Introduction
Leishmaniasis is an infectious disease caused by
protozoa of the genus Leishmania, transmitted by various
species of sandflies. The parasite affects the skin, mucous
membranes and viscera of vertebrate and invertebrate hosts as part
of its life cycle (1,2).
Leishmaniasis may present with varying clinical
forms, depending on the Leishmania species involved and the
relationship between the parasite and its host. Visceral
leishmaniasis (VL) is a serious chronic disease whose fatality rate
may reach 10% when no proper treatment is instituted (3). Cutaneous leishmaniasis (CL) is
characterized by lesions which develop at the site of the bite of
the vector (4).
According to the Pan American Health Organization
(5), it is estimated that 2
million new cases of leishmaniasis occur each year worldwide, of
which 1.5 million are cases of cutaneous leishmaniasis. It is
estimated that >12 million individuals are infected with
leismaniasis, which is considered one of the five most infectious
and parasitic diseases of major relevance worldwide (6).
American cutaneous leishmaniasis (ACL) is a public
health issue in Brazil and other countries of the New World. In the
state of Paraná, where >98% of the cases in Southern Brazil
occur, ACL has been observed since the beginning of the twentieth
century and notified since the 1980’s. In the identification of the
circuits of the disease, the North and West of Paraná were
considered areas of epidemiological importance (7) and, according to the Ministry of
Health, >11,500 new cases were reported between 1990 and 2010
(8). The cutaneous lesions of ACL
are painless, singular or multiple areas, which develop in regions
exposed to the bite of the vector, including the ears, face, upper
and lower limbs. The mucosal lesions affect the nose, mouth, throat
and laryngeal cartilaginous tissue leading to destruction and
disfigurement of the face (4).
Due to the wide spectrum of clinical and
immunopathological manifestations, ACL has been examined in order
to understand the mechanisms of host defense, which play a crucial
role in the pathogenesis of the disease. It has been
well-established that a protective immune response against
Leishmania is dependent on the efficient development of a
Th1 response, but on its own, the presentation of an antigen to a
class II MHC is not sufficient to stimulate a T-cell response
(9).
Chemokines play a significant role in the
recruitment of host cells to the site of infection. The various
chemokines act on different cells, thus controlling the nature of
the inflammatory infiltrate. The chemokine CCL4, for example, acts
on the CCR5 receptor, which plays a significant role in the
recruitment of macrophages, monocytes and T cells (10–13).
For this reason, chemokines are considered promising targets for
the treatment of inflammatory, allergic, infectious and autoimmune
diseases (14,15).
CCR5 is a member of the family of transmembrane
receptors coupled to the G protein (16). One mutation of this receptor gene
consists of a deletion of 32 base pairs, designated as Δ32
(17), which results in a
non-functional receptor, thus preventing its interaction with
chemokine ligands (18,19). Since the CCR5 molecule is typically
expressed in cells that direct the immune response to Th1, which is
associated with inflammation, the CCR5/Δ32 non-functional allele
results in a less effective Th1, consequently leading to a milder
inflammatory state (20).
Findings of a previous study showed that the
presence of the deletion allele is associated with a resistance to
HIV infection and that it confers protection against asthma,
rheumatoid arthritis, multiple sclerosis and renal graft rejection
(21). Renal transplant recipients
who are homozygous for a deletion have a significantly higher
survival rate than recipients heterozygous or homozygous for the
wild-type allele (22).
The CCR5 receptor is involved in directing the
immune response towards a Th1, thus affecting the development of
infectious diseases. As the various clinical forms of ACL depend on
the host immune response, the CCR5 receptor may play a role in the
development of the various forms of the disease. Thus, the aim of
the present study was to investigate the effect of the CCR5
chemokine receptor in the development of ACL, in a population from
the endemic areas in the north and northwest of the state of
Paraná.
Materials and methods
Study design
A retrospective study was carried out on the
epidemiological records of patients attending the Laboratory of
Teaching and Research in Clinical Analysis of the State University
of Maringá for laboratory diagnosis of ACL between 2000 and 2008.
The diagnosis consisted of a search for parasites in the lesion and
also a Montenegro skin test. An aliquot of 20 ml peripheral blood
was collected from all the patients and controls.
Control group
The control group comprised 218 healthy individuals.
The inclusion criterion was being exposed to the same environmental
risks as the ACL patients, i.e., those who had the habit of
frequenting rivers in the endemic areas of the region but did not
develop ACL. Blood relatives were excluded to reduce the likelihood
of obtaining biased data.
