Introduction
Fumonisins (FBs) are the toxic metabolites produced
mainly by Fusarium verticillioides, one of the most common
molds found on maize and other agricultural products worldwide
(1–3). FB1 is the most abundant
and toxic fumonisin. Previous studies in broilers and rats have
shown that FB1 administered in the diet is hepatotoxic,
nephrotoxic and hepatocarcinogenic, and induces severe symptoms,
including a decrease of body weight, an increase of relative
weights of the liver and kidney, and liver necrosis (4). Gelderblom et al(5) reported that FB1 induces
the formation of liver tumors in laboratory rodents. FB1
has also been shown to alter the gene expression and signal
transduction pathways in monkey kidney cells (CV-1) (6), increase the mitogenic action of
insulin in Swiss 3T3 fibroblasts (7), inhibit the proliferation of IPEC-1
and LLC-PK1 kidney cells (8),
induce oxidative damage in primary culture rat hepatocytes
(9) and lipid peroxidation in
rabbit kidney RK13 cells (10). In
addition, studies have demonstrated that FB1 is able to
increase the initial disruption of sphingolipid metabolism and the
accumulation of sphinganine in LLC-PK1 kidney cells (11,12),
cause DNA damage of apoptotic type in rat astrocytes (13), induce apoptosis, DNA fragmentation
and hypermethylation (14), and to
alter the collagen secretion pattern in primary human lung
fibroblasts and human kidney epithelial cells (15). However, there is inadequate
evidence with regard to the carcinogenicity of FB1 in
humans. Several studies have investigated the toxicity of
FB1 in human cell lines to retrieve more information
regarding the effects of FB1 on humans. Exposure to
FB1 has been shown to cause DNA damage in human
fibroblasts (16), inhibit clonal
expansion of human keratinocytes and human esophageal epithelial
cells, and inhibit proliferation of human fibroblasts (15). Furthermore, FB1 has been
demonstrated to induce apoptosis of human proximal tubule-derived
cells (17) and cause oxidative
stress in the human intestinal cell line Caco-2 (18).
Although the toxic effects of FB1 on
mammalian cells have been extensively investigated, the potential
mechanism of action and the involved signaling pathways have not
been identified. In recent years, numerous studies have shown that
FB1 affects the cell cycle of certain cells. Cell cycle
progression is known to be controlled by cyclin-dependent kinases
(CDKs) and cyclins (19,20), while cyclin E is necessary for
entry into the S phase (21). CDK
inhibitors, including P21, bind to CDK-cyclin complexes and inhibit
CDK activity (22,23). P21 expression has been shown to be
upregulated by various types of antiproliferative stimuli and the
upregulation of P21 expression results in cell cycle arrest or
apoptosis (24,25).
The liver is the critical organ for the metabolism
and degradation of chemicals, food contaminants and natural toxins.
Therefore, the aim of the present study was to investigate the
effect of FB1 on the cell cycle and the expression of
cell cycle-related genes P21 and cyclin E in the normal human liver
cell line HL-7702.
Materials and methods
Materials
FB1,
3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT),
propidium iodide (PI), dimethyl sulfoxide (DMSO), ethidium bromide
(EB) and diethyl pyrocarbonate were purchased from Sigma (St.
Louis, MO, USA). RPMI-1640 medium and trypsin were purchased from
Gibco-BRL (Rockville, MD, USA). A stock solution of FB1
for cellular assays was prepared in phosphate-buffered saline (PBS)
and then diluted in the optimal medium (≤10 μl/ml).
Ethylenediaminetetraacetic acid was purchased from Calbiochem (La
Jolla, CA, USA). Fetal bovine serum (FBS) was purchased from
Sijiqing Biological Engineering Materials Co., Ltd. (Hangzhou,
Zhejiang, China). RNA TRIzol was purchased from Invitrogen Life
Technologies (Carlsbad, CA, USA). The RevertAid™ First Strand cDNA
Synthesis kit was purchased from Fermentas Life Sciences (Glen
Burnie, MD, USA). Reagents and membranes used for protein assay,
electrophoresis and western blot analysis were obtained from
Bio-Rad (Hercules, CA, USA). Antibodies for P21 (mouse monoclonal),
cyclin E (mouse monoclonal) and horseradish peroxidase
(HRP)-conjugated goat secondary anti-mouse antibody were purchased
from Santa Cruz Biotechnology, Inc. (Santa Cruz, CA, USA). Antibody
for GAPDH (polyclonal monoclonal) was purchased from KangChen
Bio-tech (Shanghai, China).
Cell culture
The normal human liver cell line HL-7702 was
obtained from the Cell Bank of the Chinese Academy of Sciences
(Shanghai, China). The cells were cultured in RPMI-1640 medium
supplemented with 5% FBS at 37°C, 95% humidity and 5%
CO2 in a humidified incubator. Cell growth was observed
using an inverted microscope daily; RPMI-1640 medium was replaced
according to its color every 2–3 days. The study was approved by
the ethics committee of the School of Public Health, Southeast
University, Nanjing, China.
