Introduction
Esophageal carcinoma (EC) is a public health issue
in China (1). It has a poor
overall prognosis and treatment outcomes for patients with EC have
not improved over the last few decades. Significant prognostic
indicators include the extent of invasion and lymph node and distal
metastases (2). However, specific
patients with the same stage have different prognosis (3). Therefore, molecular markers to
improve the outcome prediction for patients with EC must be
identified.
Classified as type I programed cell death, apoptosis
is involved in a number of cancer-related processes, including
tumorigenesis, tumor progression and cellular responses to
chemotherapy and radiotherapy (4–6). The
well-studied tumor suppressor protein, p53, is key to apoptosis and
cancer development. Wild-type p53 inhibits tumor growth by inducing
apoptosis and inhibiting angiogenesis and metastasis; however, the
TP53 gene undergoes inactivating mutations in a variety of
cancer types. More recently, p53 has been demonstrated to repress
autophagy (7), indicating that
apoptosis and autophagy may interact. Type II programmed cell
death, or autophagy, is an evolutionarily conserved eukaryotic
process important for maintaining homeostasis by recycling stable
proteins and long-lived organelles (8). Autophagy is important in a number of
physiological processes, including adaptation to hypoxia,
prevention of tumorigenesis and antigen presentation (9–11).
Although the role of autophagy in cancer is unclear, the current
view is that it functions as a cytoprotective mechanism during
tumor progression (12).
Previously, microtubule-associated protein 1 light
chain 3A (LC3A), one of three microtubule-associated protein light
chain isoforms, was revealed to be an essential component of
autophagosomes (13). Using the
immunohistochemical detection of LC3A protein as a marker of
autophagic activity, autophagy was identified to be upregulated in
urothelial cell carcinoma, non-small cell lung cancer and
endometrial, colorectal and cutaneous squamous cell carcinomas
(14–18). In addition, LC3A overexpression is
linked to the aggressiveness of these tumors (16). However, the clinicopathological and
prognostic significance of LC3A overexpression in esophageal
squamous cell carcinomas (ESCCs) has not been established.
Cancer-related TP53 mutations are associated with the
overexpression of inactive p53 (19). The combined prognostic effect of
p53 and LC3A overexpression has not been investigated in patients
with ESCC. In the current study, the expression of p53 and LC3A
proteins was measured by immunohistochemistry and the prognostic
significance of p53 and LC3A overexpression was assessed in
patients with ESCC.
Materials and methods
Patients and tissue specimens
From our clinical archives, 114 patients with stage
II/III (Tany N+M0 or T3,4
Nany M0) ESCC were assessed. Patient were
treated with surgery followed by adjuvant concurrent
chemoradiotherapy (CRT) in the Department of Oncology at the
Affiliated Hospital of Binzhou Medical College (Shandong, China)
between January 2006 and December 2008. The study was approved by
the ethics committee of the Affiliated Hospital of Binzhou Medical
College (Shandong, China). Prior to surgery, all patients underwent
computed tomography staging of regional lesions and metastases of
the neck, thorax and abdomen. Characteristics of the 114 patients,
including gender, cell differentiation, weight loss, T, N and
clinical stages and location, are presented in Table I. The median patient age was 58.1
years (range, 40–71 years). Clinical staging was assessed according
to the 2002 TNM Staging of the International Union Against Cancer
(20). Informed consent was
obtained from all patients. Following surgery, tissue specimens
obtained from all the patients were fixed in formalin and embedded
in paraffin. Patients did not undergo chemotherapy or radiotherapy
prior to surgery. The median follow-up was 57.5 months (range, 4–70
months) and survival information was available for 107
patients.
 | Table Ip53 and LC3A expression and
clinicopathological characteristics. |
Table I
p53 and LC3A expression and
clinicopathological characteristics.
