Introduction
Diabetes is a chronic disease that poses a serious
threat to human health. Diabetic patients (~85%) commonly have
concurrent bladder dysfunction symptoms, which are often referred
to as diabetic cystopathy. In general, patients lose
bladder-filling sensation and have decreased bladder contraction
activity, which leads to increases in bladder capacity and late
residual urine volume. In the late stages of this disease, urinary
tract function is impaired, causing hydronephrosis and uremia
(1). The pathogenesis of diabetic
bladder dysfunction occurs via diabetes-induced damage to the main
sensory and autonomic nerve system in the bladder (2).
In previous years, the correlation between nerve
growth factor (NGF) and diabetes with neuropathy has caused
widespread concern. Hellweg et al(3) reported that NGF is important for
reducing the incidence of diabetic neuropathy from the sensory
nerve pathways to the target organ axons antiporter. Sasaki et
al(4) showed that NGF levels
decreased with increasing time in the bladder and L6 S1 dorsal root
ganglia and function of Aδ and C fibers in the afferent nerve was
impaired, causing bladder voiding dysfunction. Preliminary results
of the study revealed that exogenous NGF may represent an effective
treatment for diabetic neuropathy. In 2001, Goins et
al(5) reported the first use
of defective recombinant herpes simplex virus (HSV) as a carrier of
the NGF gene into the rat bladder wall and identified an increase
in NGF levels in the bladder and dorsal root ganglion. In 2004,
Sasaki et al(4) reported
that HSV vector-mediated NGF treatment of the diabetic urinary
bladder in animal studies confirmed that gene therapy significantly
increased expression of NGF in the bladder and bladder afferent
pathways and improved urinary bladder function (6).
Stem cells are a class of cells with self-renewal
capacity and a high degree of proliferation and differentiation
potential. Stem cell research in urology has gradually increased.
Yokoyama et al(7) reported
that skeletal muscle-derived stem cells injected into the bladder
and urethra wall may be capable of long-term survival. Strasser
et al(8) treated 20 stress
urinary incontinence patients with autologous stem cell injections
into skeletal muscle. Complete remission of urinary incontinence
symptoms was reported in 18 patients. A bladder wall injection of
stem cells improves bladder function by the differentiation of stem
cells into smooth muscle cells and these cells may also function as
vectors for gene therapy. Huard et al(9) incorporated the β-gal gene into
skeletal muscle stem cells and injected these stem cells into the
bladder wall. The authors reported that cells expressing this gene
were able to differentiate into smooth muscle cells and improved
contractility was observed in the damaged bladder. These studies
indicate that transgenic technology coupled with cell engineering
technology has potential application in the treatment of urinary
system dysfunction.
Diabetic bladder dysfunction is an extremely
difficult clinical issue and currently lacks effective treatment.
Preliminary results have indicated that NGF gene transfer improves
bladder voiding function. Since mesenchymal stem cells (MSCs) have
multi-lineage differentiation potential to differentiate into
smooth muscle and also act as a vehicle for gene therapy, a
recombinant lentiviral NGF vector was constructed and transfected
into MSCs to generate stable expression of NGF in the present
study. NGF gene-modified MSCs were injected into bladder tissues
and MSC survival rate and expression of the NGF gene were
observed.
Materials and methods
Materials
An inbred strain of Sprague Dawley (SD) rats was
purchased from Shanghai Silaike Experimental Animals Co., Ltd.
(QZ:SCXK; Shanghai, China). This study was performed in strict
accordance with the recommendations in the Guide for the Care and
Use of Laboratory Animals of the National Institutes of Health. The
animal use protocol was reviewed and approved by the Institutional
Animal Care and Use Committee of Zhejiang Cancer Hospital.
Lentivirus for NGF recombination
A β-NGF sequence was amplified from pGC-E1-NGFB by
PCR and cloned into the pGC-FU lentivirus vector to construct
pGC-FU-β-NGF. The plasmid was confirmed by digestion and
sequencing. pGC-FU-β-NGF and pHelper 1.0 and 2.0 were transfected
into the human embryonic kidney cell line 293T to obtain
recombinant lentivirus GU-FU-β-NGF, which encodes the β-NGF
gene.
Rat MSCs
Rat MSCs were separated, cultivated and identified
by whole bone marrow adherent culture. The third generation of rat
MSCs was separated to detect osteogenesis- and lipogenesis-induced
differentiation by Alizarin red staining and Oil red O staining.
The assay was performed using flow cytometry to detect surface CD
markers of MSCs.
Lentivirus infection
Rat MSCs were infected with lentiviruses carrying
the GU-FU-β-NGF and GFP vectors. GFP expression was observed by
fluorescence microscopy. NGF mRNA was detected using real-time
polymerase chain reaction (PCR), while NGF expression was examined
by ELISA.
Diabetic rat model
SD rats (weight, 180–200 g) were selected and fasted
for 12 h. Rats were divided into the diabetic and control groups.
The diabetic group was treated with intraperitoneal injections of
60 mg/kg/time STZ, while the control group was treated with citric
acid buffer at the same volume. Venous blood glucose (BG) was
analyzed 72 h following injection. BG level >16.7 mmol/l was
considered to indicate the successful induction of diabetes.