Cell viability assay
HL-7702 cells (1×104 cells/100 μl/well)
were seeded in 96-well plates (Becton-Dickinson, Franklin Lakes,
NJ, USA) with 100 μl culture medium containing 5% FBS, and
incubated for 24 h to allow cells to attach to the bottom of the
plate. The cells were incubated with FB1 (0.0, 0.1, 1.0,
10.0 and 100.0 μmol/l) for 24, 48, 72 and 96 h. After 100 μl MTT (5
mg/ml in PBS) was added in the culture medium, the cells were
incubated for 4 h at 37°C in a humidified atmosphere with 5%
CO2. The medium was aspirated and the cells were
suspended in 150 μl DMSO. The absorption was measured at 490 nm
with a Mithras LB 940 Multimode Microplate reader (Berthold
Technologies, Bad Wildbad, Germany). The inhibition rate of cell
proliferation was calculated as follows: 1- [optical density (OD)
of the experimental samples/OD of the control] × 100%. The
experiment and assay were repeated at least three times.
Cell harvesting
HL-7702 cells in a logarithmic growth phase were
plated at a density of 105 cells/ml in 50-cm2
culture flasks and allowed to grow in 4 ml culture medium.
Following cell attachment, the culture medium was discarded. The
cells were then treated with FB1 (0.0, 0.1, 1.0, 10.0
and 100.0 μmol/l) for 24, 48, 72 and 96 h. Then, the cells were
trypsinized and collected for cell cycle analysis, or washed twice
with ice-cold PBS and removed from the surface of the flask by
using a rubber scraper for RT-PCR and western blot analysis.
Cell cycle analysis
The cell cycle phase was examined using a
Becton-Dickinson FACSCalibur flow cytometer. The cells were stained
with Vindelov's reagent (40 mmol/l Tris, pH 7.6; 100 mmol/l NaCl;
10 mg/ml RNase A; 7.5% PI and 0.1% Nonidet P-40), and data from
10,000 cells were collected for each data file. The experiment and
assay were repeated three times.
Semiquantitative RT-PCR
Semiquantitative RT-PCR was used to assess the mRNA
expression of cyclin E and P21. Briefly, after the HL-7702 cells
were treated with FB1, total RNA was extracted using
TRIzol, followed by chloroform re-extraction and isopropanol
precipitation. Purified RNA was dissolved in RNase-free water and
quantitated by spectrophotometry. Reverse transcription was
performed using the RevertAid First Stand cDNA Synthesis kit with
oligo(dT) priming under standard conditions suggested by the
supplier. PCR was performed in a 50-μl reaction containing PCR mix
SYBR-Green (Takara Bio, Inc., Dalian, China), each primer and cDNA.
PCR was performed under the following conditions: 94°C for 2 min
for initial denaturation, followed by 28 cycles of 94°C for 1 min,
55°C for 1 min and 72°C for 2 min. The primer sequences used were:
5′-ATACAGACCCACAGAGACAG-3′ and 5′-TGCCATCCACAGAAATACTT-3′ for
cyclin E; 5′-CAGGGGACAGCAGAGGAAGA-3′ and 5′-GGGCGGCCAGGGTATGTAC-3′
for P21 and 5′-ACGGATTTGGTCGTATTG-3′ and 5′-TGATCTTGAGGCTGTTGTC-3′
for GAPDH. The size of the predicted product was visualized by 1.7%
agarose gel electrophoresis with EB staining under ultraviolet (UV)
illumination. The amounts of PCR products were determined by
densitometry analysis using the GelDoc-It™ imaging system (UVP,
Upland, CA, USA). Semiquantitative PCR results were generated by
grading a ratio between the densitometry results of the target
cytokines and GAPDH.
Western blot analysis
The cells were detached by scraping and were
centrifuged for 10 min at 16,000 RCF at 4°C. The cell pellets were
lysed in mammalian cell protein extraction reagent (20 mmol/l Tris,
0.1% SDS, 1% Triton X-100, 1% sodium deoxycholate, pH 7.4) and
mammalian protease inhibitor mixture. The supernatant was collected
after centrifugation for 20 min at 10,000 × g at 4°C. The protein
concentration was determined using Pierce® BCA protein
assay kit (Thermo Fisher Scientific Inc., Rockford, IL, USA).
Lysates (30 μl total protein) were separated by SDS-PAGE. Following
electrophoresis, the proteins were transferred onto polyvinylidene
fluoride membranes (PVDF). The membranes were blocked in
Tris-buffered saline with 0.1% Tween-20 containing 5% non-fat dry
milk for 1 h at room temperature, incubated with the corresponding
primary antibodies (1:200) at 4°C overnight. These antibodies
included anti-cyclin E, anti-P21 and anti-GAPDH. The membranes were
incubated at room temperature with goat anti-mouse IgG-HRP
(1:2,000). Following subsequent washing with TBST, incubation with
chemiluminescence reagents and detection by Kodak In Vivo
imaging systems (Carestream Health, Inc., Rochester, NY, USA)
allowed for visualization of proteins, which were then quantitated
by strip densitometry. GAPDH was used as an internal control.
Statistical analysis
The data are expressed as the means ± SD.
Statistical analysis of the data was performed by one-way analysis
of variance (ANOVA) using the SPSS package (version 13.0).
Differences among the groups were evaluated by the parametric Least
Significant Difference (LSD) experiment and differences between
experimental groups and the negative control group were evaluated
by Dunnett's t-test. P<0.05 was considered to indicate a
statistically significant difference.
