Introduction
Gliomas are the most common type of malignant
primary brain tumor. Despite advances in neurosurgical resection,
radiation therapy and chemotherapy, the prognosis of patients with
gliomas has not significantly improved in the last 30 years. The
median life expectancy for patients with malignant gliomas is ~12
months and <5% of patients survive for 5 years following
diagnosis (1,2). Consequently, investigating methods to
improve the efficacy of glioma therapy has become an important
issue. Differentiation therapy, which uses agents to induce cancer
cell differentiation, has been proposed as a potential novel
approach to treat malignant solid tumors (3). Cholera toxin (CT) has been reported
to be a differentiation agent that induces the differentiation of
malignant gliomas. The underlying mechanisms of CT-induced glioma
cell differentiation have been shown to involve the activation of
the PKA/cyclic adenosine monophosphate (cAMP)/CREB and
PI3K/AKT/GSK3β pathways (4,5).
Phosphatase and tensin homolog deleted on chromosome 10 (PTEN) is a
commonly mutated tumor suppressor gene in gliomas and is closely
associated with glioma tumorigenesis, development and
differentiation. However, the underlying mechanisms of PTEN in
glioma cell differentiation remain unclear.
In order to understand the mechanisms of PTEN on
glioma differentiation, we studied the relationship between PTEN
and SWO-38 cell differentiation.
Materials and methods
Cell line
The human glioma cell line SWO-38 was established by
the Department of Pathology, Medical College of Jinan University
(Guangzhou, China) (6) and grown
in RPMI-1640 medium (Invitrogen, Carlsbad, CA, USA) supplemented
with 10% newborn bovine serum (TBD Biotechnology Development,
Tianjin, China), 100 U/ml penicillin and 100 mg/ml streptomycin.
The cells were cultured at 37°C in a humidified incubator with 5%
CO2 and were regularly examined using an inverted
microscope.
Morphological evaluation
The morphology of CT-treated and untreated cells was
investigated during a time course of 48 h using an inverted
microscope (TE2000; Nikon Corporation, Tokyo, Japan).
Cell cycle assessment using flow
cytometry
SWO-38 cells in culture flasks were treated with 20
ng/ml CT alone for 72 h or pretreated with the MAPK inhibitor
PD0325901, the PI3K inhibitor LY294002 or DMSO (as a solvent
control) for 2 h. The cells were harvested and washed with PBS.
Cells (1×106) were fixed in 70% ethanol at 4°C for 24 h,
washed in PBS, resuspended in 0.5–1.0 ml PBS containing 50 mg/ml
RNase A at 37°C for 15 min and incubated in 50 mg/ml propidium
iodide (PI; Sigma-Aldrich, St. Louis, MO, USA) in the dark at room
temperature for 15 min. Flow cytometry was performed using a
FACScan flow cytometer (Becton-Dickinson, San Jose, CA, USA).
Cell migration and invasion
Cell migration and invasion capacity was examined
using a 24-well Transwell plate with 8-mm pore polycarbonate
membrane inserts, according to the manufacturer's instructions
(Corning, Inc., Corning, NY, USA). Matrigel (14.8 μg/ml; BD
Biosciences, Franklin Lakes, NJ, USA) was employed for the invasion
assays and applied to the upper surface of the membranes. Cells
(2×105 and 1×105/well for invasion and
migration, respectively) were seeded in the top chamber in
serum-free media and this was replaced with complete growth media
for 20 h for invasion and 12 h for migration. The cells that
invaded or migrated through the surface of the membrane were fixed
with methanol and stained with 0.1% hexamethylpararosaniline.
Migrating or invasive cells from three random microscopic fields
for each filter were selected for cell counting.
PTEN silencing in SWO-38 cells using RNA
interference
The human PTEN ShortCut siRNA and negative siRNA
(control) were purchased from Invitrogen. The coding strand
sequence of the PTEN siRNA was sense, 5′-GGCGCUAUGUGUAUUAUUAdTdT-3′
and antisense, 3′-dTdTCCGCGAUACACAUAAUAAU-5′. SWO-38 cells with
30–50% confluence were transfected using Lipofectamine™ 2000
reagent (Invitrogen), according to the manufacturer's instructions.
