Introduction
Hypoxic-ischemic encephalopathy (HIE) is one of the
primary causes of cerebral damage and long-term neurological
sequelae in the perinatal period in term and preterm infants
(1). Moderate to severe HIE occurs
at a rate of 1–2 per 1,000 full-term live births, with a total HIE
incidence of 3–5 per 1,000 (2–4); in
developing countries, the incidence of HIE is up to 10-fold higher
(5). Almost 50% of term-born
infants suffering from severe HIE die within weeks of birth, while
up to 25% of the survivors exhibit long-term complications
(6), including reductions in
cerebellar (7), cortical and
hippocampal volumes (8). These
reductions are associated in turn with cognitive and behavioral
deficits, deficits in the verbal and language domains (9,10), a
reduced IQ (11), cerebral palsy
and mental retardation (12).
However, no specific or successful neuroprotective strategies
existed until recently. Protecting the brain of a newborn remains a
challenging priority and represents an unmet medical need.
Hypoxia, which occurs in the brain when oxygen
availability drops below normal levels, is a major cause of
perinatal HI injury and plays a central role in its pathogenesis.
Additionally, insufficient blood flow to the brain, known as brain
ischemia, may lead to a poor oxygen supply. The molecular
mechanisms underlying the brain’s response to oxygen deprivation
are extremely complex. Hypoxia inducible factor-1α (HIF-1α)
(13–18) is a transcription factor that is
crucial for normal brain development and in the development of
injuries. MicroRNAs (miRs), small (18–25 nts), non-coding RNAs that
regulate gene expression by blocking the translation of target
mRNAs or by accelerating their degradation, have recently been
reported to be induced by hypoxia (19). In particular, miR-210, which is
activated by HIF-1α (20), is a
unique miR that has been evolutionarily conserved and ubiquitously
expressed in hypoxic cell and tissue types (21–27).
While miR-210 was initially considered to be intergenic, a more
recent study has revealed that it is contained within the sequence
of a hypoxia-inducible transcript with an unknown function
(AK123483) (28). miR-210 plays
multiple critical roles in the cellular regulation of responses to
low oxygen levels, including during ischemic brain injury. Recent
studies using a rat model have suggested that miR-210 is expressed
in both the brain and blood of middle cerebral artery occlusions
(MCAOs) (29,30). Fasanaro et al(31) demonstrated that miR-210 was a
critical element in endothelial cell function in response to
hypoxia and that it had considerable influence on migration,
capillary network formation and differentiation capabilities.
Therefore, we hypothesized that miR-210 may play an essential role
in HIE, which would identify miR-210 as a potential therapeutic
target.
In order to understand the mechanisms of neuronal
cell death after HI injury and to identify potential protective
agents, an in vitro cell culture model using rat
pheochromocytoma (PC12) cells has been previously developed to
mimic HI-induced cell death (32)
using oxygen-glucose deprivation (OGD). This OGD model was
extensively employed to understand the importance of the modulation
of cell death pathways in neuroprotection (33,34).
In this study, we investigated the effect of miR-210 on neuronal
cell apoptosis caused by HI injury.
Materials and methods
Cell culture
Rat PC12 cells were obtained from the American
Tissue Culture Collection (Rockville, MD, USA) and cultured in DMEM
supplemented with 10% v/v horse serum (HS), 5% v/v fetal bovine
serum (FBS) and appropriate antibiotics in a humidified chamber (5%
CO2 and 37°C). The study was approved by the ethics
committee of Nanjing Children’s Hospital of Nanjing Medical
University (Nanjing, China).
miRNA transfection
Fifty microliters of pre-miR hsa-miR-210 or
pre-miR-negative control #1 (pre-miR-NC1; Ambion, Foster City, CA,
USA) in OptiMEM I (Invitrogen, Carlsbad, CA, USA), with a final
concentration of 100 nM, was mixed with 50 μl Lipofectamine 2000
(Invitrogen; 25X dilution in OptiMEM I) and incubated at room
temperature for 20 min, prior to being added to each well of a
24-well plate (100 μl per well). PC12 cells (400 μl of
6.25×105 cells/ml) were subsequently added to each well.
The transfection mixture was incubated (5% CO2 and 37°C)
for 24 h, and the cells were either used immediately in assays or
the media was replaced (500 μl DMEM/10% v/v HS/5% v/v FBS) and
incubated further.