Results
Effect of FB1 on the
proliferation of HL-7702 cells
As shown in Fig. 1,
the inhibition rate of the cells was gradually increased and then
significantly decreased with increasing treatment time after the
HL-7702 cells were treated with 0.1, 1.0, 10.0 and 100.0 μmol/l
FB1 for 24, 48, 72 and 96 h compared with the control
cells. The inhibition rates of HL-7702 cells induced by treatment
with 10.0 and 100.0 μmol/l FB1 for 72 h were
significantly decreased by 41.8±2.6 and 44.1±1.2%, respectively,
compared with the control cells. The proliferation of HL-7702 cells
was significantly inhibited by treatment with FB1 (0.1,
1.0, 10.0 and 100.0 μmol/l) for 48 h compared with the
proliferation of the control cells; however, the proliferation of
HL-7702 cells was significantly increased by treatment with
FB1 (0.1, 1.0, 10.0 and 100.0 μmol/l) for 96 h compared
with the control cells. The proliferation of HL-7702 cells was
significantly increased by ~15% following treatment with 0.1 μmol/l
FB1 for 96 h compared with the control cells.
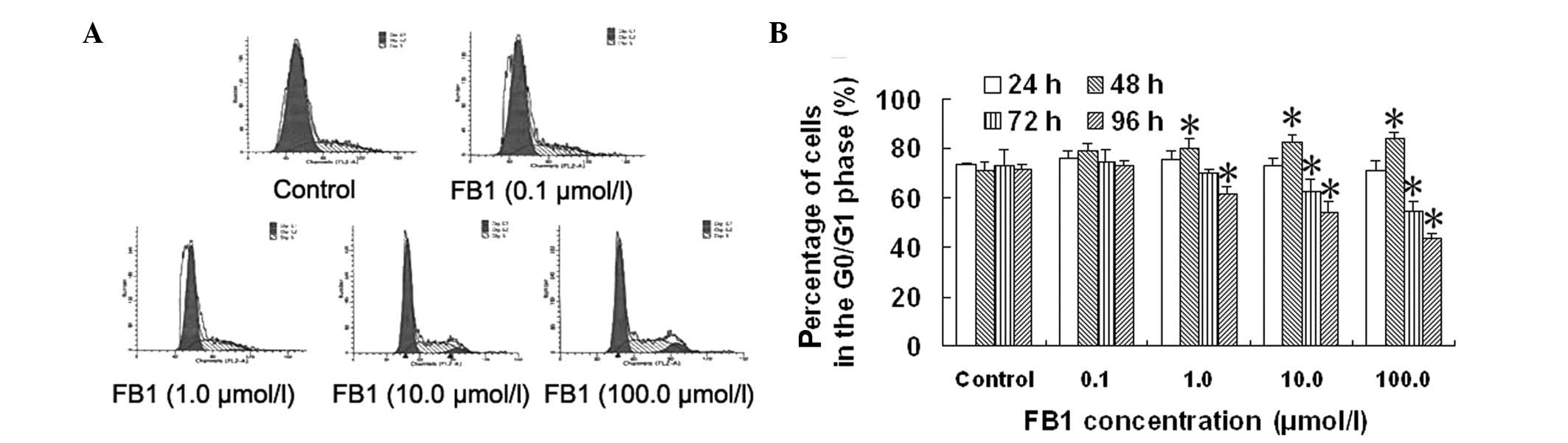
Effect of FB1 on the cell
cycle of HL-7702 cells
In order to investigate the mechanism by which
FB1 affects the proliferation of normal human liver
cells, the cell cycle was determined by flow cytometry and the
percentages of HL-7702 cells in the different phases of the cell
cycle were calculated. As shown in Fig. 2 and Table I, the cell cycle progression of
HL-7702 cells was blocked in the G2/M phase following treatment
with FB1 for 72 and 96 h. Cell progression was also
blocked in the G0/G1 phase following treatment with FB1
for 48 h. The percentages of HL-7702 cells in the G0/G1 phase were
54.5 and 43.6%, respectively, following treatment with 10.0 and
100.0 μmol/l FB1 for 96 h.
 | Table 1Effect of FB1 on the cell
cycle distribution of HL-7702 cells (%, n=3). |
Table 1
Effect of FB1 on the cell
cycle distribution of HL-7702 cells (%, n=3).