The inhibition of PTEN protein expression was assessed using
western blot analysis 6 h after transfection. The cells were then
incubated with CT for an additional 72 h for further analysis.
Western blot analysis
Protein samples were prepared with lysis buffer
containing 50 mM Tris (pH 8.0), 150 mM NaCl, 1% NP-40, 0.1% SDS,
100 ng/ml phenylmethylsulfonyl fluoride (PMSF) and 1 mg/ml
aprotinin. For western blot analysis, equal amounts of proteins
were loaded onto 10% SDS-PAGE gels for electrophoresis and then
transferred to nitrocellulose membranes (Pall Corporation,
Cortland, NY, USA). The nitrocellulose membranes were immediately
blocked with 5% non-fat milk in Tris-buffered saline (TBS)-T (0.05%
Tween-20 in TBS) buffer for 1 h at room temperature and then
incubated overnight at 4°C with primary antibodies against glial
fibrillary acidic protein (GFAP; 1:100; LabVision, Fremont, CA,
USA), PTEN (1:1,000, Cell Signaling Technology, Inc., Beverly, MA,
USA), AKT and p-AKTser473 (1:1,000; Cell Signaling Technology,
Inc.), respectively. Following incubation with horseradish
peroxidase-labeled secondary antibody at room temperature for 1 h,
the membranes were detected using SuperSignal West Pico
Chemiluminescent Substrate (Pierce, Rockford, IL, USA). GAPDH
(1:4,000; Millipore, Billerica, MA, USA) or β-actin (1:500;
Proteintech Group, Chicago, IL, USA) were used to normalize the
quantity of protein on the blots.
Statistical analysis
Data are presented as the mean ± standard deviation
(SD) from three separate experiments. Statistical analysis was
carried out by an independent samples t-test and one-way ANOVA
using the statistical package SPSS 16.0. P<0.05 was considered