| | p53 | LC3A |
|---|
| |
|
|
|---|
| Parameters | Cases | High | Low | P-value | High | Low | P-value |
|---|
| Age, years |
| ≤60 | 46 | 20 | 26 | 0.077 | 22 | 24 | 0.592 |
| >60 | 68 | 41 | 27 | | 36 | 32 | |
| Gender |
| Male | 101 | 54 | 47 | 0.979 | 50 | 51 | 0.414 |
| Female | 13 | 7 | 6 | | 8 | 5 | |
| Weight loss |
| ≤10% | 28 | 13 | 15 | 0.387 | 15 | 13 | 0.743 |
| >10% | 86 | 48 | 38 | | 43 | 43 | |
| Location |
| Ut/Mt | 61 | 36 | 25 | 0.206 | 35 | 26 | 0.136 |
| Lt | 53 | 25 | 28 | | 23 | 30 | |
| Histology |
| Well | 49 | 24 | 25 | 0.400 | 22 | 27 | 0.268 |
| Mod/poor | 65 | 37 | 28 | | 36 | 29 | |
| T stage |
| T1–2 | 37 | 15 | 22 | 0.092 | 18 | 19 | 0.741 |
| T3–4 | 77 | 46 | 31 | | 40 | 37 | |
| N stage |
| N0 | 55 | 29 | 26 | 0.017 | 24 | 31 | 0.135 |
| N+ | 59 | 32 | 27 | | 34 | 25 | |
| Clinical stage |
| II | 48 | 26 | 22 | 0.904 | 25 | 23 | 0.826 |
| III | 66 | 35 | 31 | | 33 | 33 | |
Treatment
All patients underwent radical surgical tumor
resection. Patients with upper thoracic esophageal malignancies
were subjected to transthoracic esophagectomy with three-field
lymphadenectomy and patients with middle/lower thoracic ESCC
underwent two-field lymphadenectomy. Following surgery, adjuvant
CRT consisted of three-dimensional conformal radiotherapy with 49.2
Gy (range, 40–50 Gy) concurrent with two adjuvant cycles of
cisplatin (25 mg/m2; days 1–3) and fluorouracil (600
mg/m2; days 1–3) chemotherapy.
Immunohistochemistry
Polyclonal antibodies against LC3A (1:80; Cell
Signaling Technology, Inc., Danvers, MA, USA) and p53 (1:100; Dako,
Carpinteria, CA, USA) were obtained. Immunohistological analysis of
p53 and LC3A was performed using 3-μm-thick formalin-fixed
paraffin-embedded esophageal tumor sections. Sections were
deparaffinized with xylene, rehydrated using graded alcohol
solutions and then heat-induced epitope retrieval was performed in
0.01 M citrate buffer (pH 6.0) in a microwave oven. Tissue sections
(Tany N+M0 or T3,4 Nany
M0) were incubated with fresh 0.3% hydrogen peroxide in
methanol for 30 min at room temperature. Non-specific antibody
binding was blocked by incubation with Protein Block (Dako) for 5
min at room temperature. Sections were then incubated overnight at
4°C with primary polyclonal antibody, washed in phosphate-buffered
saline (PBS) and then incubated with secondary antibody (Santa Cruz
Biotechnology, Inc., Santa Cruz, CA, USA) for 30 min at room
temperature. Following washing with PBS, sections were incubated
with 3,3′-diaminobenzidine for 5 min and counterstained with
hematoxylin. PBS was used as a negative control for the primary
antibody; no staining was detected.
Immunohistochemical analysis of p53 and
LC3A expression
For scoring, five random fields were selected from
each tissue section and the mean score for each slide was used for
subsequent analyses. Assessment of p53 expression was based on the
percentage of tumor cells revealing p53 immunoreactivity and
immunointensity. Tumor staining was classified into three
categories; >10, 10–50 and >50% of cells with positive
staining. p53 immunointensity was classified into two categories;
no or weak immunostaining and marked immunostaining. Tumors with
>50% of cells revealing marked p53 and LC3A immunostaining were
defined as exhibiting high p53 and LC3A expression, respectively.