| Duration of
treatment (h) | Cell cycle
phase | FB1
concentration (μmol/l) |
|---|
|
|---|
| 0 | 0.1 | 1.0 | 10.0 | 100.0 |
|---|
| 24 | G0/G1 | 73.3±0.7 | 76.1±3.2 | 75.5±3.7 | 72.6±3.6 | 71.3±3.6 |
| S | 24.0±0.9 | 21.1±1.1 | 22.4±2.9 | 24.8±1.9 | 25.5±2.1 |
| G2/M | 2.6±1.2 | 2.8±2.1 | 2.1±1.0 | 2.6±1.9 | 3.2±1.6 |
| 48 | G0/G1 | 71.3±3.3 | 79.1±3.1 | 80.3±3.8a | 82.7±2.6a | 83.9±2.5a |
| S | 25.3±1.3 | 16.9±1.8a | 16.3±2.5a | 15.9±1.4a | 14.9±2.4a |
| G2/M | 3.4±2.0 | 4.0±1.6 | 3.4±1.4 | 1.4±1.2 | 1.3±0.1 |
| 72 | G0/G1 | 72.8±6.7 | 74.7±5.0 | 70.1±1.4 | 62.7±5.3a | 54.6±3.9a |
| S | 23.4±5.3 | 24.0±4.8 | 24.9±1.0 | 26.4±4.6 | 28.2±2.9 |
| G2/M | 4.0±1.6 | 1.3±0.6 | 5.0±1.4 | 10.9±2.5a | 17.2±1.4a |
| 96 | G0/G1 | 71.9±1.4 | 72.9±2.1 | 61.3±3.3a | 54.5±4.2a | 43.6±2.1a |
| S | 25.2±1.3 | 24.1±1.7 | 32.5±1.1a | 30.5±0.9a | 40.5±3.3a |
| G2/M | 2.8±1.4 | 2.9±0.4 | 6.3±2.3 | 15.1±3.3a | 15.9±1.2a |
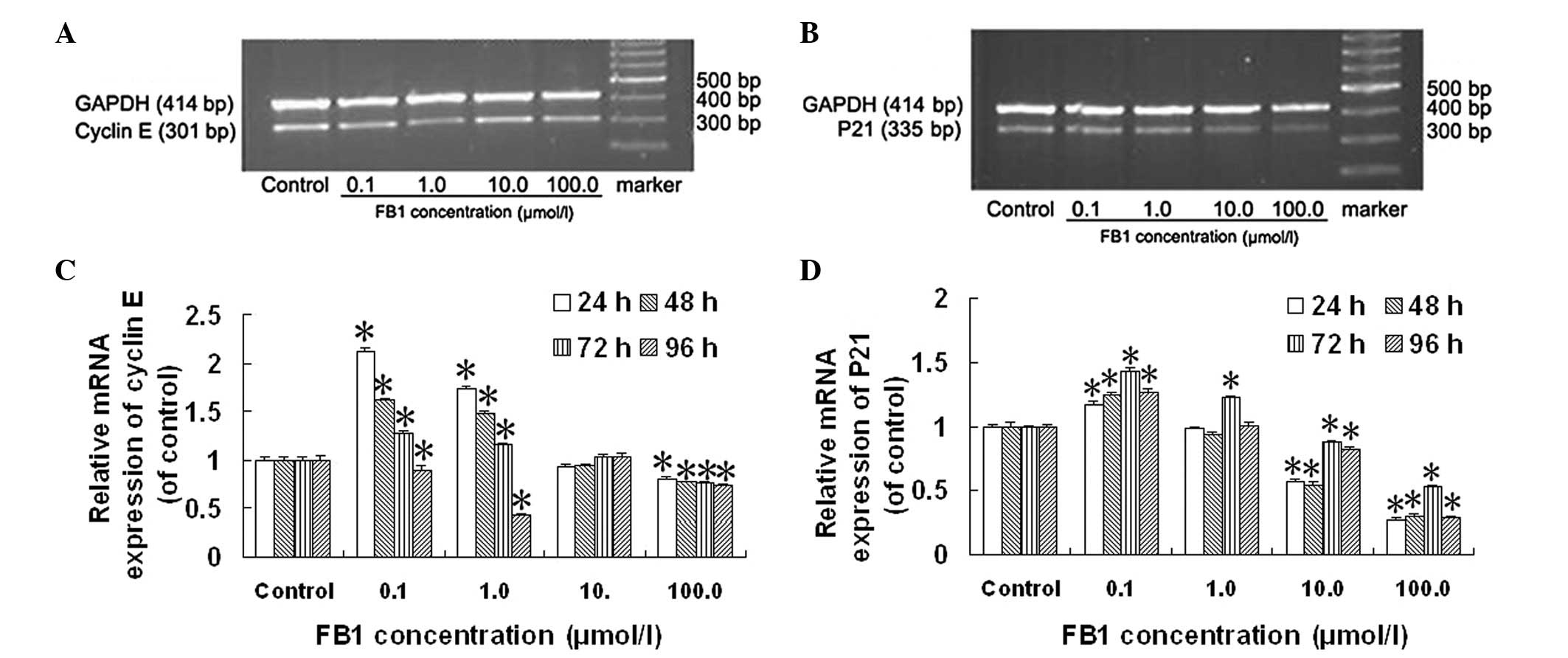
Effect of FB1 on the mRNA
expression of cyclin E and P21 in HL-7702 cells
The mRNA expression of cyclin E in HL-7702 cells was
significantly increased following treatment with 0.1 and 1.0 μmol/l
FB1 compared with the control cells (Fig. 3C). The mRNA expression of cyclin E
was initially upregulated and then gradually downregulated with
increasing treatment time following treatment with 0.1 and 1.0
μmol/l FB1 compared with the control cells; the level of
cyclin E mRNA expression was the highest following treatment for 24
h. The mRNA expression of cyclin E in HL-7702 cells was
significantly decreased following treatment with 100.0 μmol/l
FB1 for various treatment durations compared with the
control cells (Fig. 3C).
The mRNA expression of P21 in HL-7702 cells was
significantly altered following treatment with FB1 for
24, 48, 72 and 96 h in a concentration-dependent manner compared
with the control cells (Fig. 3D).
The mRNA expression of P21 in HL-7702 cells was significantly
increased following treatment with 0.1 μmol/l FB1 for
various treatment durations compared with the control cells;
however, the mRNA expression of P21 in HL-7702 cells was
significantly decreased following treatment with 10.0 and 100.0
μmol/l FB1 for the various treatment durations compared
with the control cells (Fig.