to indicate a statistically significant difference.
Results
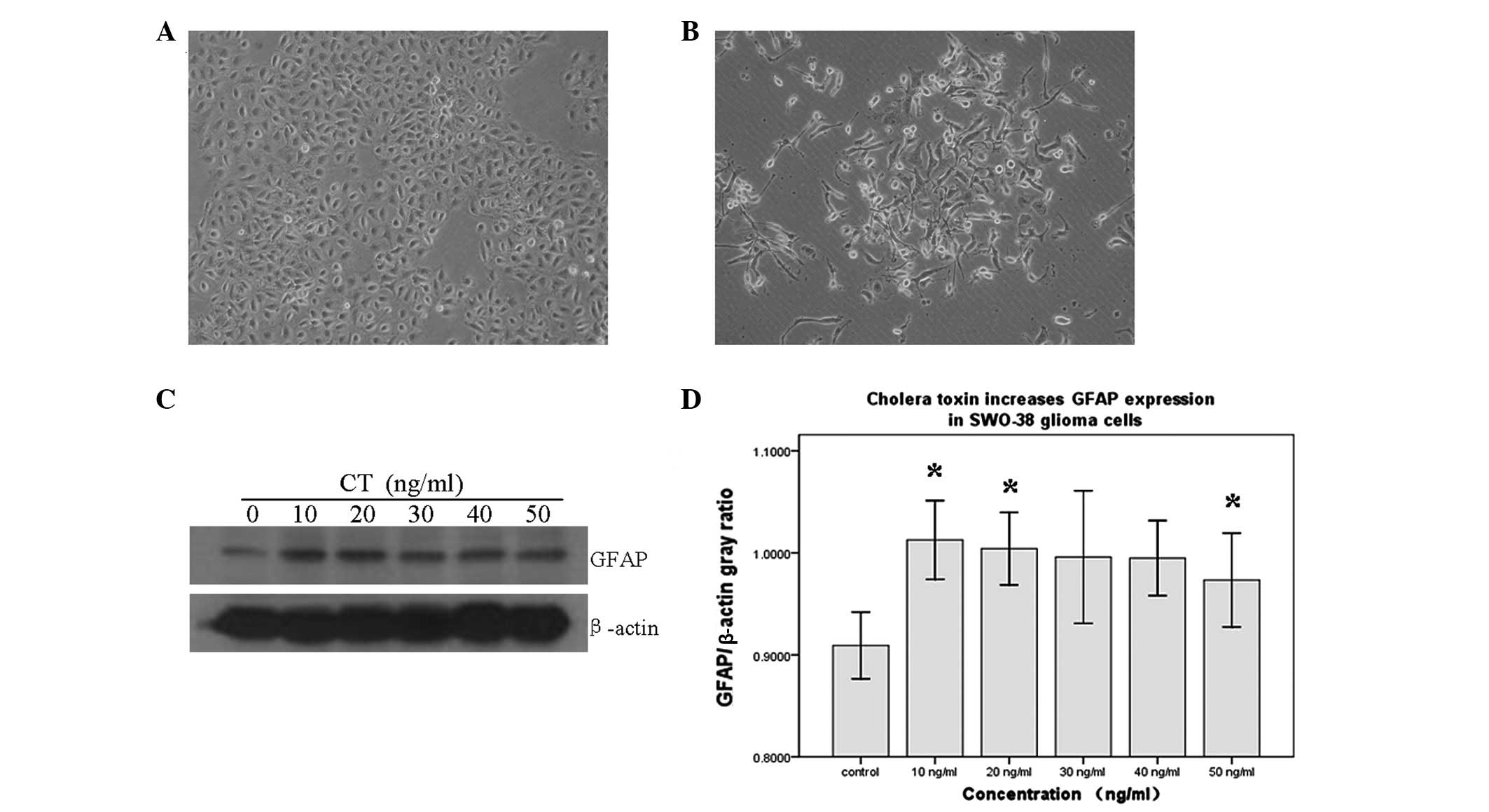
CT induces morphological transformation
and an increased expression of GFAP in SWO-38 cells
The differentiation of human SWO-38 glioma cells
towards an astrocytic type was characterized by a marked
morphological transformation from a flat polygonal appearance to
multiple elongated cytoplasmic processes, similar to those of
mature astrocytes (Fig. 1A and B).
Microscopic observation of SWO-38 glioma cells treated with 10
ng/ml CT revealed major alterations in their morphology. We further
examined the expression of GFAP, a well-documented marker of mature
astrocytes. As shown in Fig. 1C and
D, western blot analysis demonstrated a significant
upregulation of GFAP protein expression in CT-treated cells
compared with the control cells. However, this upregulation was not
dose-dependent or time-independent.
CT induces cell cycle arrest in SWO-38
cells
CT induced significant cell cycle arrest in SWO-38
cells. Incubation with 20 ng/ml CT for 72 h caused an accumulation
of cells in the G0/G1 phase; the percentage
of cells in this phase reached 73.01±2.55% compared with
65.24±3.74% in the control group (Fig.
2A and B). Furthermore, there was a significant decrease in the
percentage of cells in the S phase. The percentage of the cells in
the S phase was 17.19±2.53% in the CT-treated group and 25.58±3.18%
in the control group. Western blot analysis confirmed that
treatment with 20 ng/ml CT for 72 h downregulated the cyclin D1
protein levels (Fig. 2C and D).
Based on these results, CT appears to block cell-cycle progression
from the G1 to the S phase.