BrdU-labeled rat MSCs
BrdU (1 g) was dissolved in 100 ml citric acid
buffer. NGF-transfected rat MSCs were digested with trypsin and
plated onto pre-treated 6-well plates at a density of
1×105 cells/ml. MSCs were incubated with 10 μmol/l BrdU
for 40 h when cell density reached 40%.
BrdU-labeled diabetic rat bladders
BrdU was incubated with NGF-transfected rat MSCs for
48 h. Cells were digested with trypsin and centrifuged at 157 × g
for 5 min. Cells were suspended in phosphate-buffered saline (PBS)
to a concentration of 2×105 cells/ml. Rats were
anesthesized using intraperitoneal injections of 10% chloral
hydrate (0.3 ml/100 g body weight). Following, disinfection, a
vertical incision (~1.0 cm) was made in the rat abdomen and the
bladder was exposed. Suspended cells (10 μl) were injected into the
bladder muscle using a microsyringe; a total of 10–20 points on the
muscle were injected. The incision was closed and the rats were
revived.
Immunohistochemistry
Rat bladders were removed 4 weeks following
transplantation of NGF-transfected MSCs labeled by BrdU. Samples
were fixed with 10% formaldehyde, immersed, flooded, sliced,
spread, dried at 75°C for 1 h and waxed. The samples were then
incubated with 3% H2O2 for 20 min to block
endogenous catalase and antigens were repaired by microwave. The
samples were incubated with 0.5% Triton X-100 and washed with PBS.
Next, the samples were incubated with 1% bovine serum albumin for
10 min to block non-specific antigens following 2N HCl incubation
for 30 min. The samples were incubated with a monoclonal BrdU
antibody (1:1,000) at 37°C for 2 h. Samples were incubated with
reagent 1 at 37°C for 2 h and then reagent 2 for 2 h. Next, the
samples were developed with 3,3′-diaminobenzidine and stained with
hematoxylin for 30 sec. Observations were performed by microscopy.
Rat bladder MSC samples without BrdU incubation were set as the
negative control group.
Reverse transcription RT-PCR
RNA was extracted from rat bladder using TRIzol.
Reverse transcription and PCR were performed according to the
manufacturer's instructions. Primer sequences were as follows: NGF,
upstream AACAGGACTCACAGGAGCAA and downstream CTTCCTGCTGAGCACACACA
(369 bp); and GAPDH (control), upstream CAAGGTCATCCATGACAACTTTG and
downstream GTCCACCACCCTGTTGCTGTAG (496 bp). The annealing
temperature was set at 58°C and the reaction was repeated for 35
cycles. PCR products were confirmed by 2% agarose gel
electrophoresis. Gel images were captured using the Quantity One
image analysis system. Expression of NGF/GAPDH was scored by the
grayscale value of each band. NGF gene expression was determined by
the ratio of NGF and GADPH expression.
ELISA assay
Rat bladders were removed 8 weeks following MSC
transplantation. Experimental subgroups were the control (normal
rat bladder), diabetic (diabetic rat bladder) and treatment groups
(diabetic rat bladder transplanted with MSCs transfected with the
NGF gene). Rat bladder tissue samples were thawed gradually and
dried following rinsing with cold saline. The bladders were weighed
and placed into 10 times volume saline. The samples were sheared,
homogenized with a tissue homogenizer and centrifuged for 12 min.
Supernatant was assayed using an ELISA kit according to the
manufacturer's instructions.
Statistical analysis
Data are presented as the mean ± SD and were
analyzed using SPSS 13.0. Data associated with NGF lentivirus
transfection and diabetic rat modeling were analyzed by a t-test
(independent samples test). ELISA and RT-PCR results were analyzed
by one-way ANOVA. Comparison analysis between groups was performed
with Student-Newman-Keuls law. P<0.05 was considered to indicate
a statistically significant difference.
Results
Lentivirus preparation
Agarose gel electrophoresis of PCR products is
presented in Fig. 1A, which
demonstrates that 3FLAG and β-BGF genes were successfully
amplified. PCR product bands were of the expected sizes; 102, 792
and 872 bp. Agarose gel electrophoresis of the double digested
products of lentivirus vector pGC-FU are demonstrated in Fig. 1B. The digestion was successful
since there were 2 bands of ~10 and 749 kb, which were the expected
product sizes.