3D).
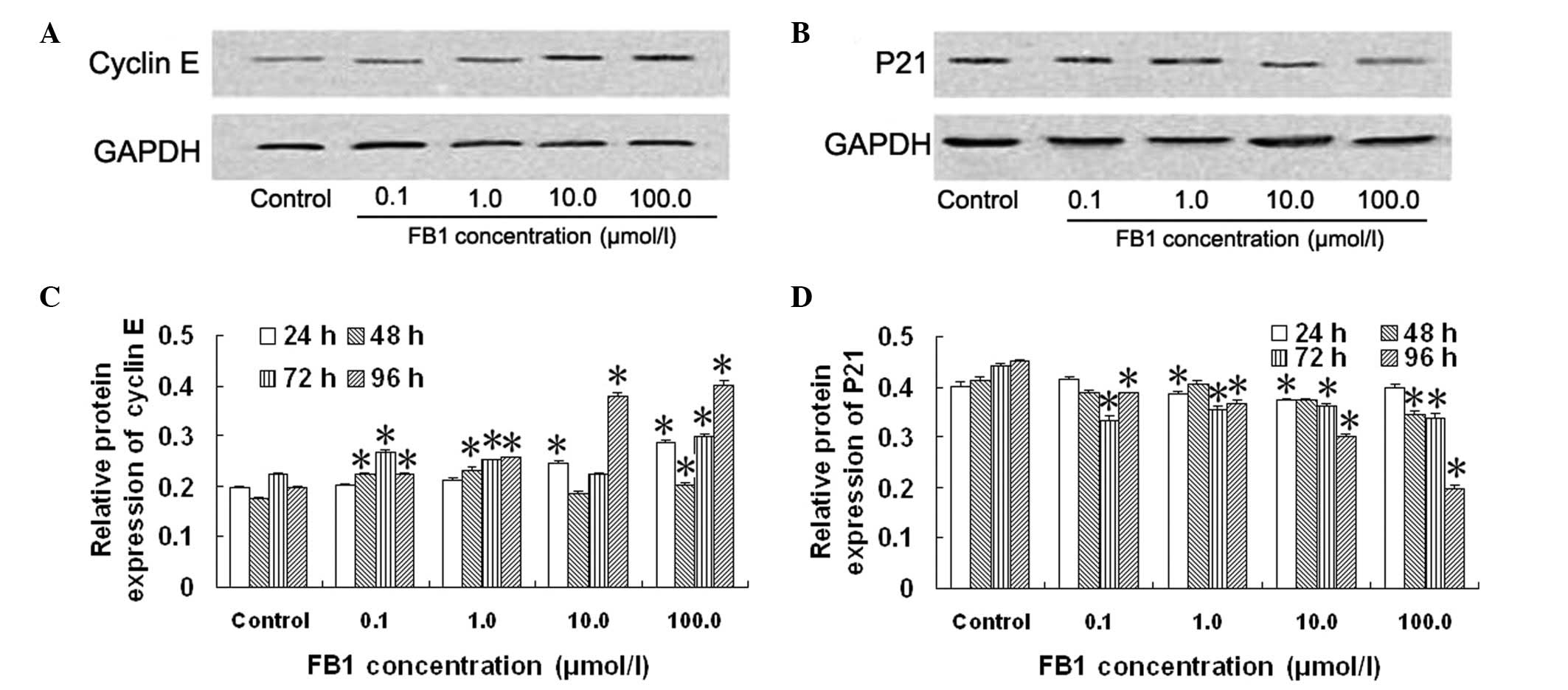
Effect of FB1 on the protein
expression of P21 and cyclin E in HL-7702 cells
The protein expression of cyclin E and P21 in
HL-7702 cells was significantly affected following treatment with
FB1 for 96 h compared with the control cells (Fig. 4). The protein expression of cyclin
E was increased on average 14.3±2.4, 30.8±1.2, 93.7±3.2 and
105.0±2.2% following treatment with 0.1, 1.0, 10.0 and 100.0 μmol/l
FB1 for 96 h, respectively, compared with the control
cells (Fig. 4C). The protein
expression of P21 was decreased on average 14.2±0.2, 19.0±0.3,
33.6±0.2 and 56.6±0.5% following treatment with 0.1, 1.0, 10.0 and
100.0 μmol/l FB1 for 96 h, respectively, compared with
the control cells (Fig. 4D). These
results demonstrated that FB1 significantly increased
the protein expression of cyclin E (Fig. 4C) and significantly decreased the
protein expression of P21 (Fig.
4D) in HL-7702 cells.
Discussion
Fumonisins are a family of cytotoxic and
carcinogenic mycotoxins. The International Agency for Research on
Cancer (IARC) has clarified that FB1 is a class 2B
carcinogen and a potential human carcinogen. In order to
investigate the effect of FB1 on the human liver, a
liver cell line derived from the normal human liver was used in
this study.
