Introduction
Hepatocellular carcinoma (HCC) is a primary
malignancy of the liver. It is the fifth most common type of cancer
worldwide and a leading cause of cancer mortality, particularly in
countries with a high prevalence of chronic hepatitis B virus (HBV)
and HCV (1). Due to a poor
therapeutic response to chemotherapy and radiotherapy, liver
resection and transplantation are currently the main curative
therapies for HCC. However, the long-term prognosis of the majority
of patients with HCC remains extremely poor due to early recurrence
and metastasis (2,3). The majority of recurrences are
associated with tumor cell invasion and intrahepatic metastasis
(4). Understanding the molecular
mechanisms associated with HCC invasion and metastasis and
identifying potential biological markers of these processes are
critical for the development of new therapeutic strategies.
Focal adhesion kinase (FAK), a cytoplasmic
non-receptor tyrosine kinase, plays a central role in a number of
cellular events, including cell proliferation, survival, migration
and invasion (5). FAK is
overexpressed and activated in a variety of human tumors, including
HCC (6–9). Elevated FAK expression levels and
activity are frequently associated with tumor metastasis and poor
HCC patient prognosis (10).
Krüppel-like factor 8 (KLF8) is a GT-box (CACCC)-binding
dual-transcription factor that contains the C2H2 zinc-finger motif
and is critical for the regulation of cell cycle progression, cell
differentiation and oncogenic transformation (11–13).
Previous studies have demonstrated that KLF8 is markedly
overexpressed in several types of human cancer, including breast
cancer (11), renal carcinoma
(12), gastric cancer (14) and HCC (15). Zhao et al(16) previously identified that KLF8 is a
downstream target of FAK and the expression levels of KLF8 are
specifically regulated by FAK-Src-phosphatidylinositol 3 kinase
(PI3K) signaling in human ovarian cancer cells (17). KLF8 directly binds and represses
the E-cadherin promoter, markedly inducing
epithelial-to-mesenchymal transition (EMT), and enhances motility
and invasiveness in breast cancer cells. Blocking KLF8 expression
by RNA interference restores E-cadherin expression in the cancer
cells and markedly inhibits cell invasion (11). KLF8 overexpression has also been
found to induce an increase in MMP-9 expression and activity. KLF8
directly binds and activates the human MMP-9 gene promoter
(18). These observations indicate
that the FAK-KLF8-MMP-9/E-cadherin signaling axis is important for
cancer progression, enabling tumor cells to gain EMT phenotypes and
increase MMP-9 levels, leading to increased invasion and
metastasis.
KLF8 has also been reported to play an oncogenic
role in HCC. Li et al(15)
found that KLF8 promotes HCC cell proliferation and invasion,
inhibits apoptosis and induces EMT. However, the molecular
mechanisms by which KLF8 enhances HCC cell invasion and metastasis
remain poorly understood. We previously hypothesized that the
FAK-KLF8-MMP-9/E-cadherin signaling axis plays a vital role in HCC
progression. In the present study, KLF8 expression and its
clinicopathological significance in HCC was investigated using
quantitative real-time reverse transcription polymerase chain
reaction (qRT-PCR) and immunohistochemistry. In addition, the
correlation between KLF8 and FAK, MMP-9 and E-cadherin expression
was determined in HCC.
Materials and methods
Patients and specimens
Sixty fresh tumor samples for qRT-PCR and
immunohistochemistry were randomly collected from HCC patients who
underwent curative resection between 2008 and 2010 at the First
Affiliated Hospital of Medical College, Xi’an Jiaotong University
(Xi’an, China). For western blot analysis, 10 invasive tumor
tissues were selected from the 60 cases. Resected tumors and
corresponding non-tumor tissues specimens (>2 cm away from the
tumor) were immediately cut from the resected liver and subdivided
into 2 portions. One portion was rapidly frozen in liquid nitrogen
and stored at −80°C until use and the other was fixed in buffered
paraformaldehyde for immunohistochemical staining. None of the
patients had received radiotherapy or chemotherapy prior to
sampling. Clinical data were obtained from medical records.
Histopathological Edmonson classification, clinical
tumor-node-metastasis (TNM) grading, maximum tumor diameter and
normal tumor-adjacent tissues were all confirmed by an experienced
pathologist who was blinded to the clinical information. Written
informed consent was obtained from all patients. The Xi’an Jiaotong
University Ethics Committee approved all protocols according to the
Helsinki Declaration of 1975.