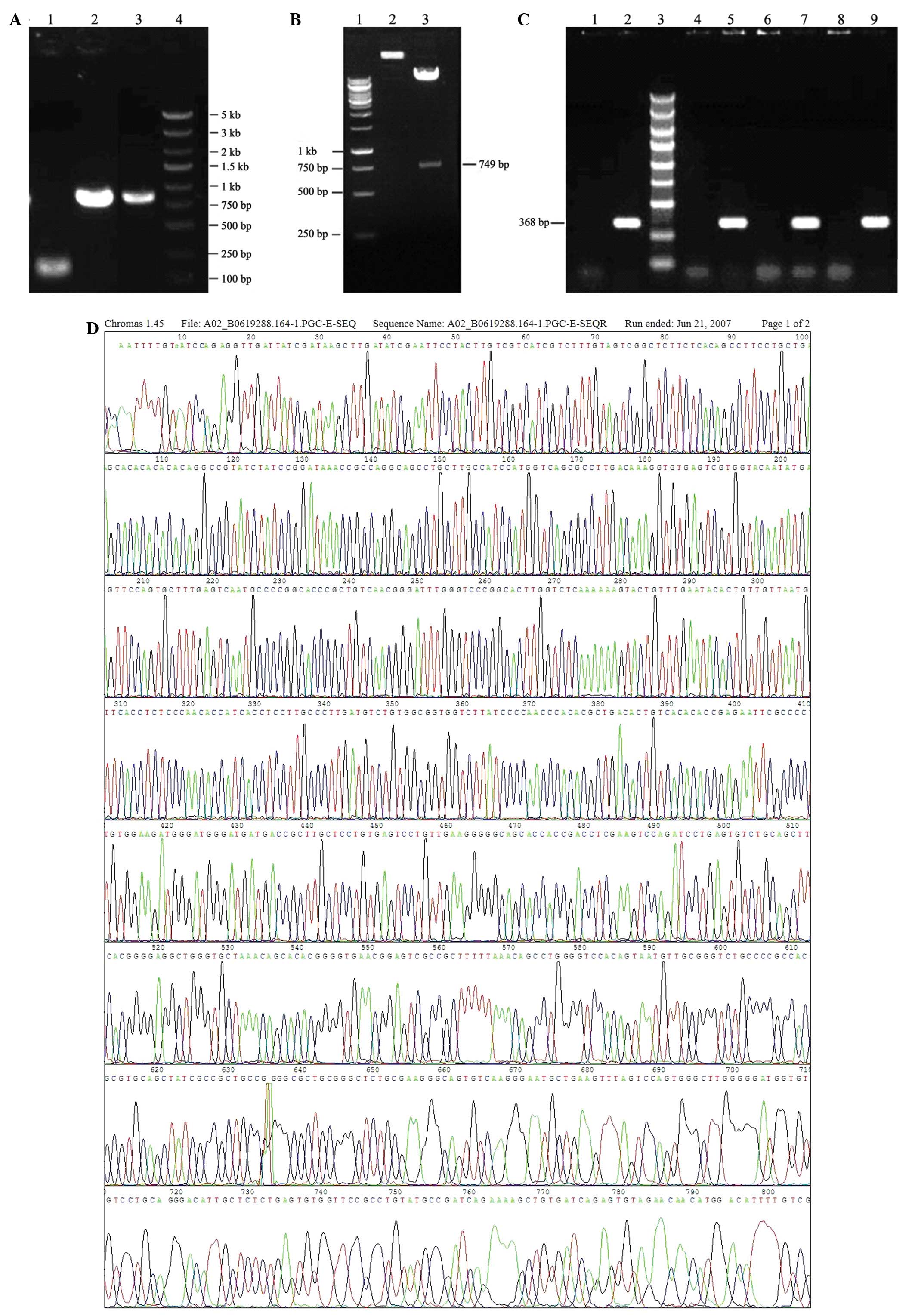 | Figure 1Preparation of lentivirus for NGF
reorganization. (A) Electrophoresis of PCR products: Lanes 1,
3FLAG; 2, β-NGF; 3, 3FLAG + β-NGF; and 4, marker. 3FLAG and β-BGF
genes were successfully amplified. PCR product bands were of the
expected sizes; 102, 792 and 872 bp. (B) pGC-FU was identified by
double digestion: Lanes 1, 1 kb marker; 2, pGC-FU without
digestion; and 3, pGC-FU digested with Age I and EcoR I. (C)
pGC-FU-β-NGF was identified by PCR: Lanes 1, negative control
(vector); 2, positive control (vector inserted with fragments which
had been sequence-identified); 3, marker; and 4–9, PCR products of
recombination plasmid. (D) Sequencing of the positive clone; (E)
BLAST analysis between sequence of the positive clone and standard
sequence. (F) Co-transfection of 293T cells with three plasmids
(bright field; magnification, ×100). NGF, nerve growth factor. |
Confirmation of recombination of pGC-FU-β-NGF by PCR
is revealed in Fig. 1C. Bands at
~368 bp in lanes 5, 7 and 9 were consistent with predicted PCR
products. Therefore, the plasmid recombination was considered to be
successful.
The positive plasmid was purified and sequenced.
BLAST analysis between the plasmid and standard sequences was
consistent, which further confirmed plasmid recombination (Fig. 1D). BLAST analysis between sequences
of the positive clone and standard is presented in Fig. 1E. Query represents the positive
clone plasmid sequence and subject represents the standard sequence
from GenBank. The identity of BLAST analysis was 99% (727/728),
while the missing base revealed in BLAST does not exist. Since the
coding DNA sequence of β-NGF is located between 170 and 895 bp, the
location revealed in BLAST represents a termination codon (UGA).
Therefore, the sequence for the recombinant plasmid was correct.
Fig. 1F demonstrates lentiviral
packaging by co-transfection to 293T cells with three plasmids.