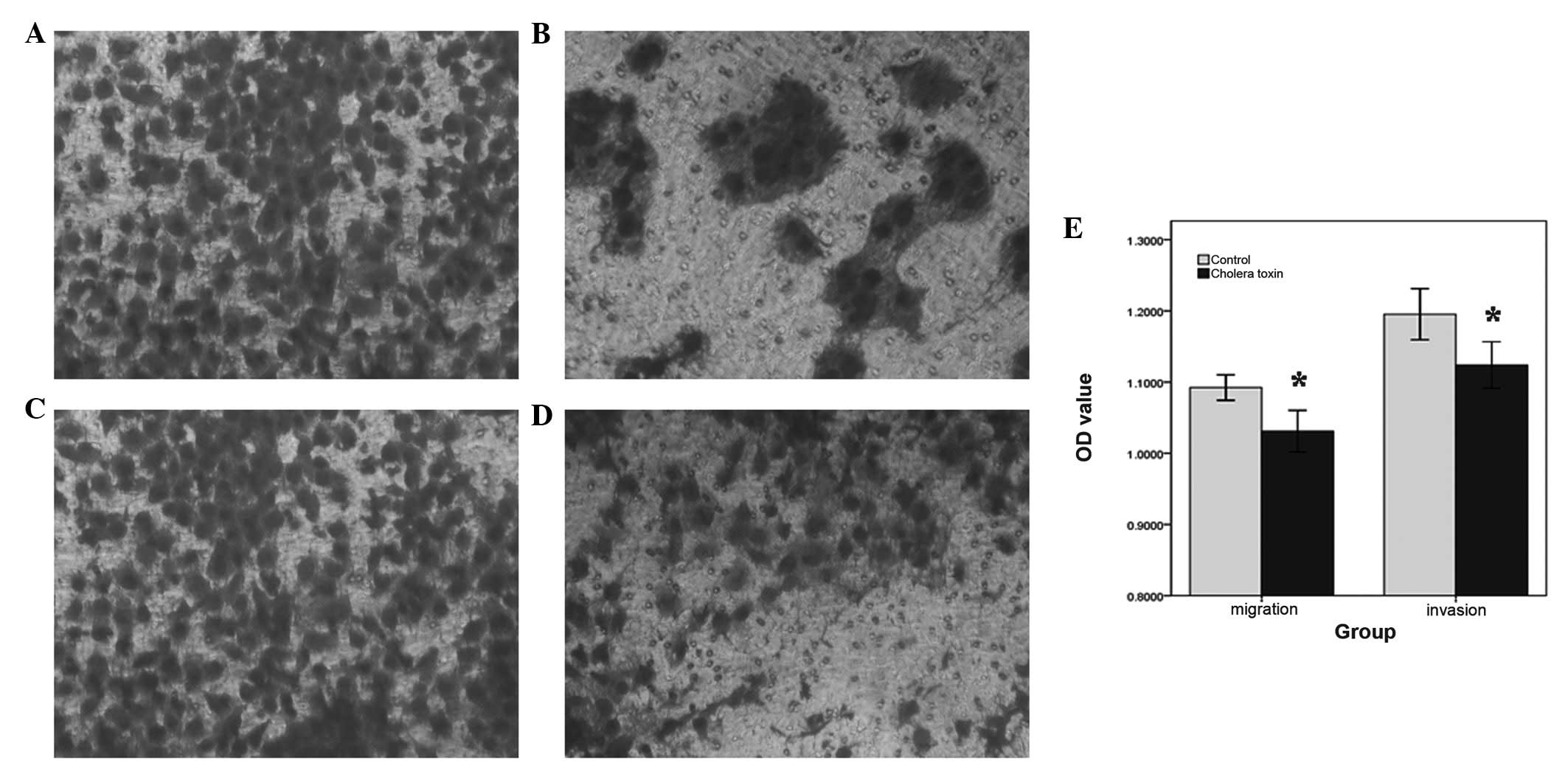
CT decreases the migration and invasion
capacity of SWO-38 cells
CT-treated SWO-38 cells exhibited a significantly
decreased invasion (Fig. 3A and B)
and migration (Fig. 3C and D)
capacity compared with the control cells (P<0.05; Fig. 3E).