Immunohistochemical staining
Immunohistochemical staining was performed on
paraformaldehyde-fixed paraffin sections (4 μm), as described
previously (19). In brief,
sections were dewaxed and rehydrated, and antigens were retrieved
in citrate buffer. Endogenous peroxidase activity was blocked for
10 min using 3.0% hydrogen peroxide. Goat serum (10%) was applied
to the sections to prevent non-specific binding. The sections were
then separately incubated with the following primary antibodies at
4°C overnight: rabbit anti-FAK (1:200; Cell Signaling Technology,
Inc., Danvers, MA, USA), rabbit anti-KLF8 (1:200; Aviva Systems
Biology, San Diego, CA, USA), mouse anti-E-cadherin (1:100) and
rabbit anti-MMP-9 (1:200; both Santa Cruz Biotechnology, Santa
Cruz, CA, USA). The primary antibody was detected using
biotinylated secondary antibodies (Beijing Zhongshan Golden Bridge
Biotechnology Co., Ltd., Beijing, China) according to the
manufacturer’s instructions. Sections were visualized with
diaminobenzidine, counterstained with hematoxylin, dehydrated in
alcohol and xylene and mounted onto glass slides. For negative
controls, primary antibody was replaced with PBS.
All sections were assessed independently by two
experienced pathologists. The staining results for the four
proteins (FAK, KLF8, E-cadherin and MMP-9) were semi-quantitatively
expressed by an immunohistochemical score combined with the
percentage of liver cells revealing specific immunoreactivity.
Staining intensity was expressed by four grades as follows: 0,
none; 1, weak; 2, moderate; and 3, strong. The percentage of
positive liver cells was expressed by the following grades: 0,
<10%; 1, 10–25%; 2, 26–50%; 3, 51–75%; and 4, >75%. The
staining intensity and average percentage of positive liver cells
were assayed for 5 independent high magnification fields. The total
score was calculated by multiplying the staining intensity and the
percentage score of positive liver cells. Specimen staining was
assessed as total score: negative (−), score 0; weakly positive
(+), score 1–4; positive (++), score 5–8; strongly positive (+++),
score 9–12.
Western blot analysis
Proteins were extracted from the portal vein cancer
emboli and corresponding HCC tissues of 5 patients and lysed in
RIPA buffer containing protease inhibitor cocktail and PMSF on ice.
Protein concentration was quantified using a BCA Protein Assay kit
(Pierce Biotechnology, Inc., Rockford, IL, USA) according to the
manufacturer’s instructions. Proteins suspended in loading buffer
were heated at 95°C for 5 min for denaturation. Equal amounts of
proteins (20 μg/lane) were separated by sodium dodecyl
sulfate-polyacrylamide gel electrophoresis and transferred to
polyvinylidene fluoride membranes (Millipore, Billerica, MA, USA).
Following blocking for 1 h with 5% non-fat milk and TBST washes,
the membranes were incubated overnight at 4°C with primary
antibodies against FAK (1:1,000), KLF8 (1:500), E-cadherin
(1:1,000), MMP-9 (1:1,000) and β-actin (1:2,000; Santa Cruz
Biotechnology). Subsequently, the membranes were incubated with
goat anti-mouse or anti-rabbit horseradish peroxidase-conjugated
secondary antibodies (1:10,000; Santa Cruz Biotechnology) for 1 h
at room temperature. Blots were then visualized using the Super
Signal West Pico chemiluminescent substrate kit (Millipore) and
exposed to X-ray films.
qRT-PCR
Total RNA was isolated from the frozen specimens
stored in liquid nitrogen using the RNAfast 200 purification kit
(Fastagen Biotech, Shanghai, China). A260/280 optical
density values of the RNA samples were between 1.8 and 2.1. RNA
integrity was confirmed by the presence of intact 18S and 28S bands
on 2% agarose gels. Next, total RNA was reverse transcribed at 37°C
for 15 min and incubated at 85°C for 5 sec using PrimeScript RT
reagent (Takara Biotechnology, Shiga, Japan). cDNA was used for
qRT-PCR analysis with SYBR-Green PCR master mix (Takara
Biotechnology) and the BD IQ5 cycler (BD Biosciences, Franklin
Lakes, NJ, USA) according to the manufacturer’s instructions. The
primer sequences used for the detection of FAK, KLF8, MMP-9,
E-cadherin and β-actin are summarized in Table I. PCR was performed under the
following conditions: 1 cycle at 95°C for 5 min, then 40 cycles at
95°C for 30 sec and 60°C for 30 sec. β-actin was used as an
internal control. Relative mRNA levels were calculated based on the
threshold cycle (CT) values, which were corrected based on β-actin
expression, according to the equation: 2−ΔCT (ΔCT =
CTtarget gene − Ctβ-actin).