Patients with ACL
The study included 111 patients who had been
diagnosed positive for ACL and whose lesions did not return in the
first 2 years subsequent to treatment. Blood relatives were not
included in the study.
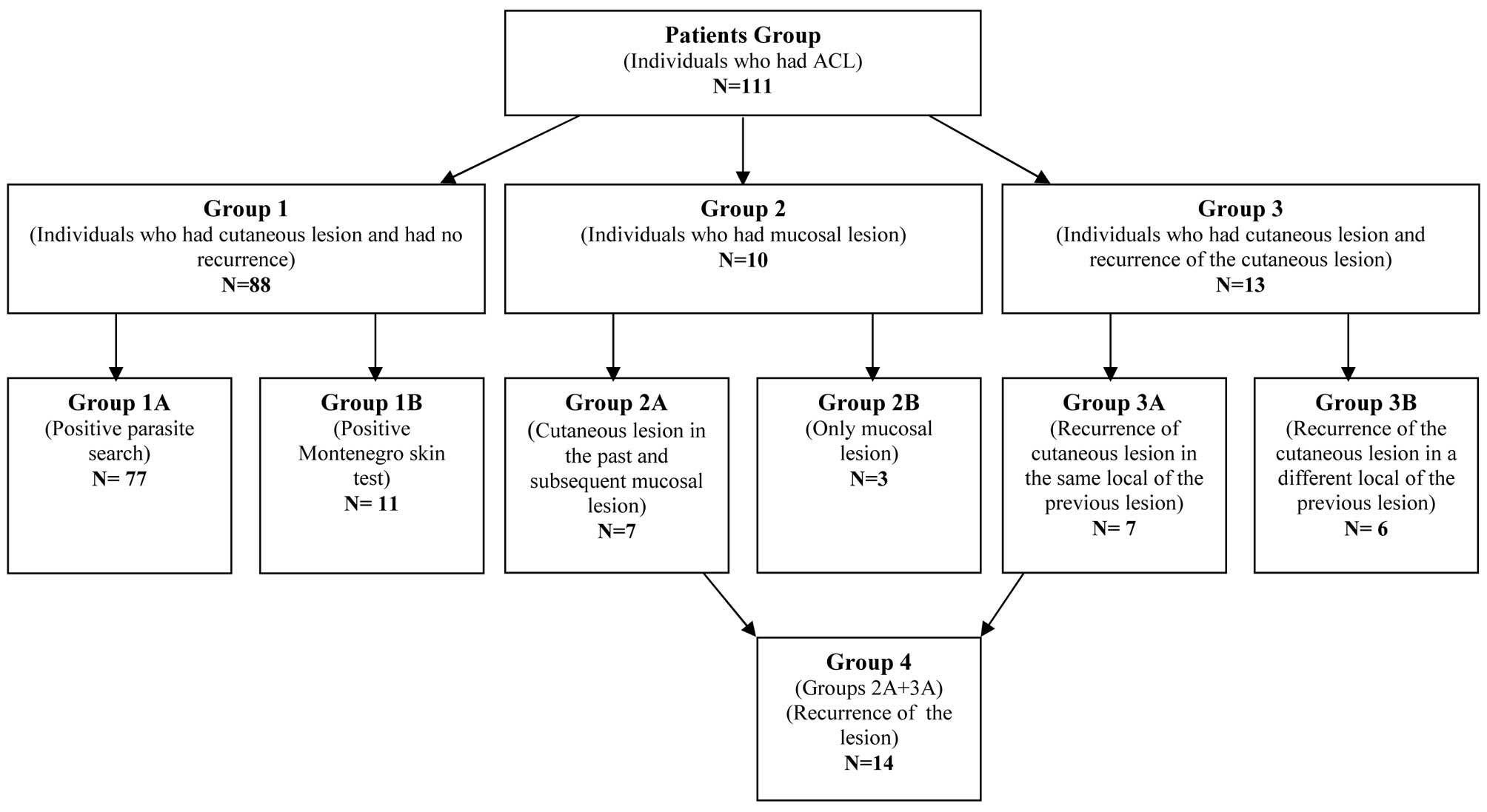
Composition of patient groups
The ACL patient group was divided into groups
according to the clinical form of ACL, the results of laboratory
tests and the clinical history (Fig.
1).