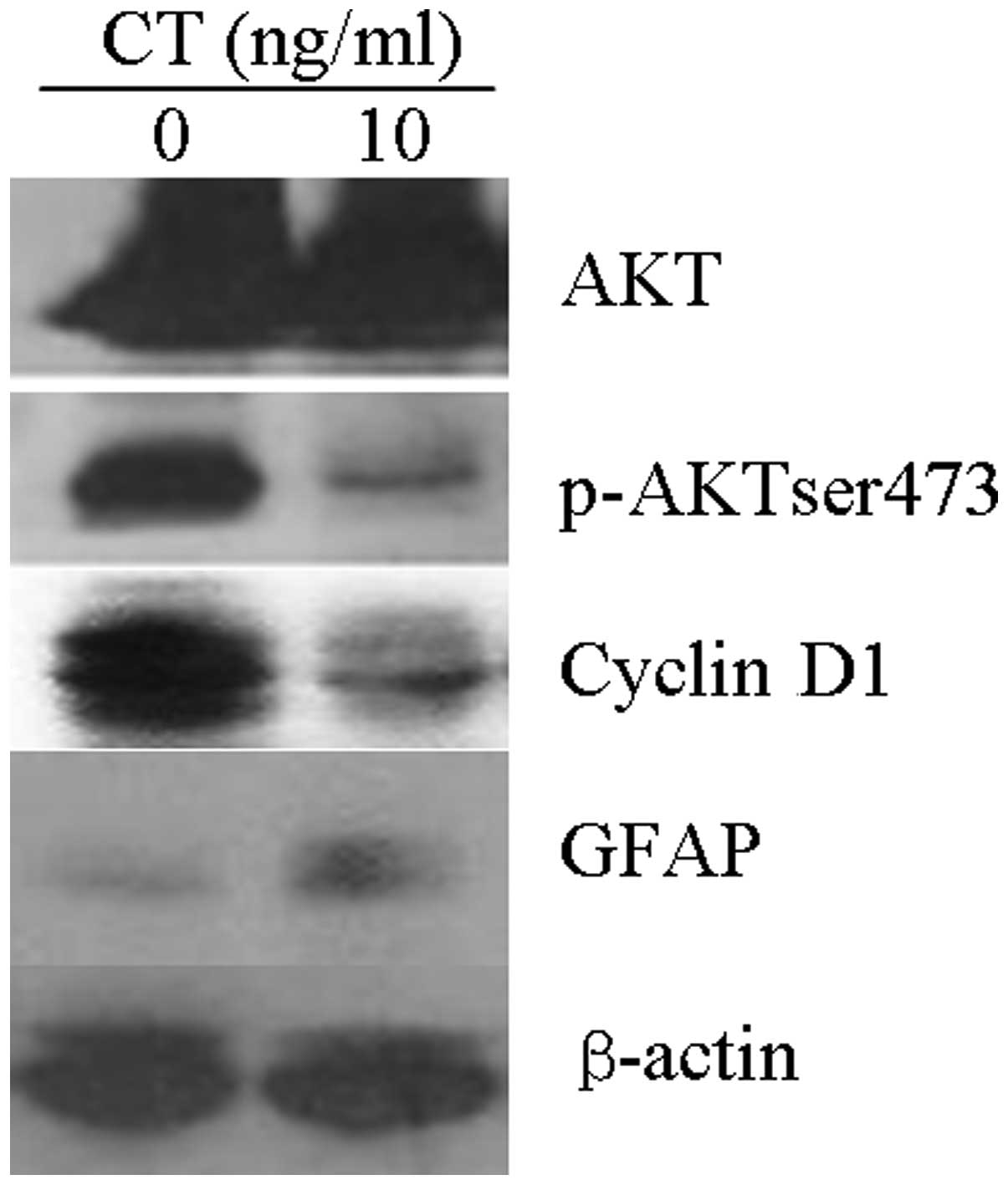
p-AKTser473 activity is decreased in
CT-induced SWO-38 glioma cell differentiation
To elucidate the underlying molecular mechanism of
CT-induced SWO-38 glioma cell differentiation, we determined the
expression of the main proteins involved in the PI3K/AKT signaling
pathway. As shown in Fig. 4,
p-AKTser473 activity decreased after SWO-38 cells were treated with
10 ng/ml CT for 72 h, whereas the AKT activity was not altered.
Additionally, CT treatment upregulated GFAP and downregulated
cyclin D1 expression in the SWO-38 cells.