 | Table IPrimer sequences for qRT-PCR. |
Table I
Primer sequences for qRT-PCR.
| Gene | Forward (5′-3′) | Reverse (5′-3′) | Products (bp) |
|---|
| FAK |
ACATTATTGGCCACTGTGGATGAG |
GGCCAGTTTCATCTTGTTGATGAG | 126 |
| KLF8 |
CTGGACAGTTGCTTATGGCATC |
CTGCCTAATCTACCCAATCAGTTCA | 169 |
| E-cadherin |
AGTGGGCACAGATGGTGTGA |
TAGGTGGAGTCCCAGGCGTA | 93 |
| MMP-9 |
CCTGGGCAGATTCCAAACCT |
GCAAGTCTTCCGAGTAGTTTTGGAT | 89 |
| β-actin |
TGGCACCCAGCACAATGAA |
CTAAGTCATAGTCCGCCTAGAAGCA | 186 |
Statistical analysis
Statistical analyses were performed with the SPSS
16.0 software (SPSS, Inc., Chicago, IL, USA). For continuous
variables, data are expressed as the mean ± SD. KLF8 mRNA
expression levels in the tumor and adjacent non-tumorous tissues
were compared by the paired t-test. Clinicopathological parameters
and KLF8 mRNA expression correlations were analyzed using the
Mann-Whitney U test. Clinicopathological parameters and KLF8