OGD
PC12 cells were washed once with glucose-free DMEM
previously bubbled through with a mixture of 95% nitrogen and 5%
CO2. Cells were maintained in this deoxygenated
glucose-free medium. The plates were then placed in a modular
incubation chamber (Billups-Rothenberg, Del Mar, CA, USA) and
flushed with 95% nitrogen/5% CO2 for 4 min at a flow
rate of 10 l/min. The chamber was then sealed and kept in an
incubator for 4 h at 37°C. Control cells were washed with
glucose-containing DMEM and incubated in a normoxic incubator for 4
h.
Real-time quantitative PCR
Total RNA was prepared using TRIzol (Invitrogen).
miR was purified using the mirVana kit according to the
manufacturer’s instructions (Applied Biosystems, Foster City, CA,
USA). Using a specific miR-210 and endogenous control U6 stem-loop
primer, reverse transcription was performed according to the
manufacturer’s instructions for the TaqMan miRNA RT kit (Applied
Biosystems). Total RNA (10 ng) was reverse transcribed to cDNA with
1 mM dNTPs (with dTTP), 50 units reverse transcriptase (RT; 1 μl),
4 units RNase inhibitor in the presence of specific miR-210 or U6
stem loop RT primers in a 15 μl system buffered by RT Buffer and
diethyl-pyrocarbonate (DEPC) water. Following the thermal cycle
program of 16°C for 30 min, 42°C for 30 min and 85°C for 5 min,
cDNA was stored at −20°C. Real-time quantitative PCR was performed
by a fast real-time PCR system (7900HT, Applied Biosystems) using a
TaqMan miRNA assay kit. The 20 μl reaction volume contained the
following components: miR-210 or U6 RT reaction product (1.33 μl),
20X TaqMan® MicroRNA assay (miR-210 or U6; 1 μl), TaqMan
2X universal PCR master mix (10 μl) and DEPC water (7.67 μl). A
96-well plate was then run using the following protocol: 95°C for
10 min, followed by 43 cycles of 95°C for 15 sec and 60°C for 1
min. Finally, the relative miR-210 level was normalized to the
endogenous control U6 expression for each sample in triplicate and
calculated using the 2−ΔCt method.
Evaluation of apoptotic index
Cells were harvested using trypsin/EDTA, washed with
PBS, resuspended in 100 μl binding buffer and stained with 5 μl
annexin V-FITC and 1 μl propidium iodide (PI) at room temperature
for 1 min (Biovision, Milpitas, CA, USA). The fluorescence of FITC
and PI was analyzed using flow cytometry after adding 400 μl
binding buffer.
Western blot analysis
Cells were washed with ice-cold PBS and lysed in
protein lysis buffer (50 mM Tris, 150 mM NaCl, 10 mM EDTA, 1%
Triton X-100, 200 mM NaF and 4 mM sodium orthovanadate-containing
protease inhibitors; pH 7.5) for 1 h on ice. Proteins were
quantified using the bicinchoninic acid (BCA) protein assay kit
(Pierce, Rockford, IL, USA) according to the manufacturer’s
instructions. After separation by 10% sodium dodecyl
sulfate-polyacrylamide gel electrophoresis (SDS-PAGE), the proteins
(20 μg/lane) were electrophoretically transferred onto a
nitrocellulose membrane (Whatman, London, UK), which was blocked
with non-fat dry milk in buffer. The membrane was incubated with
primary antibodies against caspase-3, caspase-9, Bax and Bcl-2
(Santa Cruz Biotechnology, Inc., Santa Cruz, CA, USA) and goat
anti-mouse IgG conjugated with horseradish peroxidase secondary
antibody (Santa Cruz Biotechnology, Inc.). Thereafter, the proteins
were visualized by an electrochemiluminescence detection system (GE
Healthcare Bio-Sciences, Uppsala, Sweden) and analyzed using
Quantity One Analysis Software (Bio-Rad Laboratories, Hercules, CA,
USA). β-actin was used as a protein loading control.
Statistical analysis
All data are expressed as the mean ± SD. Statistical
analysis was performed using the Student’s t-test of the SPSS 10.0
statistical software package (SPSS, Chicago, IL, USA). P<0.05
was considered to indicate a statistically significant
difference.