Group 1 comprised individuals who had presented with
cutaneous lesions and showed no signs of a recurrence of the
disease. This group was subdivided into group 1A consisting of 77
individuals who had received a positive parasitological diagnosis
and group 1B consisting of 11 individuals who only had a positive
Montenegro skin test.
Group 2 included individuals who had mucosal
lesions. This group was subdivided into group 2A composed of seven
individuals with mucosal lesions who had previously had cutaneous
lesions with a positive laboratory diagnosis (parasitological or
the Montenegro skin test) and group 2B composed of three
individuals who had mucosal lesions, without a previous history of
cutaneous lesions.
Group 3 consisted of individuals who had cutaneous
lesions which returned subsequent to treatment. This group was
subdivided into group 3A, comprising seven individuals who had
positive laboratory diagnoses (parasitological or the Montenegro
skin test) and whose lesions had returned in the same location, as
well as group 3B, comprising six individuals who had positive
laboratory diagnoses (parasitological or the Montenegro skin test)
and whose lesions had returned in a different location.
Group 4 consisted of all the individuals with a
recurrence of the lesion (groups 2A and 3A). Recurrence was
regarded as the appearance of a new lesion in the same location as
the previous cutaneous lesion (group 3A) or in the mucosa (group
2A) subsequent to 2 years of treatment. This group comprised 14
individuals.
DNA extraction
The peripheral blood samples were placed in tubes
containing EDTA anticoagulant at a concentration of 1.8 mg/ml and
centrifuged at 960 g for 10 min. The nucleated cells were separated
(buffy-coat) and frozen at −80°C until use. Genomic DNA was
extracted from the frozen cells (100 μl) using the PureLink™
Polymerase Chain Reaction (PCR) Purification kit (Invitrogen,
Carlsbad, CA, USA) following the manufacturer’s instructions.
Quantification of DNA
The genomic DNA was quantified using the Quant-iT™
dsDNA BR Assay kit in a Qubit™ fluorometer (Invitrogen, Eugene, OR,
USA).
Optimization of PCR for CCR5
The method of genotyping was optimized in the
Laboratory of Immunogenetics of the State University of Maringa
using specific primers for CCR5 to amplify the genomic DNA. Primers
CCR5.1 (sense primer, 5′ CAA AAA ACC TCT GAA AGA 3′) and CCR5.2
(antisense primer, 5′ CAT GGT GAT GAA GAT AAG CCT CA 3′) were
designed according to the GenBank sequence AF009962 (23).
The sample from each individual was amplified by PCR
with sequence specific primers (PCR-SSP). Amplification was carried
out at 94°C for 5 min, then 35 cycles at 94°C for 1 min, 58°C for 1
min, 72°C for 1 min and 72°C for 10 min in a thermocycler GeneAmp™
PCR System 9700 (Applied Biosystems, Foster City, CA, USA) and
stored at −18°C until the analysis.
All DNA amplification reactions were performed in
parallel with negative controls to detect the possibility of
contamination. The 225 and 193 base pair products were analyzed by
electrophoresis on a 2.5% agarose gel, together with a standard
molecular ladder of 50 bp (Invitrogen) and visualized using UV
fluorescence subsequent to being stained with SYBR™ Safe
(Invitrogen).
Statistical analysis
To organize the CCR5/CCR5, CCR5/Δ32 and Δ32/Δ32
genotypes, a database was created in Excel 2007 and analysis was
performed using the Statistical 7.0 program. The gender or ethnic
group variables were analyzed by a Fisher’s exact test and the age
variable was analyzed by a Student’s t-test for independent
samples. The odds ratio (OR) was calculated with a confidence
interval (CI) of 95% for P<0.05. P<0.05 was considered to
indicate a statistically significant difference.
Ethical aspects
The present study was submitted to the Human
Research Ethics Committee of the State University of Maringa and
approved according to report No. 153/2009. All individuals were
informed of the purpose of the study. Those who agreed to
participate provided written informed consent, with full liberty to
withdraw from participation at any time without any prejudice, as
stated in resolution 196/96-CNS.
Results
The variables of age, gender and ethnicity were
analyzed (Table I) in the patients
with ACL (patient group) and the healthy subjects (control group).