protein expression correlations were analyzed using the Spearman’s
rank correlation coefficient test. The correlation between KLF8
mRNA expression and FAK, MMP-9 and E-cadherin mRNA expression was
analyzed using the Pearson correlation coefficient test and the
correlation between KLF8 protein expression and FAK, MMP-9 and
E-cadherin protein expression was analyzed using Spearman’s rank
correlation coefficient test. P<0.05 was considered to indicate
a statistically significant difference.
Results
KLF8 mRNA and protein expression in HCC
and adjacent non-tumorous tissues
KLF8 mRNA expression in 60 pairs of HCC tumor and
adjacent non-tumorous tissues was detected by qRT-PCR. The
expression of KLF8 mRNA in HCC tissues was markedly upregulated
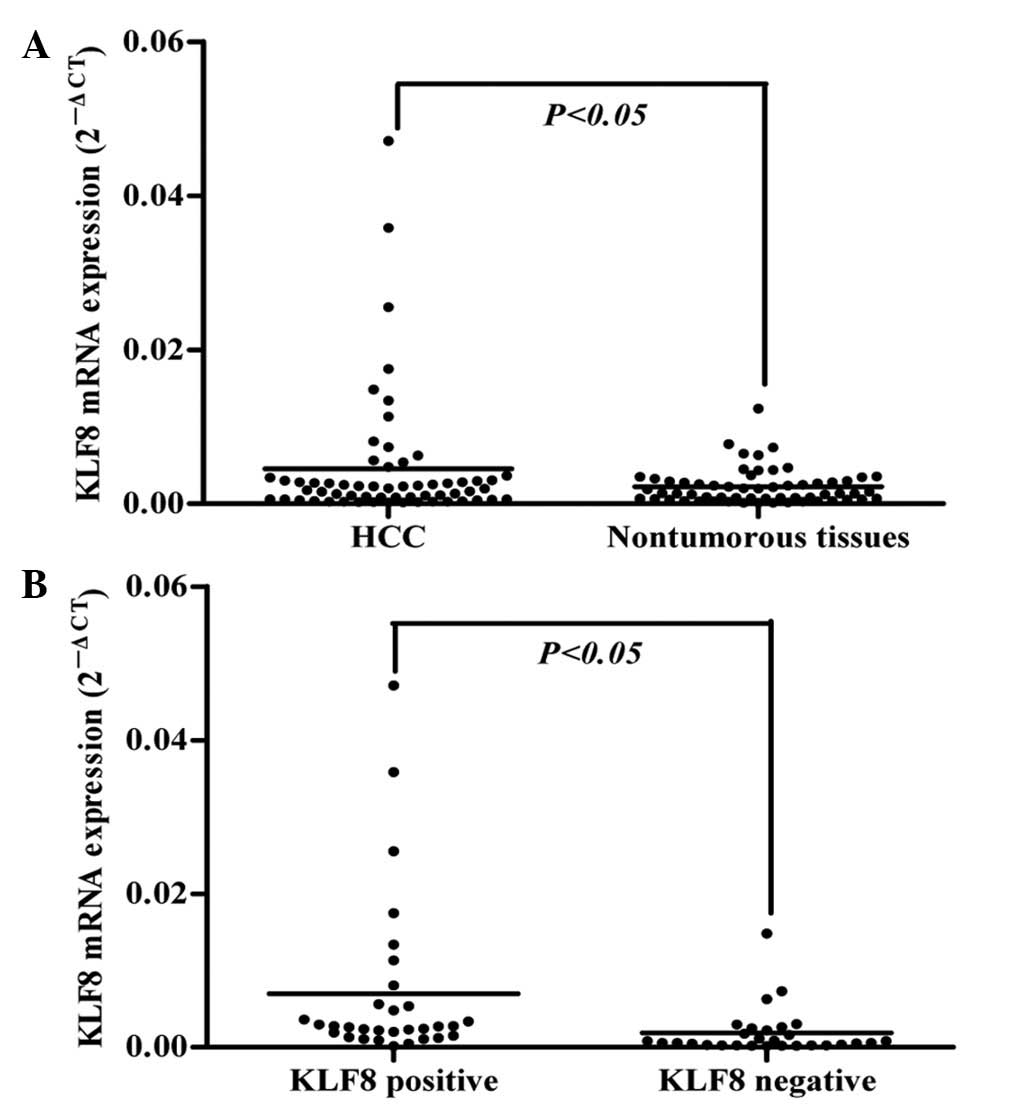
compared with adjacent non-tumorous tissues (P<0.05; Fig. 1A). Immunohistochemical staining was
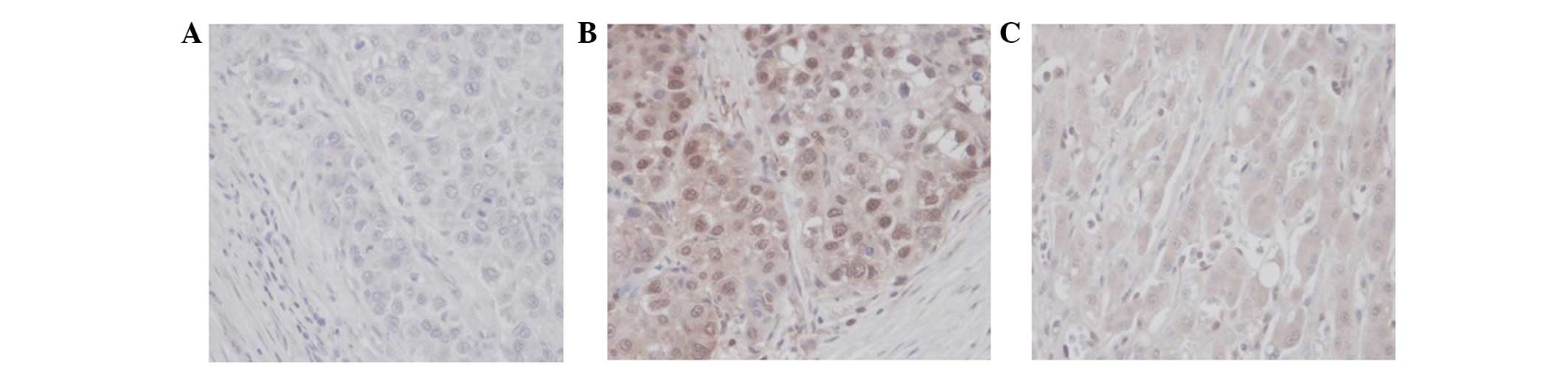
performed in the same tissues to detect KLF8 protein expression.