PTEN is requisite for the CT-induced
differentiation of SWO-38 cells
To further investigate whether PTEN is required
during the process of CT-induced SWO-38 cell differentiation, siRNA
was used to selectively knockdown the PTEN gene. After 6 h of
transfection, the PTEN level was markedly reduced compared with the
control cells (Fig. 5F). After the
addition of CT, the cells were incubated for a further 72 h. The
differentiation effects of CT on cell morphology (Fig. 5A and B), GFAP expression (Fig. 5F), cell cycle distribution
(Fig. 5C and D) and invasion and
migration capacity (Fig. 5E) in
SWO-38 cells were attenuated.
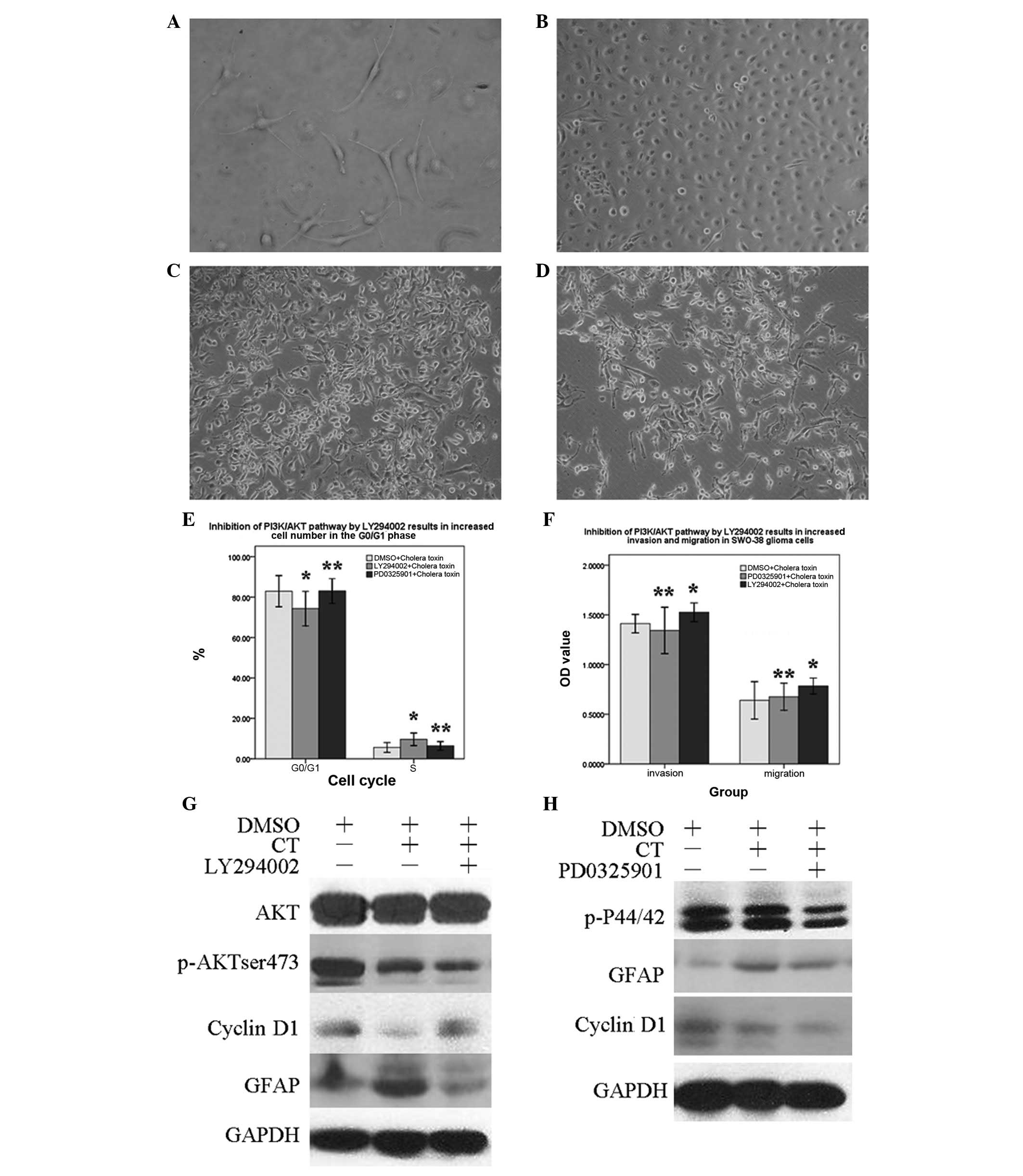
Inhibition of the PI3K pathway leads to
decreased SWO-38 cell differentiation
To provide additional evidence for the importance of
the PTEN/PI3K/AKT pathway in SWO-38 cell differentiation, we
assessed the effects of the MAPK inhibitor PD0325901 and PI3K
inhibitor LY294002 on CT-induced SWO-38 cell differentiation.