Scoring of immunohistochemical staining was performed by two
independent pathologists blind to the clinicopathological status of
the samples.
Statistical analysis
Statistical analysis was performed using SPSS
software, version 13.0 (SPSS Inc., Chicago, IL, USA). p53 and LC3A
expression levels were defined as categorical variables.
Differences between groups were estimated using the Chi-square
test. Patient survival was assessed using Kaplan-Meier curves and
differences in survival between groups of patients were analyzed
using the log-rank test. Cox’s proportional hazards model was used
for multivariable analysis. P<0.05 was considered to indicate a
statistically significant difference. The primary endpoint was
overall survival, which was defined as the time (in months) between
the date of therapy and the date of the last follow-up or
mortality.
Results
p53 and LC3A expression in archival ESCC
samples
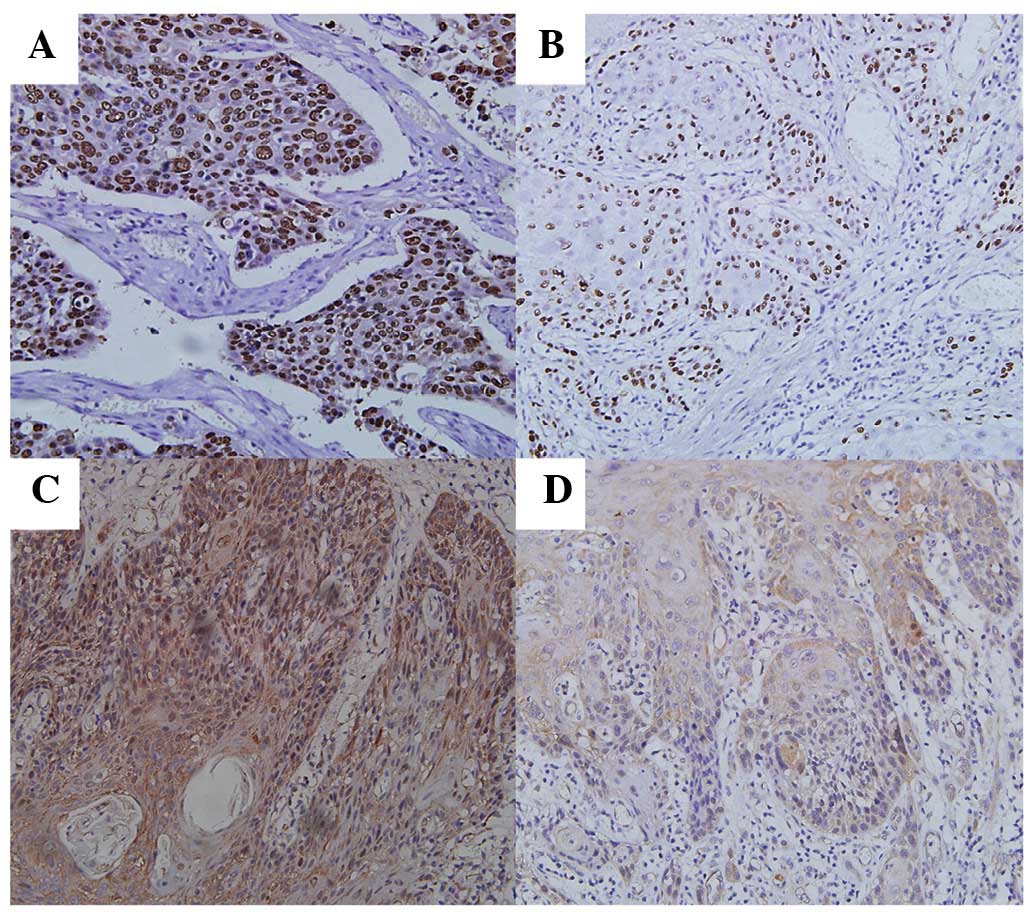
Immunohistochemical analysis identified that 53.5
(61/114) and 50.9% (58/114) of tumors exhibited p53 and LC3A
overexpression, respectively. In addition, p53 was largely
localized to the nuclei of tumor cells (Fig. 1A and B), while LC3A expression was
cytoplasmic (Fig. 1C and D). In
contrast to previous reports, LC3A expression was not observed in
perinuclear, ‘stone-like’ structures (SLS) in ESCC samples
(17).