Specifically, 51.6% (n=31) of the HCC tumor tissues and 41.6%
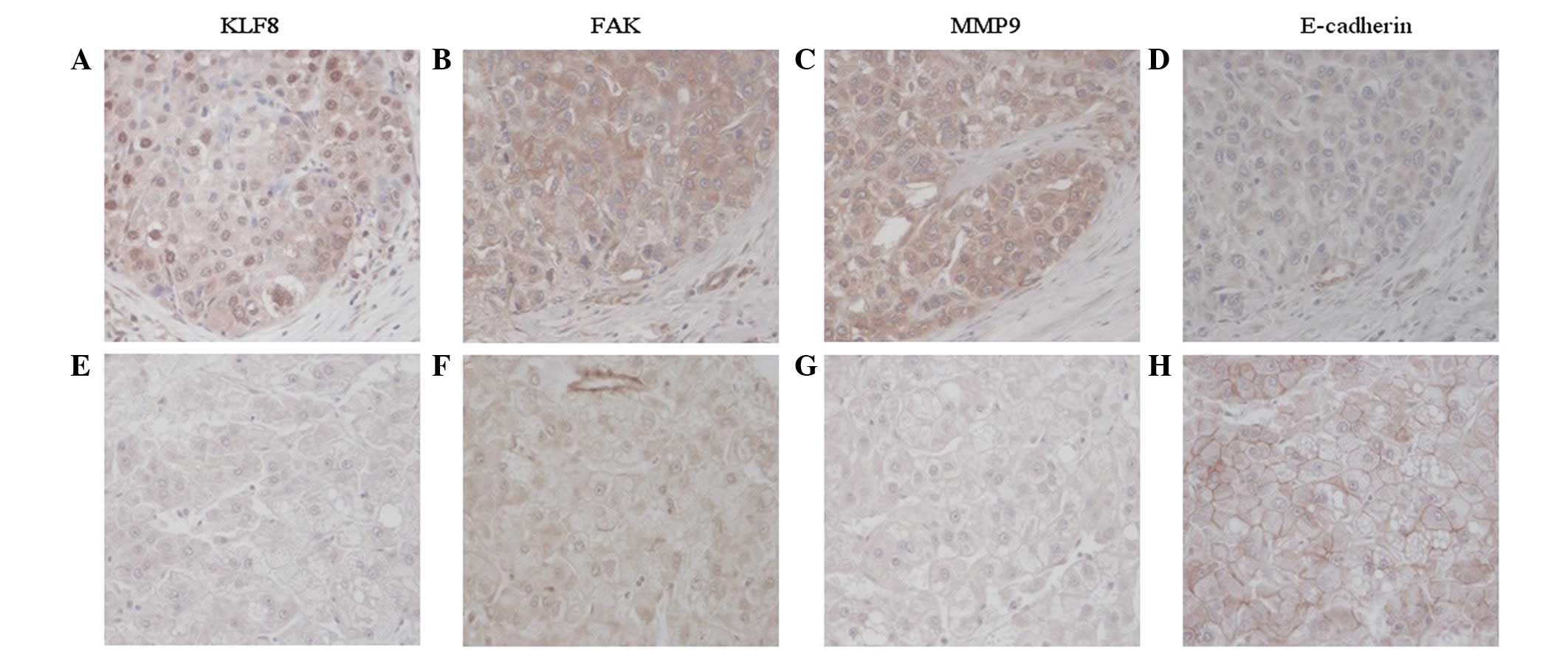
(n=25) of the adjacent non-tumorous tissues revealed KLF8-positive
staining. The majority of positive cells in HCC tumor tissues
revealed diffuse cytoplasmic staining and intense nuclear staining
of KLF8, while adjacent non-tumorous tissues mainly exhibited
diffuse cytoplasmic staining (Fig.
2). These observations indicate that KLF8 nuclear translocation
is required during HCC progression. We also investigated whether
the higher expression of KLF8 in HCC tissues was consistent at the
mRNA and protein levels. As demonstrated in Fig. 1B, by comparing the mRNA levels of
KLF8 in tumor tissues with positive staining (n=31) to those with
negative (n=29) staining, as determined by immunohistochemistry,
samples with positive protein staining were found to have
significantly higher mRNA expression than the negative group
(P<0.05), indicative of a consistency between KLF8 mRNA and
protein levels during HCC development.
Correlation between KLF8 expression in
HCC tissues and clinicopathological characteristics
To assess the clinical significance of KLF8
expression in HCC, the correlation between KLF8 expression and the
clinicopathological characteristics of the 60 HCC patients was
statistically analyzed, and the results are presented in Table II. HCC tissues with vascular
invasion exhibited higher KLF8 mRNA and protein expression levels
than those without vascular invasion (P<0.05). HCC tissues with
advanced TNM staging exhibited higher KLF8 protein expression
levels than those with a lower TNM staging (r=0.261, P<0.05).