Results
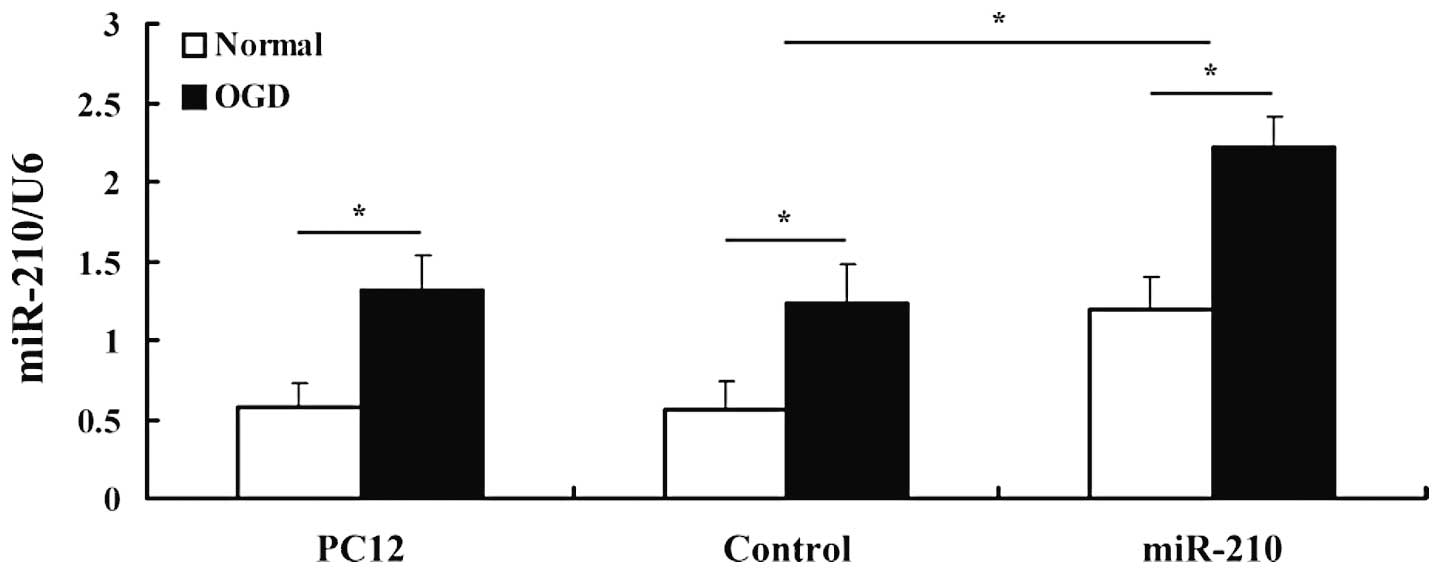
miR-210 expression
We confirmed the expression level of miR-210 using
quantitative real-time PCR. U6 was used as the endogenous control
since it was the most stably expressed miR in both the control and
experimental groups. miR-210 was robustly upregulated in cells
transfected with the miR-210 vector, which confirmed a successful
preparation. A clear upregulation in miR-210 expression was
detected in cells after 4 h of exposure to OGD, revealing that
hypoxia induces the expression of miR-210 in PC12 cell lines
(Fig. 1).
Effects of miR-210 on cell apoptosis
Cells subjected to OGD demonstrated a higher cell
death rate compared with control cells that were not deprived of
glucose and had been kept under normoxic conditions (Fig. 2). However, cells overexpressing
miR-210 demonstrated reduced apoptosis after OGD, indicating that
miR-210 protects PC12 cells from OGD-induced cell death.