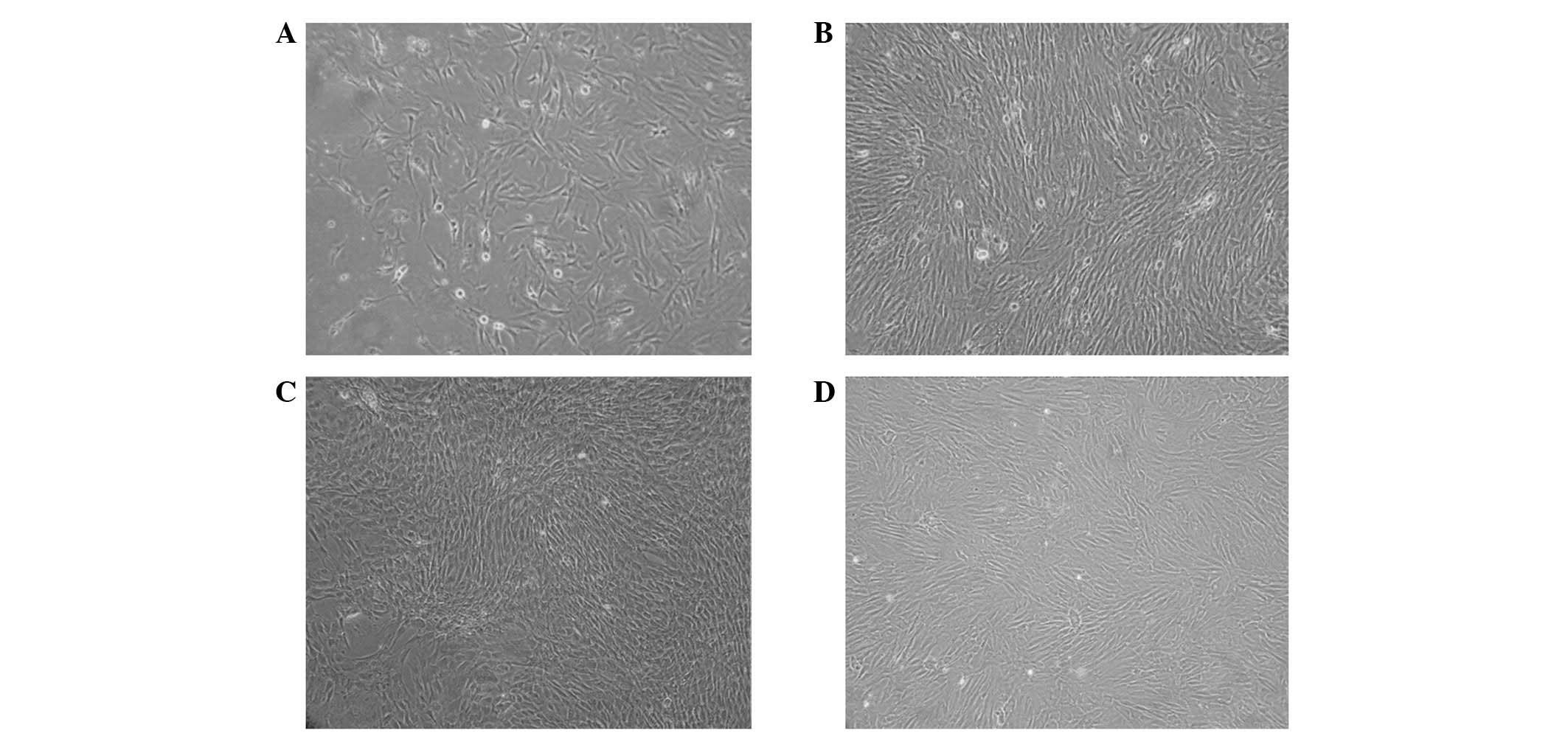
Cell culture and identification
Cells were round up when first inoculated into
culture flasks, while the majority of cells were suspended in cell
culture medium. The majority of cells were attached to the surface
after 48 h and unattached cells were removed. The morphologies of
attached cells were not homogeneous, exhibiting fusiform
fibroblastic or polygonal appearances. The cell nucleus was larger
and located at the center or edge of the cell. Dispersive attached
fibroblasts appeared at days 3 and 4 and cells formed colonies
following days 5 and 6; the colonies proliferated rapidly (Fig. 2A). Each colony was formed of
100–200 cells (Fig. 2D). Cells
demonstrated a spindle fibroblastic appearance, while certain cells
exhibited a broad and flat polygonal appearance. After 9–11 days,
cells formed a monolayer indicating >90% fusion and the vertical
axis of spindle cells were arranged parallel to each other
(Fig. 2B).
Cells were completely attached by 24 h and reached
complete fusion in 3–4 days, exhibiting a unique spindle appearance
(Fig. 2C and D). After 10
generations, cells maintained vigor in growth and expansion. No
significant change in fusion time was identified.