However, no significant correlation was observed between KLF8
expression and age, gender, HBV infection, fetoprotein (AFP), tumor
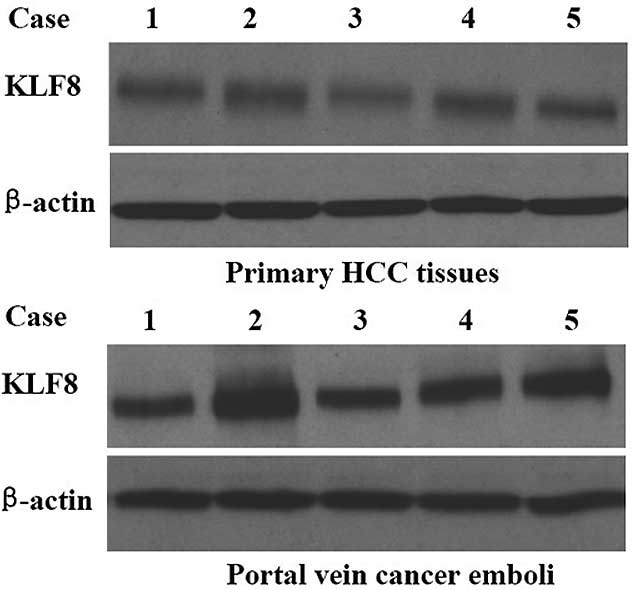
number and tumor size. In addition, western blot analysis revealed
that KLF8 protein levels were higher in portal vein cancer emboli
compared with their corresponding primary HCC tissues (Fig. 3).
 | Table IICorrelation between KLF8 expression
and clinicopathological characteristics in 60 HCC patients. |
Table II
Correlation between KLF8 expression
and clinicopathological characteristics in 60 HCC patients.
| Clinicopathological
parameters | n | KLF8 mRNA (mean ±
SD) | P-value | KLF8 protein
expression | r-value | P-value |
|---|
|
|---|
| − | + | ++ | +++ |
|---|
| Age, years |
| ≤55 | 27 | 0.0053±0.0084 | 0.181 | 11 | 7 | 7 | 2 | −0.119 | 0.367 |
| >55 | 33 | 0.0039±0.0084 | | 18 | 6 | 7 | 2 | | |
| Gender |
| Male | 49 | 0.0043±0.0080 | 0.731 | 23 | 11 | 12 | 3 | 0.043 | 0.745 |
| Female | 11 | 0.0056±0.0105 | | 6 | 2 | 2 | 1 | | |
| Tumor size, cm |
| <5 | 18 | 0.0044±0.0069 | 0.729 | 8 | 4 | 3 | 3 | −0.088 | 0.503 |
| ≥5 | 42 | 0.0046±0.0090 | | 21 | 9 | 11 | 1 | | |
| Tumor number |
| Solitary | 53 | 0.0049±0.0089 | 0.276 | 26 | 12 | 12 | 3 | 0.081 | 0.540 |
| Multiple | 7 | 0.0014±0.0012 | | 3 | 1 | 2 | 1 | | |
| Edmonson’s
staging |
| I–II | 45 | 0.0023±0.0029 | 0.069 | 22 | 11 | 10 | 2 | 0.082 | 0.531 |
| III–IV | 15 | 0.0104±0.0143 | | 7 | 2 | 4 | 2 | | |
| TNM staging |
| I | 40 | 0.0029±0.0045 | 0.198 | 23 | 7 | 9 | 1 | 0.261 | 0.044 |
| II–III | 20 | 0.0078±0.0127 | | 6 | 6 | 5 | 3 | | |
| Vascular
invasion |
| Absent | 47 | 0.0027±0.0042 | 0.047 | 27 | 8 | 11 | 1 | 0.346 | 0.007 |
| Present | 13 | 0.0111±0.0148 | | 2 | 5 | 3 | 3 | | |
| AFP, ng/ml |
| ≤20 | 23 | 0.0045±0.0086 | 0.637 | 11 | 4 | 7 | 1 | −0.027 | 0.84 |
| >20 | 37 | 0.0045±0.0084 | | 18 | 9 | 7 | 3 | | |
| HBsAg |
| Positive | 44 | 0.0045±0.0086 | 0.688 | 21 | 12 | 9 | 2 | –0.076 | 0.563 |
