the feeder layer in order to maintain the
self-renewal ability of ESCs (23–27).
Transcription factors Oct4, Sox2 and Nanog have been shown to be
important in the maintenance of pluripotency in ESCs (28,29).
These marker genes are also expressed in adult stem cells (30,31).
In the present study, we investigated the effect of cell culture
using chitosan membranes on the development and self-renewal
ability of undifferentiated MSCs. MSCs were demonstrated to rapidly
form 3D spheroids following culture using chitosan membranes, and
the expression levels of marker genes (Oct4, Sox2 and Nanog) either
remained stable or were upregulated.
3D multicellular states have been previously
described in a number of cell types, including ESC-derived embryoid
bodies and neurosphere-derived neurons (32). This information was useful for the
development of ESCs. MSCs have been shown to form 3D spheroids when
cultured using micropore membranes (33) or other types of surfaces (34,35).
Human MSCs cultured using micropore membranes formed spheroids and
exhibited increased anti-inflammatory properties in a previous
study (35). Spheroid formation
following 2D cell culture and the expression of
differentiation-related genes have not previously been
investigated. In the present study, the increased expression of
stemness-related genes indicated an improved plasticity of MSCs.
The accumulation of MSCs created a suitable environment for
cell-cell interactions, and promoted the early differentiation of
MSCs into nerve cells and chondrocytes (36). Various natural and synthetic 3D
scaffolds have been demonstrated to be beneficial for the formation
of nerve cells and cartilage (37,38).
The spheroid formation of MSCs that occurred during culture using
chitosan membranes not only enhanced cellular plasticity, but also
improved nerve cell and cartilage formation. The disadvantage of 3D
multicellular status may be the finite spreading of center.
However, our results indicated that the cell survival ratio was
>70%, even 7 days after spheroid formation, and that ≥90% cells
had survived 3 days after spheroid formation.
In conclusion, 3D MSC spheroids were observed in 2D
cultures within 1–2 days when using chitosan membranes. The
expression levels of stemness marker genes remained stable in these
spheroids; however, decreased expression levels of stemness marker
genes suppressed spheroid formation. These results indicate that
undifferentiated MSCs are able maintain their original state while
forming 3D spheroids when cultured using chitosan membranes.
Furthermore, spheroids exhibited improved nerve cell formation and
chondrogenic differentiation abilities following induction. 3D
spheroid formation on biomaterials may constitute a novel strategy
to maintain the self-renewal ability of MSCs and increase the
differentiation potential of MSCs into cartilage, and has the
potential to be applied in the treatment of diseases of the nervous
system and cartilage tissue engineering. However, the underlying
mechanism regulating spheroid formation requires further
investigation.
References
|
1
|
Phinney DG and Prockop DJ: Concise review:
mesenchymal stem/multipotent stromal cells: the state of
transdifferentiation and modes of tissue repair - current views.
Stem Cells. 25:2896–2902. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Pittenger MF, Mackay AM, Beck SC, et al:
Multilineage potential of adult human mesenchymal stem cells.
Science. 284:143–147. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Pittenger MF and Martin BJ: Mesenchymal
stem cells and their potential as cardiac therapeutics. Circ Res.
95:9–20. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Prockop DJ: Marrow stromal cells as stem
cells for nonhematopoietic tissues. Science. 276:71–74. 1997.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Wu Y, Chen L, Scott PG and Tredget EE:
Mesenchymal stem cells enhance wound healing through
differentiation and angiogenesis. Stem Cells. 25:2648–2659. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Wu Y, Zhao RC and Tredget EE: Concise
review: bone marrow-derived stem/progenitor cells in cutaneous
repair and regeneration. Stem Cells. 28:905–915. 2010.PubMed/NCBI
|
|
7
|
Chen L, Tredget EE, Wu PY and Wu Y:
Paracrine factors of mesenchymal stem cells recruit macrophages and
endothelial lineage cells and enhance wound healing. PLoS One.
3:e18862008. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Nagai A, Kim WK, Lee HJ, et al:
Multilineage potential of stable human mesenchymal stem cell line
derived from fetal marrow. PLoS One. 2:e12722007. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Aggarwal S and Pittenger MF: Human
mesenchymal stem cells modulate allogeneic immune cell responses.
Blood. 105:1815–1822. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Jiang XX, Zhang Y, Liu B, Zhang SX, Wu Y,
Yu XD and Mao N: Human mesenchymal stem cells inhibit
differentiation and function of monocyte-derived dendritic cells.
Blood. 105:4120–4126. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Baxter MA, Wynn RF, Jowitt SN, Wraith JE,
Fairbairn LJ and Bellantuono I: Study of telomere length reveals
rapid aging of human marrow stromal cells following in vitro
expansion. Stem Cells. 22:675–682. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Krampera M, Pasini A, Rigo A, et al:
HB-EGF/HER-1 signaling in bone marrow mesenchymal stem cells:
inducing cell expansion and reversibly preventing multilineage
differentiation. Blood. 106:59–66. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Wagner W, Horn P, Castoldi M, et al:
Replicative senescence of mesenchymal stem cells: a continuous and
organized process. PLoS One. 3:e22132008. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Pelttari K, Winter A, Steck E, et al:
Premature induction of hypertrophy during in vitro chondrogenesis
of human mesenchymal stem cells correlates with calcification and
vascular invasion after ectopic transplantation in SCID mice.