SWO-38 cells were incubated with LY294002 (100 μmol/l) or PD0325901
(40 μmol/l) for 2 h. CT was subsequently added and the cells were
incubated for an additional 72 h. As shown in Fig. 6A and B, CT-induced morphological
transformation was attenuated via the inhibition of PI3K activity
by LY294002, while exposure to the MAPK inhibitor PD0325901 and CT
did not alter PI3K activity (Fig. 6C
and D). Furthermore, the upregulation of GFAP and p-AKTser473
and the downregulation of cyclin D1 induced by CT were reverted by
LY294002, but not by PD0325901 (Fig.
6G and H). The cell cycle alterations
(G0/G1 cell cycle arrest and accelerated S
phase entry; Fig. 6E) and cell
migration and invasion capacity were markedly counteracted by
LY294002, indicating that the PTEN/PI3K/AKT pathway rather than the
PTEN/MAPK/ERK pathway was directly involved in the process of
differentiation in SWO-38 cells (Fig.
6F).
Discussion
The PTEN gene is an important tumor suppressor which
encodes a protein that carries out lipid and protein phosphatase
activities. The PTEN gene is one of the most commonly mutated tumor
suppressor genes in human tumors (7,8). In
gliomas, particularly glioblastomas, mutation of the PTEN gene has
been demonstrated to be as high as 70–80% and the mutation rate
increases with decreasing tumor differentiation, which indicates
that a putative suppressor gene involved in early glial
tumorigenesis may exist (9).
However, the role and mechanism of PTEN in glioma cell
differentiation has not yet been fully elucidated.
CT is the major virulence factor of Vibrio
cholerae and a specific agonist of G protein-coupled receptor.
CT has been shown to upregulate the level of intracellular cAMP and
activate the PKA/CREB signaling pathway, which plays a role in
regulating cell proliferation, apoptosis and differentiation
(10). The upregulation of the
cAMP/PKA/CREB pathway has been revealed as one of the underlying
molecular mechanisms that mediates the effect of CT on glioma cell
differentiation (4).
In the present study, CT was used as a cell
differentiation agent to investigate its effect on the induction of
SWO-38 glioma cell differentiation. CT was shown to induce SWO-38
cell differentiation; this was characterized by a morphological
transformation from a flat polygonal appearance to multiple
elongated cytoplasmic processes, the upregulation of GFAP protein
expression, an accumulation of cells in the
G0/G1 phase of the cell cycle, the
downregulation of cyclin D1 expression and a decrease in invasion
and migration capacity. Furthermore, the silencing of PTEN protein
using RNA interference resulted in suppressed cell differentiation.
This indicates that CT-induced SWO-38 cell differentiation may be
associated with PTEN.
The PTEN/PI3K/AKT pathway is involved in the
regulation of cell proliferation, differentiation and apoptosis,
and PTEN is an important upstream suppressor gene in this pathway.
Thus, we hypothesized that the mechanism of action of PTEN in
CT-induced SWO-38 cell differentiation may involve the PI3K/AKT
pathway. To test this hypothesis, the specific PI3K/AKT pathway
inhibitor LY294002 and the specific MAPK/ERK pathway inhibitor
PD0325901 were applied to block the two signaling pathways. The
previously mentioned indicators of SWO-38 cell differentiation were
detected again. Inhibition of the PI3K/AKT pathway by LY294002 was
shown to attenuate cell differentiation, whereas differentiation
remained stable with the inhibition of the MAPK/ERK pathway by
PD0325901. These results confirmed our hypothesis and suggest that
PTEN exerts its effect on SWO-38 cell differentiation through lipid
phosphatase activity.