The chronological age of the patient group was statistically
different from the control group (P<0.00001). The patients were
47 years old on average and the controls were 31 years old on
average. With regard to gender, the two groups did not differ
statistically (P=0.0696), but the majority of the subjects studied,
78% of the patients and 68% of the controls, were male. There was
no statistical difference in ethnicity between the patient and
control groups (P=0.2944). The populations examined showed no
significant deviation from the Hardy-Weinberg equilibrium.
 | Table IDistribution of the control and
patient groups according to age, gender and ethnicity. |
Table I
Distribution of the control and
patient groups according to age, gender and ethnicity.
| | Genderb | Ethnic groupc |
|---|
| |
|
|
|---|
| Group | Average agea (years) | Female n (%) | Male n (%) | White n (%) | Mulatto n (%) | Oriental n (%) | Black n (%) | Indian n (%) | Total (n) |
|---|
| Control | 31.08 | 69 (31.65) | 149 (68.35) | 171 (78.44) | 32 (14.75) | 6 (2.75) | 8 (3.67) | 1 (0.46) | 218 |
| Patient | 47.85 | 24 (21.62) | 87 (78.38) | 84 (75.68) | 24 (21.62) | 2 (1.80) | 1 (0.90) | 0 (0.00) | 111 |
The CCR5/Δ32 genotype was observed in 10.81%
(12/111) of the subjects in the patient group, but in only 7.34%
(16/218) of the control group. The Δ32/Δ32 deletion was not
observed in the studied population. The frequency of genotypes
CCR5/CCR5 and CCR5/Δ32 did not differ between the patient and
control groups (P=0.3009; Table
II).
 | Table IIDistribution of genotypes CCR5/CCR5
and CCR5/Δ32 in the groups studied. |
Table II
Distribution of genotypes CCR5/CCR5
and CCR5/Δ32 in the groups studied.
| Frequency | |
|---|
|
| |
|---|
| Genotypes | Patients | Control | P-value |
|---|
| CCR5/CCR5 | 99 | 202 | |
| CCR5/Δ32 | 12 | 16 | 0.3009 |
The comparison of the frequency of the genotypes
between the ACL patients who showed no recurrence of the lesion
subsequent to treatment (group 1A) and the other groups revealed no
difference in the genotype distribution (Table III).
 | Table IIIAbsolute distribution and percentage
of the homozygous (CCR5/CCR5) and mutated (CCR5/Δ32) gentotypes in
the patient groups and subgroups (2B, 4, 3A, 2 and 3) compared with
subgroup 1A. |
Table III
Absolute distribution and percentage
of the homozygous (CCR5/CCR5) and mutated (CCR5/Δ32) gentotypes in
the patient groups and subgroups (2B, 4, 3A, 2 and 3) compared with
subgroup 1A.
| Group | CCR5/CCR5
n (%) | CCR5/Δ32
n (%) | Total
n | P-value |
|---|
| 1A | 70 (91) | 7 (9) | 77 | |
| 2B | 2 (67) | 1 (33) | 3 | 0.2741 |
| 4 | 14 (100) | 0 (0) | 14 | 0.5899 |
| 3A | 7 (100) | 0 (0) | 7 | 1.0000 |
| 2 | 9 (90) | 1 (10) | 10 | 1.0000 |
| 3 | 11 (85) | 2 (15) | 13 | 0.6127 |
The analysis of the genotypes of the control group
versus the patient groups and subgroups showed that only the group
of individuals who had cutaneous lesions with a positive laboratory
diagnosis and whose lesions returned in a different location (Group
3B) presented with a different distribution than the control group.
This meant that the CCR5/Δ32 mutation was more frequent in this
group (P=0.020; Table IV).
 | Table IVAbsolute distribution and percentage
of the homozygous (CCR5/CCR5) and mutated (CCR5/Δ32) genotypes in
the patient groups and subgroups compared with the control
group. |
Table IV
Absolute distribution and percentage
of the homozygous (CCR5/CCR5) and mutated (CCR5/Δ32) genotypes in
the patient groups and subgroups compared with the control
group.