Expression of surface markers, CD90, CD44 and CD106,
was detected with flow cytometry. The expression levels of CD90,
CD44 and CD106 were 91.50, 99.87 and 78.02%, respectively. CD34,
CD45 and CD11 expression levels were low, at 0.07, 0.15 and 0.21%,
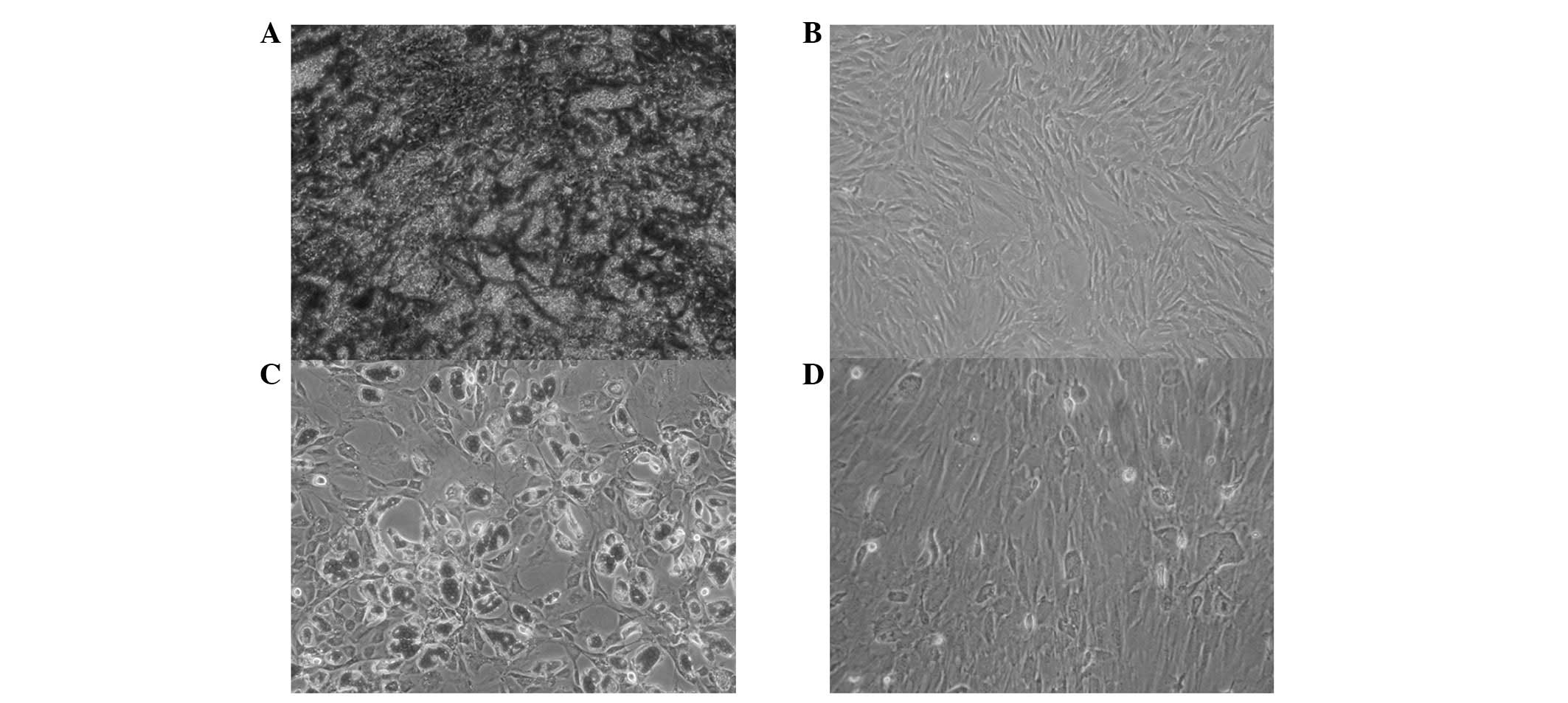
respectively (Fig. 3).
MSCs were stained with alizarin red following 21
days of induction. The induced group revealed a large number of
cells with an orange mineralization nodal morphology, while control
cells were negative for these properties (Fig. 4A and B).
Oil red O staining of MSCs following 2 weeks of
induction demonstrated numerous intracellular lipid droplets around
the nucleus and lined up in bright columns, while the cells
revealed various long spindle appearances. Partial lipid droplets
merged into larger conglomerations and moved the nucleus aside. A
low number of red lipid droplets were observed in the control group
(Fig. 4C and D).