Arthritis Rheum. 54:3254–3266. 2006. View Article : Google Scholar
|
|
15
|
Crisostomo PR, Wang M, Wairiuko GM,
Morrell ED, Terrell AM, Seshadri P, Nam UH and Meldrum DR: High
passage number of stem cells adversely affects stem cell activation
and myocardial protection. Shock. 26:575–580. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Lin SJ, Jee SH, Hsaio WC, Lee SJ and Young
TH: Formation of melanocyte spheroids on the chitosan-coated
surface. Biomaterials. 26:1413–1422. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Yang TL and Young TH: The specificity of
chitosan in promoting branching morphogenesis of progenitor
salivary tissue. Biochem Biophys Res Commun. 381:466–470. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Yeh LK, Chen YH, Chiu CS, Hu FR, Young TH
and Wang IJ: The phenotype of bovine corneal epithelial cells on
chitosan membrane. J Biomed Mater Res A. 90:18–26. 2009.PubMed/NCBI
|
|
19
|
Chatelet C, Damour O and Domard A:
Influence of the degree of acetylation on some biological
properties of chitosan films. Biomaterials. 22:261–268. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Chen YH, Wang IJ and Young TH: Formation
of keratocyte spheroids on chitosan-coated surface can maintain
keratocyte phenotypes. Tissue Eng Part A. 15:2001–2013. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Chiao E, Kmet M, Behr B and Baker J:
Derivation of human embryonic stem cells in standard and chemically
defined conditions. Methods Cell Biol. 86:1–14. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Richards M, Fong CY, Chan WK, Wong PC and
Bongso A: Human feeders support prolonged undifferentiated growth
of human inner cell masses and embryonic stem cells. Nat
Biotechnol. 20:933–936. 2002. View
Article : Google Scholar : PubMed/NCBI
|
|
23
|
Lee ST, Yun JI, Jo YS, et al: Engineering
integrin signaling for promoting embryonic stem cell self-renewal
in a precisely defined niche. Biomaterials. 31:1219–1226. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Brafman DA, Chang CW, Fernandez A, Willert
K, Varghese S and Chien S: Long-term human pluripotent stem cell
self-renewal on synthetic polymer surfaces. Biomaterials.
31:9135–9144. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Nur-E-Kamal A, Ahmed I, Kamal J, Schindler
M and Meiners S: Three-dimensional nanofibrillar surfaces promote
self-renewal in mouse embryonic stem cells. Stem Cells. 24:426–433.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Gerecht S, Burdick JA, Ferreira LS,
Townsend SA, Langer R and Vunjak-Novakovic G: Hyaluronic acid
hydrogel for controlled self-renewal and differentiation of human
embryonic stem cells. Proc Natl Acad Sci USA. 104:11298–11303.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Abraham S, Eroshenko N and Rao RR: Role of
bioinspired polymers in determination of pluripotent stem cell
fate. Regen Med. 4:561–578. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Kopp JL, Ormsbee BD, Desler M and Rizzino
A: Small increases in the level of Sox2 trigger the differentiation
of mouse embryonic stem cells. Stem Cells. 26:903–911. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Sokolov MV, Panyutin IV, Onyshchenko MI,
Panyutin IG and Neumann RD: Expression of pluripotency-associated
genes in the surviving fraction of cultured human embryonic stem
cells is not significantly affected by ionizing radiation. Gene.
455:8–15. 2010. View Article : Google Scholar
|
|
30
|
Baer PC, Griesche N, Luttmann W, Schubert
R, Luttmann A and Geiger H: Human adipose-derived mesenchymal stem
cells in vitro: evaluation of an optimal expansion medium
preserving stemness. Cytotherapy. 12:96–106. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Fortunel NO, Otu HH, Ng HH, et al: Comment
on ‘Stemness: transcriptional profiling of embryonic and adult stem
cells’ and ‘a stem cell molecular signature’. Science.
302:3932003.
|
|
32
|
Kim SY and Im GI: Control of the
chondrogenesis during the development and its application for
cartilage tissue engineering. J Tissue Eng Regen Med. 6:595–602.
2009.
|
|
33
|
Bartosh TJ, Ylöstalo JH, Mohammadipoor A,
et al: Aggregation of human mesenchymal stromal cells (MSCs) into
3D spheroids enhances their antiinflammatory properties. Proc Natl
Acad Sci USA. 107:13724–13729. 2010. View Article : Google Scholar
|
|
34
|
Wang W, Itaka K, Ohba S, Nishiyama N,
Chung UI, Yamasaki Y and Kataoka K: 3D spheroid culture system on
micropatterned substrates for improved differentiation efficiency
of multipotent mesenchymal stem cells. Biomaterials. 30:2705–2715.
2009. View Article : Google Scholar
|
|
35
|
Miyagawa Y, Okita H, Hiroyama M, et al: A
microfabricated scaffold induces the spheroid formation of human
bone marrow-derived mesenchymal progenitor cells and promotes
efficient adipogenic differentiation. Tissue Eng Part A.
17:513–521. 2011. View Article : Google Scholar
|
|
36
|
Quintana L, zur Nieden NI and Semino CE:
Morphogenetic and regulatory mechanisms during developmental
chondrogenesis: new paradigms for cartilage tissue engineering.
Tissue Eng Part B Rev. 15:29–41. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Chang JC, Hsu SH and Chen DC: The
promotion of chondrogenesis in adipose-derived adult stem cells by
an RGD-chimeric protein in 3D alginate culture. Biomaterials.
30:6265–6275. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Martinez-Diaz S, Garcia-Giralt N, Lebourg
M, et al: In vivo evaluation of 3-dimensional polycaprolactone
scaffolds for cartilage repair in rabbits. Am J Sports Med.
38:509–519. 2010. View Article : Google Scholar : PubMed/NCBI
|