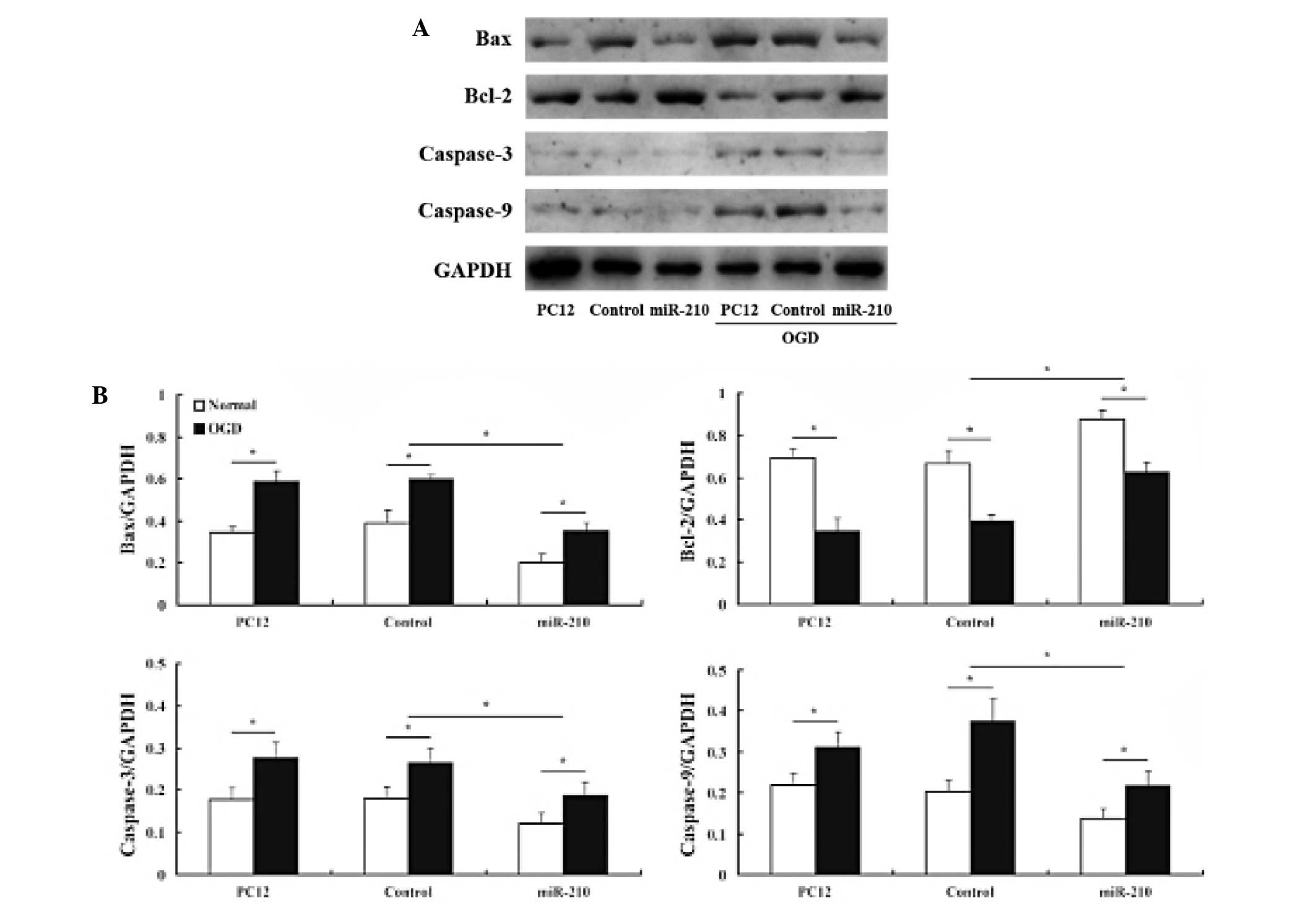
Effects of miR-210 on protein expression
of caspase-3, caspase-9, Bax and Bcl-2
We assessed the effects of miR-210 on
apoptosis-related protein expression. Western blot analysis
demonstrated that Bax, caspase-3 and caspase-9 protein levels
decreased in cells that overexpressed miR-210 compared with
controls. By contrast, anti-apoptotic Bcl-2 expression behaved in
an almost inverse manner (Fig.
3).
Discussion
miRs are a recently discovered class of naturally
occurring, non-coding RNA molecules that negatively regulate
eukaryotic gene expression by binding to complementary sequences in
the 3′-untranslated region (3′UTR) of target mRNA. There are
>400 known human miRs and >1000 predicted miR sequences
awaiting confirmation (35). The
current theory suggests that 10–30% of all human genes are targets
for miR regulation. miRs have roles in almost all aspects of cell
biology, including development, apoptosis, proliferation, adipocyte
differentiation, hematopoiesis and exocytosis, and their
deregulation has been reported in various diseases, most notably in
cancer (36). Although several
reports have demonstrated the role of specific miRs in neuronal
differentiation, neurogenesis, neural cell specification and
neurodevelopmental function (37–39),
no report is available on the importance of miRs in HIE.