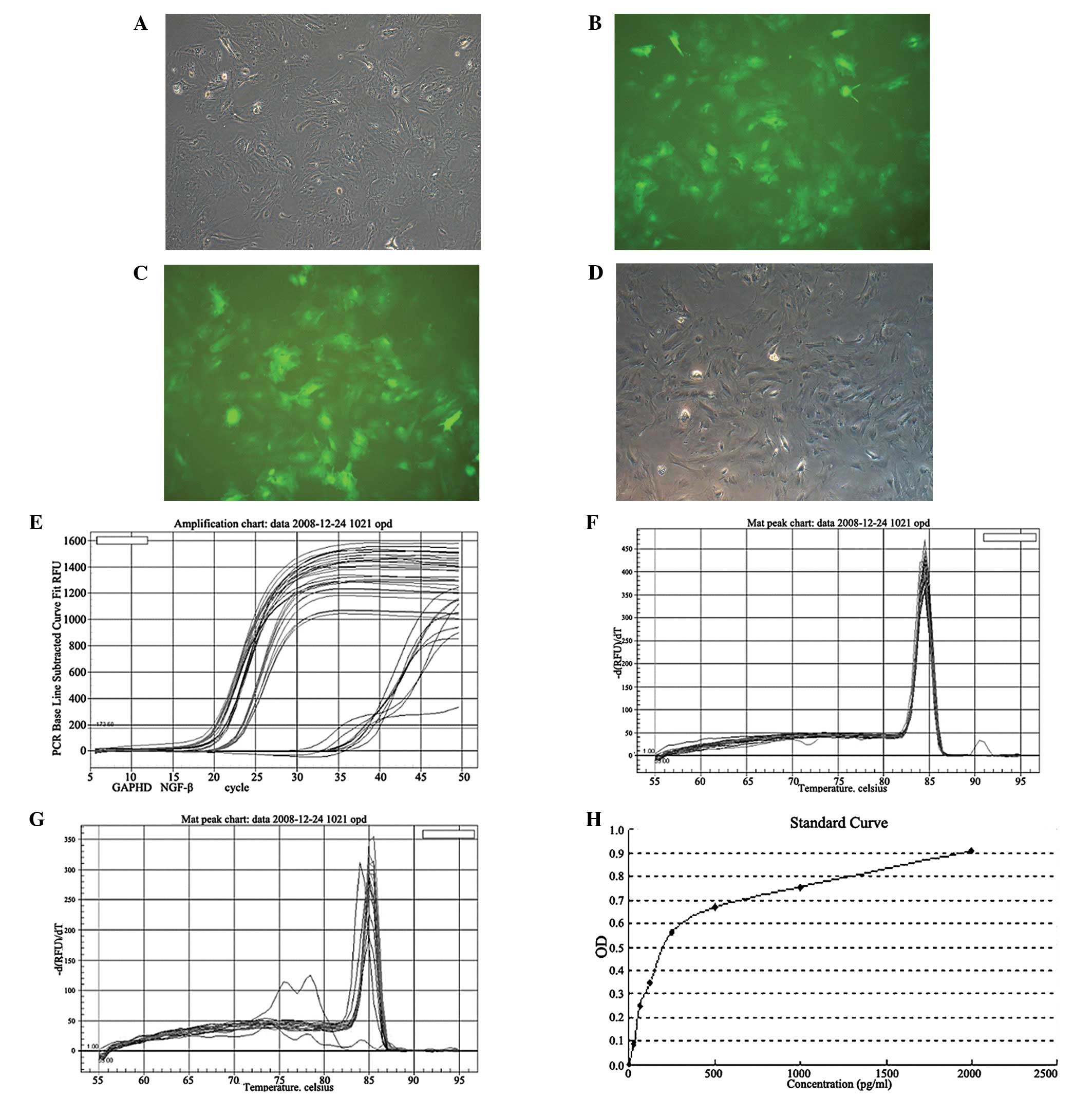
Recombinant NGF lentivirus infection of
rat MSCs
Rat MSCs infected with the lentiviral GFP-tagged
vector for 48 h revealed green fluorescence. The infection rate was
estimated to be >95% by comparing green and bright field cells
(Fig. 5A and B). Green
fluorescence was observed in whole cells and was sustainably
expressed through generations, indicative of successful GFP vector
transfection. Since the transfection conditions of the GFP vector
and recombinant NGF were the same, these observations indicated
that the NGF gene was successfully transfected into MSCs.
Reverse transcription RT PCR augmentation curves
(Fig. 5E) demonstrated that GAPDH,
which was set as a PCR control, was expressed at similar levels in
the experimental and control groups. Threshold cycle
(Ct)GADPH values in the two groups were within the same
range (Table I). Statistical
analysis revealed that there was no significant difference in GAPDH
expression between the groups.
 | Table ICt values of rat MSCs transfected with
NGF (experimental group) or empty vectors (control group). |
Table I
Ct values of rat MSCs transfected with
NGF (experimental group) or empty vectors (control group).
| Group |
CtGAPDH | CtNGF |
|---|
| Control | 16.02±0.46 | 33.02±1.40 |
| Experimental | 15.19±0.36a | 18.46±0.30b |
By contrast, a significant difference in NGF
expression was identified between the two groups. CtNGF
was >33 in the control group and there were miscellaneous peaks
in the gene melting curve (Fig.
5G), indicating that the NGF gene was not expressed in the
control group. In addition, CtNGF was 18.46 in the
experimental group, which was revealed to be significantly
different from the control (P<0.05). The melting curves were
normal (Fig. 5F and G). Therefore,
the expression of NGF was higher in the NGF lentivirus-infected
group compared with the control group. Using the
2−(ΔΔCT) method, relative expression of the NGF gene was
calculated as 213.73=13,587.57.
The MSCs were cultured for 48–72 h following
infection. Supernatants were obtained from the experimental and
control groups. An ELISA assay was performed as described. NGF
levels in the experimental and control groups were 190±41.34 and 0
pg/ml, respectively, as NGF protein was not detected in the control
group (P<0.01; Fig. 5H).
Diabetic rat modeling
Blood sugar levels of rats increased 72 h following
intraperitoneal injection with STZ. Levels remained high 8 weeks
later in diabetic rats (n=15) compared with control rats (n=15).