p53 and LC3A overexpression correlates
with ESCC clinical aggressiveness
The correlation between p53 and LC3A expression and
various clinicopathological parameters are presented in Table I. p53 overexpression was more
frequently observed in lymph node-positive than in lymph
node-negative tumors (P=0.017). There were no associations between
p53 immunostaining and other factors, including gender, weight
loss, age and tumor location. Similarly, no clinicopathological
characteristics were found to correlate with LC3A
immunoreactivity.
High p53 and LC3A co-expression inversely
correlates with patient survival
Having identified correlations between p53 and LC3A
expression and clinicopathological parameters, the correlation
between p53 and LC3A expression and patient survival was
determined. Among the clinicopathological characteristics, N stage
(N0) and clinical stage (II) were identified to
significantly correlate with survival (N0 vs.
N+, P=0.000; II vs. III, P=0.044; Table II). The overall 5-year survival
rate of 54 patients with high p53 expression was not observed to
differ significantly from that of 53 patients with low p53
expression (P>0.05). Similarly, there was no correlation between
LC3A expression and the 5-year survival rate (P>0.05). Next, 107
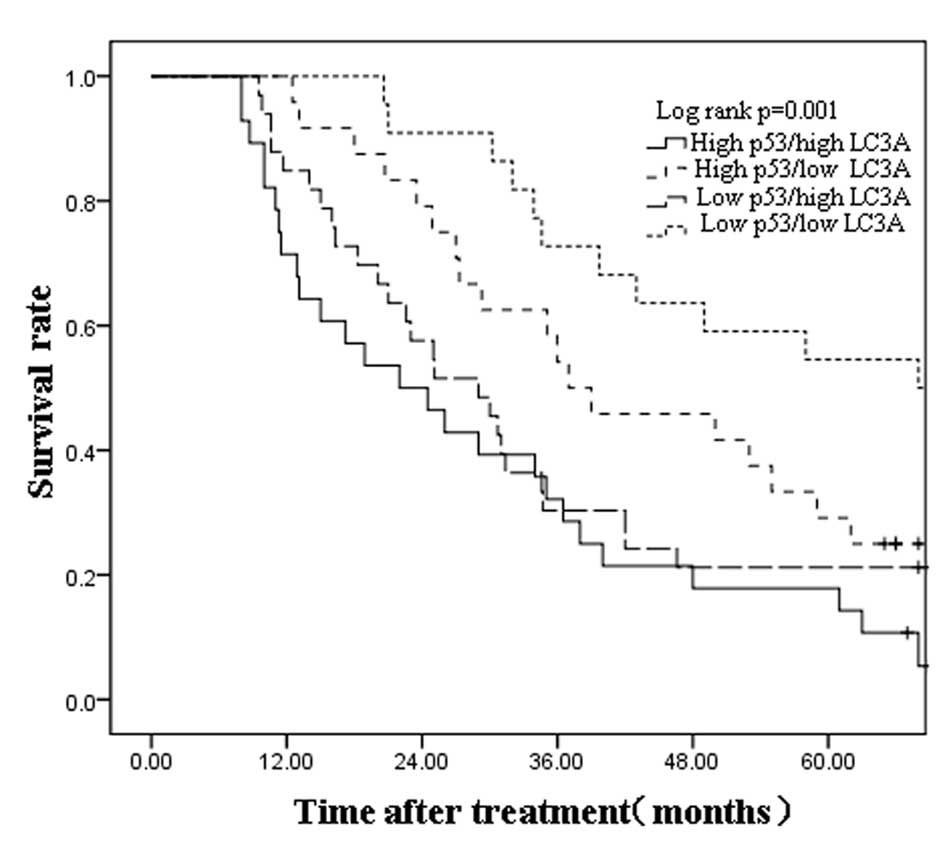
patients were divided into four subgroups according to their p53
and LC3A status (high p53/high LC3A, n=27; high p53/low LC3A, n=32;
low p53/high LC3A, n=27; and low p53/low LC3A, n=21). Kaplan-Meier
curves revealed that low p53 and LC3A co-expression in primary
ESCCs is associated with longer overall patient survival times,
whereas high p53 and LC3A co-expression is associated with shorter
patient survival time (median of 45 vs. 28 months, respectively;
log-rank test, P=0.001; Fig. 2).