| Group | CCR5/CCR5
n | CCR5/Δ32
n | Total
n | P-value |
|---|
| Control | 202 (92%) | 16 (8%) | 218 | |
| 1 | 79 (89%) | 9 (11%) | 88 | 0.4890 |
| 2 | 9 (90%) | 1 (10%) | 10 | 0.5467 |
| 3 | 11 (84%) | 2 (16%) | 13 | 0.2682 |
| 4 | 14 (100%) | 0 (0%) | 14 | 0.6063 |
| 1A | 70 (91%) | 7 (9%) | 77 | 0.6249 |
| 1B | 9 (64%) | 2 (36%) | 11 | 0.2102 |
| 2A | 7 (100%) | 0 (0%) | 7 | 1.0000 |
| 2B | 2 (67%) | 1 (33%) | 3 | 0.2144 |
| 3A | 7 (100%) | 0 (0%) | 7 | 1.0000 |
| 3B | 4 (67%) | 2 (33%) | 6 | 0.0020 |
| OR=0.1584 | OR=0.3125 | | |
| (0.03–0.93) | (1.07–37.14) | | |
Discussion
In the last 20 years the incidence of ACL has
increased in all the states of Brazil (3). This increase was primarily
attributable to behavioral and environmental changes, including
invasions in primary forests, deforestation, massive migration from
rural to urban areas and rapid and unplanned urbanization (3). Therefore, due to the increase in the
number of cases and the wide spectrum of clinical and
immunopathological manifestations of ACL, the aim of the present
study was to evaluate the potential effect of the chemokine
receptor CCR5 in the varying forms of ACL.
In the present study, the patients were older than
the controls. The ACL patients were 47 years old on average while
the controls were 31 years old on average. The age range of the
patients was as expected as the ACL occurs more frequently in older
patients (24).
It was observed that the majority of the patients
were male. These results were expected as males more commonly
conduct leisure activities, including hunting and fishing, that
involve the risk of contracting ACL (24). Thus, the control group comprised a
higher proportion of males to the remaining paired data.
CCR5 studies have demonstrated the importance of the
Δ32 mutation, particularly in the susceptibility to HIV infection,
since CCR5 is a co-receptor in the primary stage of infection that
is essential for the onset of the disease (25). Therefore, it was expected that the
populations most affected by the virus would have a higher degree
of gene deletion, however, mutations of this gene may have occurred
in fatal generalized epidemics that occurred in Europe and not due
to the HIV virus. Prior to HIV, the bubonic plague would have
affected the mutation. A recent study has demonstrated that
smallpox may also have affected the mutation since the smallpox
virus uses the CCR5 and CXCR4 as receptors to enter cells (26). Other studies have suggested that
the CCR5/Δ32 mutation may prevent the development of cerebral
malaria, slow multiple sclerosis (27) and prolong graft survival in kidney
and heart transplants (28), but
controversy remains with regard to liver transplants (29).
In the present study, which analyzed a population of
the north and northwest of the Paraná state, a frequency of ~10%
was identified for the CCR5/Δ32 genotype, corroborating the results
of other investigators who had studied the frequency of this
genotype in the northern population of the state (30,31).
A frequency of 7% was observed in an urban population of CCR5/Δ32
individuals (32). Another study
based on four tribes in the Brazilian Amazon observed that the
population was 100% homozygous for the normal allele, which
supports the hypothesis that the Δ32 allele has a European origin
and that its occurrence in the populations of South America is a
result of immigration (33). The
highest frequency of the Δ32 allele (20.93%) was recorded in Jewish
populations (with Israeli ancestry) and in Eastern Europe, which
are both known to be highly inbred (34). Among African-Americans this
frequency is <1% and among Caucasians in Asia (Pakistan and
India), there is also a very low frequency of this mutation. This
allele has not been reported in China, Japan or native African
populations (34).
There were no individuals with the homozygous
recessive genotype in the present study. The frequency of the
Δ32/Δ32 genotype is ~1% in the population of Eastern Europe
(17). Thus, the chance of
identifying a homozygous deleted individual is extremely small and
consistent with the genotypes observed in this population.
There was no difference in the frequency of the
CCR5/CCR5 and CCR5/Δ32 genotypes between the patient control groups
in the present study, showing that these alleles had no effect on
the development of ACL. These results corroborated the results of
another study which also identified no differences in the frequency
of these alleles among its study groups (30).
The analysis of the frequency of the alleles in the
patient subgroups when compared with the control group showed an
association between the CCR5/Δ32 genotype and group 3B (individuals
who had a positive laboratory diagnosis and whose lesions returned
in a different location). The recurrence of cutaneous lesions may
be due to an immune deficiency of the host or infection by
parasitic subspecies capable of inducing immunosuppression in the
host (35). The present study data
suggested that the CCR5/Δ32 genotype may be associated with the
reappearance of the cutaneous lesions in ACL, although the number
of patients in this group was small.