In the present study, the proliferative or
anti-proliferative effects of treatment with various concentrations
of FB1 for various treatment durations were observed
using the cell viability assay in HL-7702 cells. It has been
suggested that the mechanism of FB1 action is associated
with the biphasic dose responses, which is also known as
‘hormesis’, meaning that FB1 can stimulate the
proliferation of human normal liver cell at low doses and exhibit
effects of inhibition at high doses (26). Similarly, anti-proliferative
effects of FB1 have been observed in human hepatoma
(27) and swine peripheral blood
mononuclear cells (28). The
results of the present study demonstrated that the maximum
inhibition rate of HL-7702 cells was induced following treatment
with 100.0 μmol/l FB1 for 72 h. Although various
concentrations of FB1 inhibited the proliferation of
HL-7702 cells for treatment durations up to 48 h, the maximal
inhibition rate was still <26%. These results were consistent
with those reported by Fornelli et al(29), who demonstrated that the inhibition
rate of cell proliferation induced by the same concentration of
FB1 was ~20% in SF-9 cells. CD50 of
FB1 was 476.2±16.7 μg/ml (~680 μmol/l) in BEAS-2B cells,
which indicated that low doses of FB1 may have no
evident cytotoxicity (30).
Treatment with various concentrations of
FB1 for 48 h blocked G0/G1 phase arrest, while cell
proliferation was inhibited compared with the control cells. The
ratio of the cell population in the G0/G1 phase in all the
concentrations of FB1 used, was initially increased and
then gradually decreased with increasing treatment time, which was
similar with the results obtained from the MTT assay. In animal
experiments, FB1 affected the check point of the G1/S
phase, leading to alterations in the cell cycle (31). The percentage of cells blocked in
the G0/G1 phase of the cell cycle has been shown to be
significantly increased by FB1 in swine peripheral blood
mononuclear cells (28). A similar
result was also found in CV-1 cells in vitro where
FB1 blocked the cells in the G1 phase and resulted in
cell cycle arrest. However, FB1 did not exert the same
effects on COS-7 cells (32).
Consequently, different effects on the cell cycle have been
observed in different types of cells following treatment with
FB1.
In eukaryotes, the cell cycle is tightly regulated
by several protein kinases composed of cyclin-dependent kinases,
corresponding regulatory cyclins and cyclin-dependent kinase
inhibitors. The microinjection of anti-cyclin E antibody in cells
in the G1 phase inhibited normal fibroblasts to enter the S phase
(33). Additional studies in
Drosophila cyclin E mutant embryos indicated that cyclin E
is required for the progression through the S phase of the mitotic
cycle (34,35). These results suggest that cyclin E
and its associated kinase in the majority of eukaryotic cells are
needed for cell entry into the S phase. With the decrease of cyclin
E protein expression and the activation of CDK2 in the cell cycle,
the G1/S phase transition is promoted, and the cells proliferate
abnormally or tumor formation is stimulated. The decrease of cyclin
E mRNA expression may lead to the reduction of cyclin E protein
expression, interference of the normal physiological function,
transition of G1 to S phase and inhibition of normal cell
proliferation. Conversely, the increase of cyclin E expression
promotes cell proliferation.
The results of the present study showed that the
mRNA and protein expression of cyclin E in HL-7702 cells was
significantly increased following treatment with FB1
(0.1 and 1.0 mol/l) for 48 h compared with the control cells, and
that the mRNA and protein expression of cyclin E in HL-7702 cells
was not significantly altered following treatment with 10.0 μmol/l
FB1 for 48 h compared with the control cells. However,
the mRNA expression of cyclin E in HL-7702 cells was significantly
decreased and the protein expression of cyclin E was significantly
increased following treatment with 100.0 μmol/l FB1 for
48 h compared with the control cells. These results indicated that
FB1 affected the genetic transcription of cyclin E and
also the protein degradation of cyclin E in HL-7702 cells. The cell
cycle is regulated by the level of cyclin E protein expression;
however, not the level of cyclin E mRNA expression. According to
cell cycle analysis, treatment of HL-7702 cells with FB1
(1.0, 10.0 and 100.0 μmol/l) for 48 h blocked G0/G1 phase arrest.
These results were contradictory with the protein expression of
cyclin E. Therefore, these results demonstrated that FB1
affected the cell cycle of HL-7702 cells through other cell
cycle-related pathways than the cyclin E pathway.
The results showed that the protein expression of
cyclin E in HL-7702 cells was significantly increased following
treatment with FB1 (0.1, 1.0 and 100.0 μmol/l) for 72 h
compared with the control cells, and that the protein expression of
cyclin E in HL-7702 cells was significantly increased following
treatment with FB1 (0.1, 1.0, 10.0 and 100.0 μmol/l) for
96 h compared with the control cells. According to cell cycle
analysis, the ratio of cells in the G0/G1 phase treated with 1.0
and 100.0 μmol/l FB1 for 72 h was significantly
increased compared with the control cells. The ratio of HL-7702
cells in the G0/G1 phase treated with 1.0, 10.0 and 100.0 μmol/l
FB1 for 96 h was significantly increased. Similar
results were obtained following the assessment of cyclin E protein
expression. These results indicated that the protein expression of
cyclin E was increased following treatment with FB1 for
72 and 96 h and that FB1 promoted HL-7702 cells to enter
the S phase and increased cell proliferation.