Compared with the controls, the diabetic group was shown to have
higher blood sugar levels (P<0.05), decreased weight
(P<0.05), increased urine volume (P<0.05) and increased
bladder wet weight (P<0.05). In addition, the fur of diabetic
rats lost quality, while drinking water volume and food intake
increased. These observations indicate that the diabetic rat model
was established (Table II).
 | Table IIBlood glucose, body weight, urine
volume and bladder wet weight in the diabetic and control
groups. |
Table II
Blood glucose, body weight, urine
volume and bladder wet weight in the diabetic and control
groups.
| Characteristics | Diabetic group
(n=15) | Control group
(n=15) |
|---|
| Blood glucose
(mmol/l) | 23.620±5.210 | 4.727±1.321 |
| Weight (g) | 251.147±7.536 | 323.133±13.192 |
| Urine volume
(ml) | 91.100±9.463 | 22.247±4.1028 |
| Bladder wet weight
(mg) | 172.940±6.385 | 135.833±2.302 |
BrdU-labeled NGF transfected MSCs
NGF-transfected rat MSCs were incubated with 10 μM
BrdU for 48 h. The cell nucleus was stained brown by
immunohistochemistry and staining efficiency was >80%. The
nuclear negative control was stained blue (Fig. 6A and B).
Immunohistochemistry
Detection of BrdU-labeled MSCs was performed 4 weeks
following transplantation and indicated that the MSCs had survived
in the rat bladder. Sporadic brown markings could be observed in
bladder muscle cells, while positive staining was not identified in
the control group (Fig. 6C and
D).
Reverse transcription RT-PCR
NGF and GAPDH mRNA levels of the control, diabetic
and treatment groups were analyzed by reverse transcription RT-PCR
(Fig. 6E). NGF mRNA levels in the
diabetic group were lower compared with the control group
(P<0.05). Analysis was performed 4 weeks following MSC
transplantation, indicating that NGF expression was greater in the
treatment compared with the diabetic group (P<0.05; Table III).
 | Table IIINGF mRNA and protein expression
(pg/ml) in the control, diabetic and treatment groups. |
Table III
NGF mRNA and protein expression
(pg/ml) in the control, diabetic and treatment groups.
| Group | n | mRNA | Protein |
|---|
| Control | 15 | 0.183±0.004 | 113.837±3.044 |
| Diabetic | 15 | 0.032±0.139 | 70.057±1.953 |
| Treatment | 15 | 0.130±0.165 | 109.943±2.347 |
ELISA
An ELISA assay was performed using supernatants
obtained from each group. NGF protein expression in the control,
diabetic and treatment groups were 113.837±3.044, 70.057±1.953 and
109.943±2.347 pg/ml, respectively. The expression of NGF was
reduced in the diabetic compared with the control group
(P<0.05). NGF protein levels in the treatment group recovered to
control levels (Table III).
Discussion
Use of the correct vector is an important factor for
stable gene expression in gene therapy. A previous study
demonstrated low levels of transfection efficiency using plasmids,
including pCDNA-3.1, while the transfection efficiency associated
with the use of adenovirus vectors was markedly higher. However,
the use of adenovirus vectors may lead to activation of an immune
response, which is associated with a number of disadvantages during
in vivo assays (10).
Lentiviruses, an adenovirus subfamily, are able to infect cells of
the majority of organisms, regardless of whether the cell is
currently undergoing mitosis. Following insertion into the host
genome, viral genes are stably expressed. The high infection rate
and concentration of the virus enable long-term expression in
vivo, high levels of safety and reduce host immune responses in
the body (11). These advantageous
properties have led to the use of lentiviruses as a common gene
transfection vector.
The present study was performed using a novel,
simple and efficient PCR cloning method, namely the in-fusion
technology of Clontech Laboratories, Inc. (Mountain View, CA, USA).
In-fusion technology is a newly developed technology suitable for
use in the directed cloning of PCR products into linearized vectors
using recombinase. Use of this technology in research is increasing
and has been used for a number of applications, including genetic
mutation, long fragment cloning and multi-gene fusion (12). Using this lentiviral technology,
Berrow et al(13) obtained
>90% cloning efficiency during high-throughput vector
construction, which is markedly higher than methods utilizing
classic restriction ligases (14).
In the current study, 15 bases, which were isogenous with the
lentiviral vector, were connected to the ends of the NGF gene to
construct the plasmid. Correct construction of the plasmid using
the in-fusion technique was determined by PCR and sequencing.
Bone marrow MSCs were first described in 1968 by
Friedenstein et al(15).
MSCs are associated with a number of advantages, including numerous
sources, easy separation and recovery, efficient amplification
in vitro, no MHC II receptor expression and resistance to
receptors (16,17). MSCs differentiate into a wide
diversity of cells under suitable conditions and are easily
extracted autologously, avoiding immune rejection responses. These
advantages have led to the development of MSCs into an important
research tool (18–20). MSCs are easy to transfect and the
transfected gene does not generally affect MSC differentiation and
proliferation. Therefore, future applications of MSCs also include
tissue and cell engineering and gene therapy. In the present study,
rat MSCs were infected with a lentivirus transfected with a vector
plasmid encoding a GFP tag or GFP tag and NGF. Infection efficiency
and stable gene expression were confirmed by fluorescence
microscopy. In addition, the exogenous NGF gene was identified to
have been transcribed efficiently in rat MSCs and real-time PCR
demonstrated that NGF expression was significantly greater in the
NGF-transfected group, consistent with ELISA results, indicating
that the MSCs secreted functional proteins.