Recently, a specific group of hypoxia- and
HIF-1α-regulated miRs were identified; among them was miR-210,
which was found to be important for cell survival in a hypoxic
microenvironment (40), cell cycle
regulation (41), DNA damage and
repair (42) and compromised
mitochondrial function. However, no report has revealed its
functional relevance in HIE thus far. To the best of our knowledge,
this is the first study to utilize the OGD model to investigate the
link between miR-210 and HI injury.
miR-210 is currently regarded as the master miR of
the hypoxic response, as it has been found to be upregulated by
hypoxia in all cell types tested to date (20). Consistent with these data, our
results also demonstrated that miR-210 expression was upregulated
in PC12 cells after 4 h of exposure to OGD. Thus, the expression of
miR-210 may increase during HIE and its expression in vivo
requires further characterization.
During normal brain development, redundant neurons
are removed via apoptosis; this is an important physiological
process to ensure the formation of appropriate neuronal networks.
However, after HI injury, this apoptotic component is pathological
and leads to excessive neuronal loss. Previous studies have
revealed that miR-210 protects cells from hypoxia-induced apoptosis
(31,40,43).
Similarly, our data also revealed that miR-210 expression within
the first 4 h after OGD is able to prevent cell apoptosis. Thus, we
reason that miR-210 may contribute to modulating the cell apoptotic
response to HIE. There are limitations to the therapeutic
treatments for HIE, particularly anesthetics and anti-epileptic
agents, due to the fact that they induce pathological neural
apoptosis in the immature brain (44–47).
Furthermore, the blood-brain barrier (BBB) has been shown to be
more permeable to various blood-borne solutes and small
lipid-insoluble molecules in the fetal rat brain than in adults. As
miRs are only 18–25 nts in length, they readily cross the BBB to
the HI area (48,49). Although further studies are
required, we can conclude that miR-210 delivery via blood
circulation may be a novel avenue for therapeutic interventions to
combat HIE.
Apoptosis involves a series of gene activation,
expression and regulation events. For example, apoptosis is
mediated by Bcl-2 family protein members. Bcl-2 pro-survival
proteins negatively regulate pro-apoptotic Bax proteins via
interactions between their Bcl-2 homology (BH) domains. The role of
individual caspases in the developing brain is not fully
understood. Genetic analysis revealed that caspase-3 and caspase-9
execute programmed cell death in the central nervous system
(50,51). Western blot analysis in the present
study revealed that caspase-3, caspase-9 and Bax protein levels
decreased and Bcl-2 expression increased in cells overexpressing
miR-210, which suggests that miR-210 suppressed neuronal apoptosis
by inhibiting caspase activity and regulating the balance between
Bcl-2 and Bax levels.
In conclusion, this study demonstrated the effect of
miR-210 on neuronal cell apoptosis following OGD. However, HI
injury-induced events in the brain are extremely complex and
further studies are required to clarify the exact mechanism by
which miR-210 inhibits cell apoptosis in HIE in vivo.
Acknowledgements
This project was supported by grants from the
Science and Technology Development Project of Nanjing, China (No.
201001090), the Medical Science and Technology Development Project
of Nanjing Health Bureau, China (No. YKK10046).
References
|
1
|
du Plessis AJ and Volpe JJ: Perinatal
brain injury in the preterm and term newborn. Curr Opin Neurol.
15:151–157. 2002.PubMed/NCBI
|
|
2
|
Gonzalez FF and Ferriero DM: Therapeutics
for neonatal brain injury. Pharmacol Ther. 120:43–53. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Shankaran S and Laptook AR: Hypothermia as
a treatment for birth asphyxia. Clin Obstet Gynecol. 50:624–635.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Whitelaw A and Thoresen M: Clinical trials
of treatments after perinatal asphyxia. Curr Opin Pediatr.
14:664–668. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Lawn JE, Cousens S and Zupan J; Lancet
Neonatal Survival Steering Team. 4 million neonatal deaths: when?
Where? Why? Lancet. 365:891–900. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Fotopoulos S, Pavlou K, Skouteli H,
Papassotiriou I, Lipsou N and Xanthou M: Early markers of brain
damage in premature low-birth-weight neonates who suffered from
perinatal asphyxia and/or infection. Biol Neonate. 79:213–218.