P21 is a member of the CKI family and has a direct
role on cyclin E/CDK2. As an inducible growth inhibitor, the high
expression of P21 combined with cyclin E/CDK2 arrests the cell
cycle at the G1 phase and thus inhibits DNA replication, resulting
in the dysregulation of the cell cycle and the normal cell
differentiation process. Following western blot analysis, a
decrease of P21 protein expression was observed in the normal human
liver cells following treatment with various concentrations of
FB1. The mRNA expression of P21 in HL-7702 cells was
significantly decreased following treatment with 10.0 and 100.0
μmol/l FB1 for various treatment durations compared with
the control cells, and the mRNA expression of P21 in HL-7702 cells
was significantly increased following treatment with 0.1 μmol/l
FB1 for various treatment durations compared with the
control cells. These results showed that the protein expression was
decreased by treatment with FB1 for 72 and 96 h, and
that FB1 induced HL-7702 cells to enter the S phase and
increased cell proliferation.
According to the study by Zhang et
al(36), the two Sp1 binding
sites within −124 to −101 were necessary and sufficient for
FB1-induced P21 transcription in CV-1 cells (36). However, the concentration of
FB1 used in this study was 5 mmol/l, notably higher than
the dose used in our experiment. Therefore, it is suggested that
the different doses of FB1 lead to different effects on
P21 expression.
In conclusion, the effect of FB1 on the
proliferation, cell cycle and expression of cyclin E and P21 in the
normal human liver cell line HL-7702 indicates that FB1
is most likely to affect other cell cycle-related factors.
Meanwhile, the inconsistency of the mRNA and protein expression of
genes indicates different regulatory mechanisms (such as synthesis
and degradation rates) acting on the synthesized mRNA and protein,
which differentially affect the amount of the two molecules.
Therefore, further studies are needed to elucidate the underlying
mechanism of action.
Acknowledgements
This study was supported by grants from the National
Natural Science Foundation of China (30800914). The authors would
like to thank Dr Zilin Sun (Department of Endocrinology, Southeast
University, Nanjing, Jiangsu, China) for his assistance with the
western blot analysis and Dr Jia-Sheng Wang (Department of
Environmental Health Science, College of Public Health, University
of Georgia, Athens, GA, USA) for his valuable input throughout this
study.
References
|
1
|
Theumer MG, López AG, Aoki MP, et al:
Subchronic mycotoxicoses in rats. Histopathological changes and
modulation of the sphinganine to sphingosine (Sa/So) ratio
imbalance induced by Fusarium verticillioides culture
material, due to the coexistence of aflatoxin B1 in the diet. Food
Chem Toxicol. 46:967–977. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Stockmann-Juvala H and Savolainen K: A
review of the toxic effects and mechanisms of action of fumonisin
B1. Hum Exp Toxicol. 27:799–809. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Cano-Sancho G, Ramos AJ, Marín S and
Sanchis V: Occurrence of fumonisins in Catalonia (Spain) and an
exposure assessment of specific population groups. Food Addit
Contam Part A Chem Anal Control Expo Risk Assess. 29:799–808. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Ledoux DR, Broomhead JN, Bermudez AJ and
Rottinghaus GE: Individual and combined effects of the
Fusarium mycotoxins fumonisin B1 and moniliformin in broiler
chicks. Avian Dis. 47:1368–1375. 2003.PubMed/NCBI
|
|
5
|
Gelderblom WC, Marasas WF, Lebepe-Mazur S,
et al: Cancer initiating properties of fumonisin B1 in a short-term
rat liver carcinogenesis assay. Toxicology. 250:89–95. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Zhang Y, Jones C and Dickman MB:
Identification of differentially expressed genes following
treatment of monkey kidney cells with the mycotoxin fumonisin B(1).
Food Chem Toxicol. 39:45–53. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Wattenberg EV, Badria FA and Shier WT:
Activation of mitogen-activated protein kinase by the carcinogenic
mycotoxin fumonisin B1. Biochem Biophys Res Commun. 227:622–627.
1996. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Bouhet S, Hourcade E, Loiseau N, et al:
The mycotoxin fumonisin B1 alters the proliferation and the barrier
function of porcine intestinal epithelial cells. Toxicol Sci.
77:165–171. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Theumer MG, Cánepa MC, López AG, et al:
Subchronic mycotoxicoses in Wistar rats: assessment of the in vivo
and in vitro genotoxicity induced by fumonisins and aflatoxin B(1),
and oxidative stress biomarkers status. Toxicology. 268:104–110.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Rumora L, Kovacić S, Rozgaj R, et al:
Cytotoxic and genotoxic effects of fumonisin B1 on rabbit kidney
RK13 cell line. Arch Toxicol. 76:55–61. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Sharma N, He Q and Sharma RP: Sphingosine
kinase activity confers resistance to apoptosis by fumonisin B1 in
human embryonic kidney (HEK-293) cells. Chem Biol Interact.
151:33–42. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Rentz SS, Showker JL, Meredith FI, et al:
Inhibition of sphingolipid biosynthesis decreases phosphorylated
ERK2 in LLC-PK1 cells. Food Chem Toxicol. 43:123–131. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Galvano F, Campisi A, Russo A, et al: DNA
damage in astrocytes exposed to fumonisin B1. Neurochem Res.