For in vivo testing, rat MSCs expressing the
therapeutic gene, NGF, were transplanted into diabetic rat
bladders. Transplanted MSCs were revealed to exhibit markedly
higher NGF expression in bladders of the treatment rats compared
with the diabetic group. NGF gene expression in the bladder was
stable and functional for use in diabetes therapy. This treatment
is effective and safe as it was performed using autologous stem
cells to prevent immune rejection responses and risks of viral
infection, including increased active virus copies, activation of
carcinogenic genes and insertion of mutations into the host genome.
Further studies are required to investigate the differentiation of
transplanted rat MSCs in the bladder of diabetic rats and determine
variations in organizational structure and functions of the bladder
following transplantation.
References
|
1
|
Ueda T, Yoshimura N and Yoshida O:
Diabetic cystopathy: relationship to autonomic neuropathy detected
by sympathetic skin response. J Urol. 157:580–584. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Paro M, Italiano G, Travagli RA, et al:
Cystometric changes in alloxan diabetic rats: evidence for
functional and structural correlates of diabetic autonomic
neuropathy. J Auton Nerv Syst. 30:1–11. 1990. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Hellweg R, Raivich G, Hartung HD, Hock C
and Kreutzberg GW: Axonal transport of endogenous nerve growth
factor (NGF) and NGF receptor in experimental diabetic neuropathy.
Exp Neurol. 130:24–30. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Sasaki K, Chancellor MB, Phelan MW, et al:
Diabetic cystopathy correlates with a long-term decrease in nerve
growth factor levels in the bladder and lumbosacral dorsal root
ganglia. J Urol. 168:1259–1264. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Goins WF, Yoshimura N, Phelan MW, et al:
Herpes simplex virus mediated nerve growth factor expression in
bladder and afferent neurons: potential treatment for diabetic
bladder dysfunction. J Urol. 165:1748–1754. 2001. View Article : Google Scholar
|
|
6
|
Sasaki K, Chancellor MB, Goins WF, et al:
Gene therapy using replication-defective herpes simplex virus
vectors expressing nerve growth factor in a rat model of diabetic
cystopathy. Diabetes. 53:2723–2730. 2004. View Article : Google Scholar
|
|
7
|
Yokoyama T, Yoshimura N, Dhir R, et al:
Persistence and survival of autologous muscle derived cells versus
bovine collagen as potential treatment of stress urinary
incontinence. J Urol. 165:271–276. 2001. View Article : Google Scholar
|
|
8
|
Strasser H, Marksteiner R, Margreiter E,
et al: Transurethral ultrasonography-guided injection of adult
autologous stem cells versus transurethral endoscopic injection of
collagen in treatment of urinary incontinence. World J Urol.
25:385–392. 2007. View Article : Google Scholar
|
|
9
|
Huard J, Yokoyama T, Pruchnic R, et al:
Muscle-derived cell-mediated ex vivo gene therapy for urological
dysfunction. Gene Ther. 9:1617–1626. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Pedersini R, Vattemi E and Claudio PP:
Adenoviral gene therapy in high-grade malignant glioma. Drug News
Perspect. 23:368–379. 2010.PubMed/NCBI
|
|
11
|
Dreyer JL: Lentiviral vector-mediated gene
transfer and rna silencing technology in neuronal dysfunctions. Mol
Biotechnol. 47:169–187. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Benoit RM, Wilhelm RN, Scherer-Becker D
and Ostermeier C: An improved method for fast, robust, and seamless
integration of DNA fragments into multiple plasmids. Protein Expr
Purif. 45:66–71. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Berrow NS, Alderton D, Sainsbury S, et al:
A versatile ligation-independent cloning method suitable for
high-throughput expression screening applications. Nucleic Acids
Res. 35:e452007. View Article : Google Scholar
|
|
14
|
Klock HE, White A, Koesema E and Lesley
SA: Methods and results for semi-automated cloning using integrated
robotics. J Struct Funct Genomics. 6:89–94. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Friedenstein AJ, Petrakova KV, Kurolesova
AI and Frolova GP: Heterotopic of bone marrow. Analysis of
precursor cells for osteogenic and hematopoietic tissues.
Transplantation. 6:230–247. 1968.PubMed/NCBI
|
|
16
|
Porada CD, Zanjani ED and Almeida-Porad G:
Adult mesenchymal stem cells: a pluripotent population with
multiple applications. Curr Stem Cell Res Ther. 1:365–369. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Hristov M, Heussen N, Schober A and Weber
C: Intracoronary infusion of autologous bone marrow cells and left
ventricular function after acute myocardial infarction: a
meta-analysis. J Cell Mol Med. 10:727–733. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Kanazawa H, Fujimoto Y, Teratani T, et al:
Bone marrow-derived mesenchymal stem cells ameliorate hepatic
ischemia reperfusion injury in a rat model. PLoS One. 6:e191952011.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Choi H, Lee RH, Bazhanov N, Oh JY and
Prockop DJ: Anti-inflammatory protein TSG-6 secreted by activated
MSCs attenuates zymosan-induced mouse peritonitis by decreasing
TLR2/NF-κB signaling in resident macrophages. Blood. 118:330–338.
2011.PubMed/NCBI
|
|
20
|
Xu YL, Liu YL, Wang Q, Li G, Lü XD and
Kong B: Intravenous transplantation of mesenchymal stem cells
attenuates oleic acid induced acute lung injury in rats. Chin Med J
(Engl). 125:2012–2018. 2012.PubMed/NCBI
|