The 5-year survival rate of patients with high p53 and LC3A
co-expression was 18.0%, while that of patients with low p53 and
LC3A co-expression was 54.4% (P=0.001). Thus, high expression of
p53 and LC3A in ESCCs is linked to poor patient prognosis.
 | Table IIUnivariate Cox analysis of 5-year
overall survival rate following surgery in 107 patients with
ESCC. |
Table II
Univariate Cox analysis of 5-year
overall survival rate following surgery in 107 patients with
ESCC.
| Parameters | Cases | HR | 95% CI | P-value |
|---|
| Age, years |
| ≤60 | 43 | 1.000 | | 0.325 |
| >60 | 64 | 1.340 | 0.841–2.408 | |
| Gender |
| Male | 95 | 1.000 | | 0.412 |
| Female | 12 | 0.820 | 0.475–1.316 | |
| Weight loss |
| ≤10% | 25 | 1.000 | | 0.253 |
| >10% | 82 | 1.297 | 0.739–2.054 | |
| Location |
| Ut/Mt | 57 | 1.000 | | 0.844 |
| Lt | 50 | 0.902 | 0.307–3.238 | |
| Histology |
| Well | 44 | 1.000 | | 0.471 |
| Mod/poor | 63 | 1.313 | 0.712–2.951 | |
| T stage |
| T1–2 | 36 | 1.000 | | 0.298 |
| T3–4 | 71 | 1.165 | 0.414–3.977 | |
| N stage |
| N0 | 51 | 1.000 | | 0.000 |
| N+ | 57 | 4.624 | 1.153–10.356 | |
| Clinical stage |
| II | 45 | 1.000 | | 0.044 |
| III | 62 | 3.072 | 1.315–5.924 | |
| LC3A |
| High | 54 | 1.000 | | 0.063 |
| Low | 53 | 0.653 | 0.280–1.561 | |
| p53 |
| High | 59 | 1.000 | | 0.156 |
| Low | 48 | 0.705 | 0.232–1.748 | |
High p53 and LC3A co-expression is a poor
prognostic factor for ESCC patients
To determine whether p53 and LC3A represent
prognostic factors of ESCC, overall patient survival was examined
using Cox regression proportional hazard analysis on prognostic
factors (T, N and clinical stage and p53 and LC3A status) in 107
ESCC patients. High levels of p53 and LC3A expression were not
identified as independent prognostic factors (P>0.05; Table III, part A); however, N and
clinical stages were revealed to be independent prognostic factors
(P=0.006 and P=0.013, respectively). However, when defined as a
single factor, high p53/high LC3A co-expression was determined as
an independent prognostic factor (P=0.027; Table III, part B).
 | Table IIIMultivariable analysis of factors
associated with survival. |
Table III
Multivariable analysis of factors
associated with survival.