27:345–351. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Ribeiro DH, Ferreira FL, da Silva VN, et
al: Effects of aflatoxin b(1) and fumonisin b(1) on the viability
and induction of apoptosis in rat primary hepatocytes. Int J Mol
Sci. 11:1944–1955. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Schwerdt G, Konigs M, Holzinger H, et al:
Effects of the mycotoxin fumonisin B(1) on cell death in human
kidney cells and human lung fibroblasts in primary culture. J Appl
Toxicol. 29:174–182. 2009. View
Article : Google Scholar : PubMed/NCBI
|
|
16
|
Galvano F, Russo A, Cardile V, et al: DNA
damage in human fibroblasts exposed to fumonisin B1. Food Chem
Toxicol. 40:25–31. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Seefelder W, Humpf HU, Schwerdt G, et al:
Induction of apoptosis in cultured human proximal tubule cells by
fumonisins and fumonisin metabolites. Toxicol Appl Pharmacol.
192:146–153. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Kouadio JH, Mobio TA, Baudrimont I, et al:
Comparative study of cytotoxicity and oxidative stress induced by
deoxynivalenol, zearalenone or fumonisin B1 in human intestinal
cell line Caco-2. Toxicology. 213:56–65. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Czerednik A, Busscher M, Bielen BA, et al:
Regulation of tomato fruit pericarp development by an interplay
between CDKB and CDKA1 cell cycle genes. J Exp Bot. 63:2605–2617.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Yamamoto S, Kohsaka S and Nakajima K: Role
of cell cycle-associated proteins in microglial proliferation in
the axotomized rat facial nucleus. Glia. 60:570–581. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Li J, Deng M, Wei Q, et al:
Phosphorylation of MCM3 protein by cyclin E/cyclin-dependent kinase
2 (Cdk2) regulates its function in cell cycle. J Biol Chem.
286:39776–39785. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Roy HK, Koetsier JL, Tiwari AK, et al:
Involvement of p21cip1/waf1 in the anti-proliferative effects of
polyethylene glycol in colon carcinogenesis. Int J Oncol.
38:529–536. 2011.PubMed/NCBI
|
|
23
|
Chien MH, Lee TS, Liang YC and Lee WS:
β-Sitosterol inhibits cell cycle progression of rat aortic smooth
muscle cells through increases of p21cip1 protein. J Agric Food
Chem. 58:10064–10069. 2010.
|
|
24
|
Fang Y, Yu S and Braley-Mullen H: TGF-β
promotes proliferation of thyroid epithelial cells in IFN-γ(−/−)
mice by down-regulation of p21 and p27 via AKT pathway. Am J
Pathol. 180:650–660. 2011.
|
|
25
|
Zuo S, Liu C, Wang J, et al: IGFBP-rP1
induces p21 expression through a p53-independent pathway, leading
to cellular senescence of MCF-7 breast cancer cells. J Cancer Res
Clin Oncol. 138:1045–1055. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Calabrese EJ: Hormesis: changing view of
the dose-response, a personal account of the history and current
status. Mutat Res. 511:181–189. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
McKean C, Tang L, Tang M, et al:
Comparative acute and combinative toxicity of aflatoxin B1 and
fumonisin B1 in animals and human cells. Food Chem Toxicol.
44:868–876. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Marin DE, Gouze ME, Taranu I and Oswald
IP: Fumonisin B1 alters cell cycle progression and interleukin-2
synthesis in swine peripheral blood mononuclear cells. Mol Nutr
Food Res. 51:1406–1412. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Fornelli F, Minervini F and Mulè G:
Cytotoxicity induced by nivalenol, deoxynivalenol, and fumonisin B1
in the SF-9 insect cell line. In Vitro Cell Dev Biol Anim.
40:166–171. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Lewis CW, Smith JE, Anderson JG and
Freshney RI: Increased cytotoxicity of food-borne mycotoxins toward
human cell lines in vitro via enhanced cytochrome p450 expression
using the MTT bioassay. Mycopathologia. 148:97–102. 1999.
View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Ramljak D, Calvert RJ, Wiesenfeld PW, et
al: A potential mechanism for fumonisin B(1)-mediated
hepatocarcinogenesis: cyclin D1 stabilization associated with
activation of Akt and inhibition of GSK-3beta activity.
Carcinogenesis. 21:1537–1546. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Wang W, Jones C, Ciacci-Zanella J, et al:
Fumonisins and Alternaria alternata lycopersici toxins:
sphinganine analog mycotoxins induce apoptosis in monkey kidney
cells. Proc Natl Acad Sci USA. 93:3461–3465. 1996.
|
|
33
|
Zhu X, Ohtsubo M, Böhmer RM, et al:
Adhesion-dependent cell cycle progression linked to the expression
of cyclin D1, activation of cyclin E-cdk2, and phosphorylation of
the retinoblastoma protein. J Cell Biol. 133:391–403. 1996.
View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Wang ZA and Kalderon D: Cyclin E-dependent
protein kinase activity regulates niche retention of
Drosophila ovarian follicle stem cells. Proc Natl Acad Sci
USA. 106:21701–21706. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Fox PM, Vought VE, Hanazawa M, et al:
Cyclin E and CDK-2 regulate proliferative cell fate and cell cycle
progression in the C. elegans germline. Development.
138:2223–2234. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Zhang Y, Dickman MB and Jones C: The
mycotoxin fumonisin B1 transcriptionally activates the p21 promoter
through a cis-acting element containing two Sp1 binding
sites. J Biol Chem. 274:12367–12371. 1999. View Article : Google Scholar : PubMed/NCBI
|