| Negative | 16 | 0.0041±0.0071 | | 8 | 1 | 5 | 2 | | |
KLF8 expression in HCC tissues positively
correlates with FAK expression
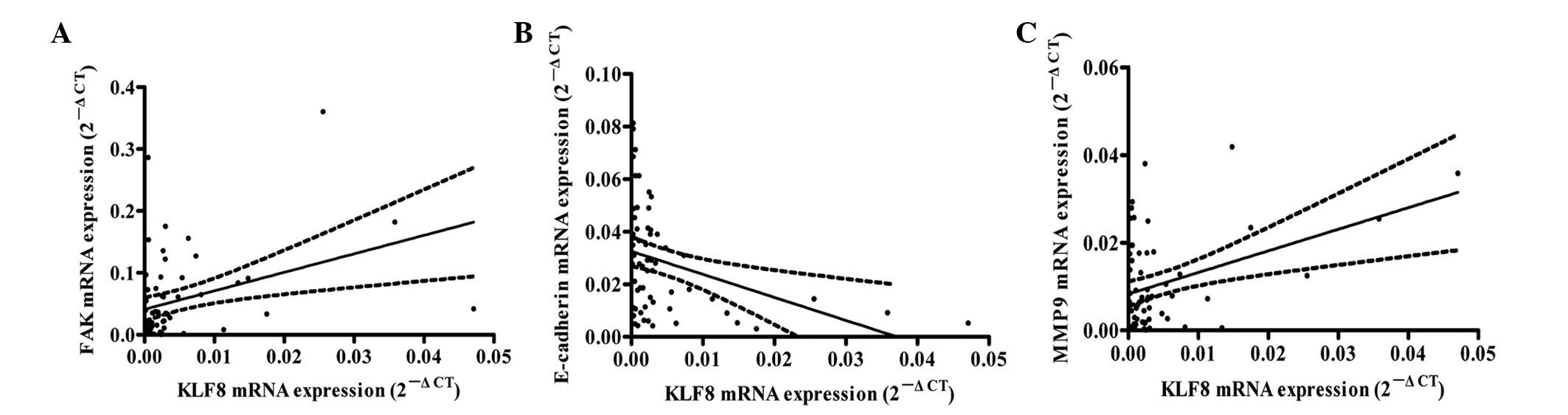
KLF8 and FAK mRNA expression levels were determined
by qRT-PCR in 60 HCC tissues. KLF8 mRNA expression was found to
positively correlate with FAK mRNA expression levels in the HCC
tissues (r=0.362, P<0.001), as assessed by Pearson correlation
coefficient test (Fig. 4A). KLF8
and FAK protein expression was determined by immunohistochemical
staining. FAK protein was positively stained in the cytoplasm of
carcinoma cells and 53.3% (n=32) of the HCC tumor samples revealed
positive staining of FAK protein. KLF8 protein expression levels
positively correlated with FAK protein expression levels in the HCC
tissues (r=0.377, P<0.01), as assessed by Spearman’s rank
correlation coefficient test (Table
III, Fig. 5).
 | Table IIICorrelation between KLF8 protein
expression and FAK, E-cadherin and MMP-9 protein expression in 60
HCC patients. |
Table III
Correlation between KLF8 protein
expression and FAK, E-cadherin and MMP-9 protein expression in 60
HCC patients.
| FAK | | | MMP-9 | | | E-cadherin | | |
|---|
|
| | |
| | |
| | |
|---|
| KLF8 | − | + | ++ | +++ | r-value | P-value | − | + | ++ | +++ | r-value | P-value | − | + | ++ | +++ | r-value | P-value |
|---|
| 0 | 18 | 7 | 3 | 1 | 0.377 | 0.003 | 9 | 9 | 9 | 2 | 0.336 | 0.009 | 11 | 7 | 7 | 4 | −0.410 | 0.001 |
| + | 6 | 7 | 0 | 0 | | | 5 | 4 | 4 | 0 | | | 6 | 3 | 2 | 2 | | |
| ++ | 4 | 4 | 5 | 1 | | | 2 | 3 | 3 | 6 | | | 12 | 1 | 1 | 0 | | |
| +++ | 0 | 2 | 1 | 1 | | | 0 | 0 | 2 | 2 | | | 4 | 0 | 0 | 0 | | |
KLF8 expression in HCC tissues positively
correlates with MMP-9 expression
qRT-PCR demonstrated that KLF8 mRNA expression
levels positively correlated with MMP-9 mRNA expression levels in
the HCC tissues (r=0.392, P<0.01), as assessed by Pearson
correlation coefficient test (Fig.