| A, High p53 and
LC3A co-expression, analyzed as two independent factors. |
|---|
|
|---|
| Variable | Hazard ratio | 95% CI | P-value |
|---|
| T stage (T3,4) | 1.5 | 0.709–3.152 | 0.234 |
| N stage (N+) | 4.2 | 1.104–9.613 | 0.000 |
| Clinical stage
(III) | 8.3 | 1.825–19.463 | 0.013 |
| p53 (high) | 0.8 | 0.254–3.182 | 0.597 |
| LC3A (high) | 1.7 | 0.571–3.467 | 0.435 |
|
| B, High p53 and
LC3A co-expression, analyzed as a single factor. |
|
| Variable | Hazard ratio | 95% CI | P-value |
|
| T stage (T3,4) | 1.2 | 0.227–3.041 | 0.485 |
| N stage (N+) | 3.1 | 1.562–8.275 | 0.000 |
| Clinical stage
(III) | 5.6 | 1.647–14.264 | 0.009 |
| p53 (high)/LC3A
(high) | 2.8 | 1.536–6.183 | 0.027 |
Discussion
In the present study, the expression of LC3A and
mutant p53 was measured by immunohistochemistry. Results indicated
that high p53 expression was linked to ESCC lymph node metastasis,
but did not correlate with the overall survival of patients with
ESCC. However, when high levels of p53 and LC3A co-expression were
considered as a single factor, it was linked to poor prognosis.
The TP53 gene is commonly mutated in human
tumors (21,22); however, associations between mutant
p53 expression and patient prognosis remain unclear. TP53
mutations often lead to a loss of tumor suppressor activity and an
extended half-life; therefore, the accumulation of mutant p53
protein is detectable by immunohistochemistry (23). Previous studies have demonstrated
that p53 expression correlates with tumor stage, lymph node
metastasis and overall survival in EC (2,23–24).
Consistent with these studies, p53 overexpression was found to be
more frequent in lymph node-positive ESCCs in the current study. By
contrast, additional studies have reported that p53 expression is
not linked to lymph node metastasis and survival (25–26).
These inconsistencies may be caused by the use of various
experimental conditions, classification standards and patient
populations between studies.
LC3A is a key mediator of autophagy and specific
studies have indicated that the immunohistochemical determination
of LC3A expression is a useful prognostic indicator (15–17).
Fujii et al(27)
demonstrated that high LC3A expression correlates with reduced
disease-free and overall patient survival. In addition, more recent
studies have found that LC3A exhibits three expression patterns in
numerous human malignancies; diffuse cytoplasmic,
cytoplasmic/juxtanuclear and SLS (14–18).
High numbers of SLS have been linked to poor patient outcome,
whereas the remaining two expression patterns have demonstrated no
prognostic significance. In the present study, only diffuse
cytoplasmic expression was observed in ESCCs. A previous study
reported that LC3A expression was restricted to the cytoplasm in
melanomas (28) and that LC3A
overexpression does not correlate with patient survival. We
therefore hypothesized that LC3A expression patterns and clinical
significance are tissue-specific.
The programmed cell death pathways, apoptosis and
autophagy, do not function independently. Environmental stressors,
including hypoxia and irradiation, induce apoptosis and autophagy,
and molecular cross-talk exists between these pathways. p53 induces
apoptosis through transactivation of Bcl-2 family proteins and
inhibits autophagy through a direct interaction with RB1CC/FIP200
(7). In addition, the autophagy
component, hAtg7, is a p53 regulator and in the absence of hAtg7,
the proapoptotic activity of p53 increases, while p53-mediated cell
cycle arrest decreases (29). In
addition, other molecules, including mTOR and Atg5, are important
for cross-talk between various cell death signaling pathways
(30,31), thus highlighting the complex
regulation of these processes. In the majority of cases, the
regulation of apoptosis and autophagy occurs in opposite
directions; however, both are inhibited by activation of the
PI3K/Akt/mTOR pathway (32).
Therefore, we hypothesized that inactivating mutations in p53
simultaneously decrease apoptosis and increase autophagy, thus
promoting tumor cell survival. This hypothesis is consistent with
results of the current study, which revealed that high p53 and LC3A
co-expression is linked to poor prognosis in patients with
ESCC.
In conclusion, observations of this study indicate
that p53 expression is linked to lymph node metastasis and that
high levels of p53 and LC3A co-expression correlate with poor
patient prognosis. Thus, immunohistochemical analysis of p53 and
LC3A co-expression represents a promising prognostic indicator for
this disease. However, these results require validation in further,
large-cohort, prospective studies.