There was also no difference in the allele frequency
between the subgroup of the individuals who presented with
cutaneous lesions and had a positive parasitological diagnosis with
no signs of recurrence of the disease within a period of at least 2
years (group 1A) and the other patient subgroups. This showed that
there was no correlation between the alleles and the various groups
of ACL patients that were studied.
In the present study, the Δ32 allele was also
observed in the patients of groups 2B (individuals who had mucosal
lesions, without a previous history of cutaneous lesions) and 3B
(individuals who had a positive laboratory diagnosis and whose
lesions returned in a different location). This result was in
contrast with a study that observed that the heterozygous patients
only had cutaneous lesions (30).
According to this study, the Th1 immune response was associated
with the non-functional allele CCR5/Δ32 genotype, leading to a
milder immune response.
The present study showed no difference in the
frequency of the CCR5/Δ32 genotype between the patient and control
groups, but there was a higher frequency of this genotype in the
subgroup of patients who had a recurrence of cutaneous lesions in
different locations to the primary lesion. However, due to the
small number of patients in each subgroup, more studies are
required to elucidate the role of CCR5 in the pathogenesis of ACL,
enabling advances in therapeutic treatments and new prophylactic
strategies for the disease.
Acknowledgements
This study was supported by grants from the
Araucaria Foundation and the Laboratory of Immunogenetics,
Department of Basic Health Sciences, State University of Maringá,
Brazil.
References
|
1
|
Rey L: Leishmania and leishmaníases:
parasites. Parasitology, Editora Guanabara Koogan. 3rd edition. Rio
de Janeiro: pp. 214–226. 2001
|
|
2
|
Condino MLF, Galati EAB, Holeman MM, Salum
MRB, Silva DC and Junior RAN: American cutaneous leishmaniasis on
the northern coastline of São Paulo, 1993 to 2005. Rev Soc Bras
Trop Med. 41:635–641. 2008.(In Portuguese).
|
|
3
|
Gontijo CMF and Melo MN: Visceral
leishmaniasis in Brazil: current status, challenges and prospects.
Rev Bras Epidemiol. 7:338–349. 2004.(In Portuguese).
|
|
4
|
Gontijo B and de Carvalho Mde L: American
cutaneous leishmaniasis. Rev Soc Bras Med Trop. 36:71–80. 2003.(In
Portuguese).
|
|
5
|
Pan American Health Organization.
Leishmaniasis: 2007 update. http://www.paho.org/English/AD/DPC/CD/leish-2007.pdf.
Accessed Feb 09, 2012
|
|
6
|
Guerra JAO, Barbosa MGV, Loureiro ACSP,
Coelho CP, Rosa GG and Coelho LIACR: American cutaneous
leishmaniasis in children: epidemiological aspects of cases treated
in Manaus, Amazonas, Brazil. Rep Public Health. 23:2215–2223.
2007.(In Portuguese).
|
|
7
|
Lima AP, Minelli L, Teodoro U and
Comunello E: Distribution of cutaneous leishmaniasis by orbital
remote sensing images, in Paraná State, Brazil. An Bras Dermatol.
77:681–692. 2002.
|
|
8
|
Brasil: Ministério da Saúde: Vigilância
Epidemiológica. 2012, http://portal.saude.gov.br/portal/saude/profissional/area.cfm?id_area=1560.
Accessed Feb 09, 2012
|
|
9
|
Alexander J and Bryson K: T helper
(h)1/Th2 and Leishmania: paradox rather than paradigm. Immunol
Letters. 99:17–23. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Abbas AK: Cytokines. Cellular and
Molecular Immunology. Abbas AK, Lichtman AH and Pillai S: 6th
edition. Elsevier; Rio de Janeiro: pp. 267–302. 2008
|
|
11
|
Murphy PM: The molecular biology of
leukocyte chemoattractant receptors. Annu Rev Immunol. 12:593–633.
1994. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Baggiolini M: Chemokines and leukocyte
traffic. Nature. 392:565–568. 1998. View
Article : Google Scholar : PubMed/NCBI
|
|
13
|
Proost P, Wuyts A and van Damme J: The
role of chemokines in inflammation. Int J Clin Lab Res. 26:211–223.