2001. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Allin M, Matsumoto H, Santhouse AM, et al:
Cognitive and motor function and the size of the cerebellum in
adolescents born very pre-term. Brain. 124:60–66. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Isaacs EB, Edmonds CJ, Chong WK, Lucas A,
Morley R and Gadian DG: Brain morphometry and IQ measurements in
preterm children. Brain. 127:2595–2607. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Casiro OG, Moddemann DM, Stanwick RS,
Panikkar-Thiessen VK, Cowan H and Cheang MS: Language development
of very low birth weight infants and fullterm controls at 12 months
of age. Early Hum Dev. 24:65–77. 1990. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Marlow N, Rose AS, Rands CE and Draper ES:
Neuropsychological and educational problems at school age
associated with neonatal encephalopathy. Arch Dis Child Fetal
Neonatal Ed. 90:F380–F387. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Steinman KJ, Gorno-Tempini ML, Glidden DV,
et al: Neonatal watershed brain injury on magnetic resonance
imaging correlates with verbal IQ at 4 years. Pediatrics.
123:1025–1030. 2009.PubMed/NCBI
|
|
12
|
Tioseco JA, Aly H, Essers J, Patel K and
El-Mohandes AA: Male sex and intraventricular hemorrhage. Pediatr
Crit Care Med. 7:40–44. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Bacon AL and Harris AL: Hypoxia-inducible
factors and hypoxic cell death in tumour physiology. Ann Med.
36:530–539. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Gordan JD and Simon MC: Hypoxia-inducible
factors: central regulators of the tumor phenotype. Curr Opin Genet
Dev. 17:71–77. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Gruber M and Simon MC: Hypoxia-inducible
factors, hypoxia, and tumor angiogenesis. Curr Opin Hematol.
13:169–174. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Harris AL: Hypoxia - a key regulatory
factor in tumour growth. Nat Rev Cancer. 2:38–47. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Kim JW, Tchernyshyov I, Semenza GL and
Dang CV: HIF-1-mediated expression of pyruvate dehydrogenase
kinase: a metabolic switch required for cellular adaptation to
hypoxia. Cell Metab. 3:177–185. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Koumenis C: ER stress, hypoxia tolerance
and tumor progression. Curr Mol Med. 6:55–69. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Pocock R: Invited review: decoding the
microRNA response to hypoxia. Pflugers Arch. 461:307–315. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Ivan M, Harris AL, Martelli F and
Kulshreshtha R: Hypoxia response and microRNAs: no longer two
separate worlds. J Cell Mol Med. 12:1426–1431. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Chan SY and Loscalzo J: MicroRNA-210: a
unique and pleiotropic hypoxamir. Cell Cycle. 9:1072–1083. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Chan SY, Zhang YY, Hemann C, Mahoney CE,
Zweier JL and Loscalzo J: MicroRNA-210 controls mitochondrial
metabolism during hypoxia by repressing the iron-sulfur cluster
assembly proteins ISCU1/2. Cell Metab. 10:273–284. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Chen Z, Li Y, Zhang H, Huang P and Luthra
R: Hypoxia-regulated microRNA-210 modulates mitochondrial function
and decreases ISCU and COX10 expression. Oncogene. 29:4362–4368.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Favaro E, Ramachandran A, McCormick R, et
al: MicroRNA-210 regulates mitochondrial free radical response to
hypoxia and krebs cycle in cancer cells by targeting iron sulfur
cluster protein ISCU. PLoS One. 5:e103452010. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Huang X, Ding L, Bennewith KL, et al:
Hypoxia-inducible mir-210 regulates normoxic gene expression
involved in tumor initiation. Mol Cell. 35:856–867. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Kushibiki T: Photodynamic therapy induces
microRNA-210 and -296 expression in HeLa cells. J Biophotonics.
3:368–372. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Pulkkinen K, Malm T, Turunen M, Koistinaho
J and Ylä-Herttuala S: Hypoxia induces microRNA miR-210 in vitro
and in vivo ephrin-A3 and neuronal pentraxin 1 are potentially
regulated by miR-210. FEBS Lett. 582:2397–2401. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Camps C, Buffa FM, Colella S, et al:
hsa-miR-210 Is induced by hypoxia and is an independent prognostic
factor in breast cancer. Clin Cancer Res. 14:1340–1348. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Jeyaseelan K, Lim KY and Armugam A:
MicroRNA expression in the blood and brain of rats subjected to
transient focal ischemia by middle cerebral artery occlusion.