Acknowledgements
The authors thank Shu-Hua Wu, Yu-Hong Zhu and Dong
Tian for their knowledge and technical assistance.
References
|
1
|
Ke L: Mortality and incidence trends from
esophagus cancer in selected geographic areas of China circa
1970–90. Int J Cancer. 102:271–274. 2002.PubMed/NCBI
|
|
2
|
Yoon HH, Khan M, Shi Q, et al: The
prognostic value of clinical and pathologic factors in esophageal
adenocarcinoma: a mayo cohort of 796 patients with extended
follow-up after surgical resection. Mayo Clin Proc. 85:1080–1089.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Bauer KR, Brown M, Cress RD, et al:
Descriptive analysis of estrogen receptor (ER)-negative,
progesterone receptor (PR)-negative, and HER2-negative invasive
breast cancer, the so-called triple-negative phenotype: a
population-based study from the California cancer Registry. Cancer.
109:1721–1728. 2007. View Article : Google Scholar
|
|
4
|
Raouf AA, Evoy DA, Carton E, Mulligan E,
Griffin MM and Reynolds JV: Loss of Bcl-2 expression in Barrett’s
dysplasia and adenocarcinoma is associated with tumor progression
and worse survival but not with response to neoadjuvant
chemoradiation. Dis Esophagus. 16:17–23. 2003.
|
|
5
|
Ito Y, Takeda T, Sasaki Y, et al: Bcl-2
expression in cholangiocellular carcinoma is inversely correlated
with biologically aggressive phenotypes. Oncology. 59:63–67. 2000.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Kunnumakkara AB, Diagaradjane P, Guha S,
et al: Curcumin sensitizes human colorectal cancer xenografts in
nude mice to gamma-radiation by targeting nuclear
factor-kappaB-regulated gene products. Clin Cancer Res.
14:2128–2136. 2008. View Article : Google Scholar
|
|
7
|
Morselli E, Shen S, Ruckenstuhl C, et al:
p53 inhibits autophagy by interacting with the human ortholog of
yeast Atg17, RB1CC1/FIP200. Cell Cycle. 10:2763–2769. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Levine B: Cell biology: autophagy and
cancer. Nature. 446:745–747. 2007. View
Article : Google Scholar : PubMed/NCBI
|
|
9
|
Rouschop KM, Ramaekers CH, Schaaf MB, et
al: Autophagy is required during cycling hypoxia to lower
production of reactive oxygen species. Radiother Oncol. 92:411–416.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Mathew R, Karp CM, Beaudoin B, et al:
Autophagy suppresses tumorigenesis through elimination of p62.
Cell. 137:1062–1075. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Tey SK and Khanna R: Autophagy mediates
transporter associated with antigen processing-independent
presentation of viral epitopes through MHC class I pathway. Blood.
120:994–1004. 2012. View Article : Google Scholar
|
|
12
|
Hu YL, Jahangiri A, Delay M, et al: Tumor
cell autophagy as an adaptive response mediating resistance to
treatments such as antiangiogenic therapy. Cancer Res.
72:4294–4299. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
He H, Dang Y, Dai F, et al:
Post-translational modifications of three members of the human
MAP1LC3 family and detection of a novel type of modification for
MAP1LC3B. J Biol Chem. 278:29278–29287. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Sivridis E, Koukourakis MI, Mendrinos SE,
Touloupidis S and Giatromanolaki A: Patterns of autophagy in
urothelial cell carcinomas-the significance of ‘stone-like’
structures (SLS) in transurethral resection biopsies. Urol Oncol.
Jan 24–2012.(Epub ahead of print).
|
|
15
|
Karpathiou G, Sivridis E, Koukourakis MI,
et al: Light-chain 3A autophagic activity and prognostic
significance in non-small cell lung carcinomas. Chest. 140:127–134.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Sivridis E, Giatromanolaki A, Liberis V
and Koukourakis MI: Autophagy in endometrial carcinomas and
prognostic relevance of ‘stone-like’ structures (SLS): what is
destined for the atypical endometrial hyperplasia? Autophagy.