Li et al(4,5)
previously reported that the cAMP/PKA/CREB pathway and
PI3K/AKT/GSK-3β-mediated cyclin D1 ubiquitin-based degradation were
the major molecular mechanisms underlying CT-induced glioma cell
differentiation (5,11). They also showed that CT-induced
SWO-38 glioma cell differentiation involved the PI3K/AKT pathway
and PTEN inhibited the PI3K/AKT pathway in this process. Therefore,
the cAMP/PKA/CREB and PI3K/AKT pathway may be the major mechanisms
of CT-induced glioma cell differentiation (4,12,13).
In conclusion, results of the present study showed
that PTEN was involved in CT-induced SWO-38 cell differentiation
via its lipid phosphatase activity, which inhibits the PI3K/AKT
pathway. However, further investigation is required to fully
elucidate the association between CT and PTEN.
Acknowledgements
This study was supported by grants from the National
Natural Science Foundation of China (no. 81072059), the National
Natural Science Foundation of China for Young Scholars (no.
81101652), the Science and Technology Innovation Key Project of
Guangdong Higher Education Institutes (no. CXZD1110), the Natural
Science Foundation of Guangdong Province (no. 10151063201000059)
and the Natural Science Foundation of Hainan Province, China (no.
309037).
References
|
1
|
Das P, Puri T, Jha P, et al: A
clinicopathological and molecular analysis of glioblastoma
multiforme with long-term survival. J Clin Neurosci. 18:66–70.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Stupp R, Mason WP, van den Bent MJ, et al;
European Organisation for Research and Treatment of Cancer Brain
Tumor and Radiotherapy Groups; National Cancer Institute of Canada
Clinical Trials Group. Radiotherapy plus concomitant and adjuvant
temozolomide for glioblastoma. N Engl J Med. 352:987–996. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Leszczyniecka M, Roberts T, Dent P, Grant
S and Fisher PB: Differentiation therapy of human cancer: basic
science and clinical applications. Pharmacol Ther. 90:105–156.
2001. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Li Y, Yin W, Wang X, et al: Cholera toxin
induces malignant glioma cell differentiation via the PKA/CREB
pathway. Proc Natl Acad Sci USA. 104:13438–13443. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Li Y, Lu H, Huang Y, et al: Glycogen
synthase kinases-3beta controls differentiation of malignant glioma
cells. Int J Cancer. 127:1271–1282. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Situ R, Wang HH, Wang JH, et al:
Establishment of human brain malignant glioma cell line (SWO-38)
and observation of its biologic properties. Chin J Cancer.
6:235–238. 1987.(In Chinese).
|
|
7
|
Li J, Yen C, Liaw D, et al: PTEN, a
putative protein tyrosine phosphatase gene mutated in human brain,
breast, and prostate cancer. Science. 275:1943–1947. 1997.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Tamura M, Gu J, Matsumoto K, et al:
Inhibition of cell migration, spreading, and focal adhesions by
tumor suppressor PTEN. Science. 280:1614–1617. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Ohgaki H and Kleihues P: Genetic pathways
to primary and secondary glioblastoma. Am J Pathol. 170:1445–1453.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Walsh DA and Van Patten SM: Multiple
pathway signal transduction by the cAMP-dependent protein kinase.
FASEB J. 8:1227–1236. 1994.PubMed/NCBI
|
|
11
|
Shu M, Zhou Y, Zhu W, et al: MicroRNA 335
is required for differentiation of malignant glioma cells induced
by activation of cAMP/protein kinase A pathway. Mol Pharmacol.
81:292–298. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Wang L, Liu F and Adamo ML: Cyclic AMP
inhibits extracellular signal-regulated kinase and
phosphatidylinositol 3-kinase/Akt pathways by inhibiting Rap1. J
Biol Chem. 276:37242–37249. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Downward J: Role of phosphoinositide-3-OH
kinase in Ras signaling. Adv Second Messenger Phosphoprotein Res.
31:1–10. 1997. View Article : Google Scholar : PubMed/NCBI
|