1996. View Article : Google Scholar
|
|
14
|
Pereira AB, Rezende NA, Teixeira AL Jr, et
al: Cytokines and chemokines in renal transplantation. J Bras
Nefrol. 31:286–296. 2009.(In Portuguese).
|
|
15
|
Spagnolo P, Renzoni EA, Wells AU, et al:
C-C chemokine receptor 5 gene variants in relation to lung disease
in sarcoidosis. Am J Respir Crit Care Med. 172:721–728. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Guignard F, Combadiere C, Tiffany HL and
Murphy PM: Gene organization and promoter function for CC chemokine
receptor 5 (CCR5). J Immunol. 160:985–992. 1998.PubMed/NCBI
|
|
17
|
Liu R, Paxton WA, Choe S, et al:
Homozygous defect in HIV-1 coreceptor accounts for resistence of
some multiply-exposed individuals to HIV-1 infection. Cell.
86:367–377. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Yang X, Ahmad T, Gogus F, et al: Analysis
of the CC chemokine receptor 5 (CCR5) Delta32 polymorphism in
Behçet’s disease. Eur J Immunogenet. 31:11–14. 2004.
|
|
19
|
Sidoti A, D’Angelo R, Rinaldi C, et al:
Distribution of the mutated delta32 allele of the CCR5 gene in a
Sicilian population. Int J Immunogenet. 32:193–198. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Chies JA and Hutz MH: High frequency of
the CCR5delta32 variant among individuals from an admixed Brazilian
population with sickle cell anemia. Braz J Med Biol Res. 36:71–75.
2003. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Zúñiga JA, Villarreal-Garza C, Flores E,
et al: Biological relevance of the polymorphism in the CCR5 gene in
refractory and non-refractory rheumatoid arthritis in Mexicans.
Clin Exp Rheumatol. 21:351–354. 2003.PubMed/NCBI
|
|
22
|
Fildes JE, Walker AH, Howlett R, et al:
Donor CCR5 Delta32 polymorphism and outcome following cardiac
transplantation. Transplant Proc. 37:2247–2249. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
GenBank. http://www.ncbi.nlm.nih.gov/nuccore/AF009962.
Accessed Apr 05, 2010
|
|
24
|
Silveira TG, Arraes SM, Bertolini DA,
Teodoro U, et al: The laboratory diagnosis of epidemiology of
cutaneous leishmaniasis in Paraná State, southern Brazil. Rev Soc
Bras Med Trop. 32:413–423. 1999.(In Portuguese).
|
|
25
|
Galvani AP and Novembre J: The
evolutionary history of the CCR5-Delta32 HIV-resistance mutation.
Microbes Infect. 7:302–309. 2005. View Article : Google Scholar
|
|
26
|
Stanford Medicine Schoool. http://www.thetech.org/genetics/news.php?id=13.
Accessed Feb 12, 2012
|
|
27
|
Lamb A: CCR5-delta32: a very beneficial
mutation. J Creation. 20:152006.
|
|
28
|
Pereira AB, Rao NA, Teixeira AL Jr,
Teixeira MM and Silva ACS: Cytokines and chemokines in renal
transplantation. J Bras Nefrol. 31:286–296. 2009.(In
Portuguese).
|
|
29
|
Li H, Xie HY, Zhou L and Zheng SS: Lack of
association of the polymorphism of the CCR5 gene in liver
recipients with acute rejection from China. Exp Clin Transplant.
9:252–257. 2011.PubMed/NCBI
|
|
30
|
Oliveira KB, Reiche EMV, Morimoto HK, et
al: Analysis of the CC chemokine receptor 5 delta32 polymorphism in
a Brazilian population with cutaneous leishmaniasis. J Cutan
Pathol. 34:27–32. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Muxel SM, Borelli SD, Amarante MK, et al:
Association study of CCR5 delta 32 polymorphism among the HLA-DRB1
Caucasian population in Northern Paraná, Brazil. J Clin Lab Anal.
22:229–233. 2008.PubMed/NCBI
|
|
32
|
Passos GA Jr and Picanço VP: Frequency of
the delta CCR5 deletion allele in the urban Brazilian population.
Immunol Lett. 61:205–207. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Leboute AP, de Carvalho MW and Simões AL:
Absence of the deltaCCR5 mutation in indigenous populations of the
Brazilian Amazon. Hum Genet. 105:442–443. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Martinson JJ, Chapman NH, Rees DC, Liu YT
and Clegg JB: Global distribution of the CCR5 gene 32-basepair
deletion. Nature Genet. 16:100–103. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Brasil. Ministério da Saúde. Manual de
vigilância da leishmaniose tegumentar Americana. Brasília, DF: pp.
1812007
|