7:74–82. 2011.PubMed/NCBI
|
|
17
|
Giatromanolaki A, Koukourakis MI, Harris
AL, Polychronidis A, Gatter KC and Sivridis E: Prognostic relevance
of light chain 3 (LC3A) autophagy patterns in colorectal
adenocarcinomas. J Clin Pathol. 63:867–872. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Sivridis E, Giatromanolaki A, Karpathiou
G, Karpouzis A, Kouskoukis C and Koukourakis MI: LC3A-positive
‘stone-like’ structures in cutaneous squamous cell carcinomas. Am J
Dermatopathol. 33:285–290. 2011.
|
|
19
|
Hofseth LJ, Hussain SP and Harris CC: p53:
25 years after its discovery. Trends Pharmacol Sci. 25:177–181.
2004.
|
|
20
|
Sobin LH and Wittekind CH; International
Union against Cancer. TNM Classification of Malignant Tumors. 6th
ed. Wiley-Liss; New York: pp. 52–56. 2002
|
|
21
|
Ishida M, Morita M, Saeki H, et al:
Expression of p53 and p21 and the clinical response for
hyperthermochemoradiotherapy in patients with squamous cell
carcinoma of the esophagus. Anticancer Res. 27:3501–3506.
2007.PubMed/NCBI
|
|
22
|
Olivier M, Eeles R, Hollstein M, Khan MA,
Harris CC and Hainaut P: The IARC TP53 database: new online
mutation analysis and recommendations to users. Hum Mutat.
19:607–614. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Han U, Can OI, Han S, Kayhan B and Onal
BU: Expressions of p53, VEGF C, p21: could they be used in
preoperative evaluation of lymph node metastasis of esophageal
squamous cell carcinoma? Dis Esophagus. 20:379–385. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Heeren PA, Kloppenberg FW, Hollema H,
Mulder NH, Nap RE and Plukker JT: Predictive effect of p53 and p21
alteration on chemotherapy response and survival in locally
advanced adenocarcinoma of the esophagus. Anticancer Res.
24:2579–2583. 2004.PubMed/NCBI
|
|
25
|
Taghavi N, Biramijamal F, Sotoudeh M, et
al: Association of p53/p21 expression with cigarette smoking and
prognosis in esophageal squamous cell carcinoma patients. World J
Gastroenterol. 16:4958–4967. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Ressiot E, Dahan L, Liprandi A, et al:
Predictive factors of the response to chemoradiotherapy in
esophageal cancer. Gastroenterol Clin Biol. 32:567–577. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Fujii S, Mitsunaga S, Yamazaki M, et al:
Autophagy is activated in pancreatic cancer cells and correlates
with poor patient outcome. Cancer Sci. 99:1813–1819.
2008.PubMed/NCBI
|
|
28
|
Sivridis E, Koukourakis MI, Mendrinos SE,
et al: Beclin-1 and LC3A expression in cutaneous malignant
melanomas: a biphasic survival pattern for beclin-1. Melanoma Res.
21:188–195. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Lee IH, Kawai Y, Fergusson MM, et al: Atg7
modulates p53 activity to regulate cell cycle and survival during
metabolic stress. Science. 336:225–228. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Budanov AV and Karin M: p53 target genes
sestrin1 and sestrin2 connect genotoxic stress and mTOR signaling.
Cell. 134:451–460. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Xia HG, Zhang L, Chen G, et al: Control of
basal autophagy by calpain1 mediated cleavage of ATG5. Autophagy.
6:61–66. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Moretti L, Cha YI, Niermann KJ and Lu B:
Switch between apoptosis and autophagy: radiation-induced
endoplasmic reticulum stress? Cell Cycle. 6:793–798. 2007.
View Article : Google Scholar : PubMed/NCBI
|