Stroke. 39:959–966. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Liu DZ, Tian Y, Ander BP, et al: Brain and
blood microRNA expression profiling of ischemic stroke,
intracerebral hemorrhage, and kainate seizures. J Cereb Blood Flow
Metab. 30:92–101. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Fasanaro P, D’Alessandra Y, Di Stefano V,
et al: MicroRNA-210 modulates endothelial cell response to hypoxia
and inhibits the receptor tyrosine kinase ligand Ephrin-A3. J Biol
Chem. 283:15878–15883. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Tabakman R, Lazarovici P and Kohen R:
Neuroprotective effects of carnosine and homocarnosine on
pheochromocytoma PC12 cells exposed to ischemia. J Neurosci Res.
68:463–469. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Guo G and Bhat NR: p38alpha MAP kinase
mediates hypoxia-induced motor neuron cell death: a potential
target of minocycline’s neuroprotective action. Neurochem Res.
32:2160–2166. 2007.PubMed/NCBI
|
|
34
|
Tabakman R, Jiang H, Schaefer E, Levine RA
and Lazarovici P: Nerve growth factor pretreatment attenuates
oxygen and glucose deprivation-induced c-Jun amino-terminal kinase
1 and stress-activated kinases p38alpha and p38beta activation and
confers neuroprotection in the pheochromocytoma PC12 Model. J Mol
Neurosci. 22:237–250. 2004. View Article : Google Scholar
|
|
35
|
Griffiths-Jones S, Grocock RJ, van Dongen
S, Bateman A and Enright AJ: miRBase: microRNA sequences, targets
and gene nomenclature. Nucleic Acids Res. 34:D140–D144. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Lee YS and Dutta A: MicroRNAs in cancer.
Annu Rev Pathol. 4:199–227. 2009. View Article : Google Scholar
|
|
37
|
Kim J, Krichevsky A, Grad Y, et al:
Identification of many microRNAs that copurify with polyribosomes
in mammalian neurons. Proc Natl Acad Sci USA. 101:360–365. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Kosik KS and Krichevsky AM: The Elegance
of the MicroRNAs: A Neuronal Perspective. Neuron. 47:779–782. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Schratt GM, Tuebing F, Nigh EA, et al: A
brain-specific microRNA regulates dendritic spine development.
Nature. 439:283–289. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Kulshreshtha R, Ferracin M, Wojcik SE, et
al: A microRNA signature of hypoxia. Mol Cell Biol. 27:1859–1867.
2007. View Article : Google Scholar
|
|
41
|
Giannakakis A, Sandaltzopoulos R, Greshock
J, et al: miR-210 links hypoxia with cell cycle regulation and is
deleted in human epithelial ovarian cancer. Cancer Biol Ther.
7:255–264. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Crosby ME, Kulshreshtha R, Ivan M and
Glazer PM: MicroRNA regulation of DNA repair gene expression in
hypoxic stress. Cancer Res. 69:1221–1229. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Hu S, Huang M, Li Z, et al: MicroRNA-210
as a novel therapy for treatment of ischemic heart disease.
Circulation. 122:S124–S131. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Bittigau P, Sifringer M, Genz K, et al:
Antiepileptic drugs and apoptotic neurodegeneration in the
developing brain. Proc Natl Acad Sci USA. 99:15089–15094. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Ikonomidou C, Bosch F, Miksa M, et al:
Blockade of NMDA receptors and apoptotic neurodegeneration in the
developing brain. Science. 283:70–74. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Jevtovic-Todorovic V, Hartman RE, Izumi Y,
et al: Early exposure to common anesthetic agents causes widespread
neurodegeneration in the developing rat brain and persistent
learning deficits. J Neurosci. 23:876–882. 2003.
|
|
47
|
Sanders RD, Ma D, Brooks P and Maze M:
Balancing paediatric anaesthesia: preclinical insights into
analgesia, hypnosis, neuroprotection, and neurotoxicity. Br J
Anaesth. 101:597–609. 2008. View Article : Google Scholar
|
|
48
|
Pardridge WM: Intravenous, non-viral RNAi
gene therapy of brain cancer. Expert Opin Biol Ther. 4:1103–1113.
2004. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Pardridge WM: shRNA and siRNA delivery to
the brain. Adv Drug Deliv Rev. 59:141–152. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Kuida K, Haydar TF, Kuan CY, et al:
Reduced apoptosis and cytochrome c-mediated caspase activation in
mice lacking caspase 9. Cell. 94:325–337. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Kuida K, Zheng TS, Na S, et al: Decreased
apoptosis in the brain and premature lethality in CPP32-deficient
mice. Nature. 384:368–372. 1996. View Article : Google Scholar : PubMed/NCBI
|