4B). Immunohistochemical staining revealed that MMP-9 protein
was diffuse with moderate or strong staining in the cytoplasm of
tumor cells. Positive staining of MMP-9 protein was found in 73.3%
(n=44) of the HCC tissues. Consistent with mRNA expression levels,
KLF8 protein expression positively correlated with MMP-9 protein
expression in the HCC tissues (r=0.336, P<0.01; Table III, Fig. 5).
KLF8 expression in HCC tissues negatively
correlates with E-cadherin expression
KLF8 mRNA expression levels were found to negatively
correlate with E-cadherin mRNA expression levels in the HCC tissues
(r=−0.364, P<0.01), as assessed by Pearson correlation
coefficient test (Fig. 2C).
Immunohistochemical analysis revealed that positive E-cadherin
protein staining was largely presented in the membranes of
carcinoma cells. The positive staining of E-cadherin protein was
found in 45% (n=27) of the HCC tissues. KLF8 protein expression
levels negatively correlated with E-cadherin protein expression
levels in the HCC tissues (r=−0.410, P<0.01; Table III, Fig. 5).
Discussion
The long-term prognosis of the majority of patients
with HCC remains extremely poor due to early recurrences and
metastases. HCC metastasis requires invasive cells to detach from
the localized tumors by EMT and degrade the extracellular matrix
using proteases, including MMPs. Following this, these cells must
survive the circulation as circulating tumor cells and colonize
distant locations (20,21). Understanding the molecular
mechanisms underlying each of these steps is crucial for targeting
metastatic cells at early stages to improve patient survival.
KLF8 has recently emerged as an important oncogenic
transcription factor and is overexpressed in several types of human
cancer (11–15). In the present study, KLF8 mRNA
expression was found to be significantly upregulated in HCC tumor
tissues compared with tumor-adjacent tissues. KLF8 expression level
was significantly higher in tumors with advanced TNM stages and
vascular invasion compared with that in tumors with early TNM stage
and absence of vascular invasion (P<0.05). In addition, higher
expression levels of KLF8 protein were detected in portal vein
cancer emboli compared with corresponding primary HCC tissues.
These results indicate that increased KLF8 expression correlates
with more invasive HCC phenotypes and a poorer prognosis.
Next, the correlation between KLF8 expression and
its possible upstream and downstream factors was analyzed in 60 HCC
tissues. FAK, a cytoplasmic non-receptor tyrosine kinase, plays a
key role in the regulation of cell proliferation, survival,
migration and invasion in HCC. High levels of FAK expression were
found to correlate with early metastasis and poor prognosis in HCC
patients who underwent curative surgery (22). KLF8 was previously identified as a
downstream target of FAK and KLF8 mRNA levels are upregulated by
FAK through the PI3K-Akt-Sp1 signaling pathway in ovarian cancer
cells (11,16). In the present study, KLF8
expression levels were found to positively correlate with FAK
expression in HCC tissues. These observations indicated that KLF8
may also be a downstream target of FAK in HCC cells and is
regulated by FAK.
MMP-9 is a member of the MMP family that degrades
denatured collagens and type IV collagen present in the basement
membrane. Thus, MMP-9 is critical for tumor cell invasion and
metastasis (23). MMP-9 is
significantly upregulated in several types of cancer, including
HCC, and is associated with tumor progression and poor survival in
HCC patients (24,25). KLF8 directly binds and activates
the MMP-9 gene promoter, leading to a marked increase in MMP-9
expression and activity in human breast cancer cells (18). In the current study, KLF8
expression levels were found to positively correlate with MMP-9
expression in HCC tissues. The positive correlation between KLF8
and MMP-9 indicated that MMP-9 may represent a downstream target of
KLF8 in HCC cells, and the increased activity of MMP-9 in HCC cells
may be caused, in part, by upregulated KLF8 expression.
During EMT, non-invasive and non-metastatic tumor
cells lose their epithelial phenotype, acquire invasive properties,
infiltrate surrounding tissues and metastasize to secondary sites,
and this process is considered to be a crucial step of metastasis
(26). Studies have demonstrated
that EMT frequently occurs in HCC and is involved in tumorigenesis
and metastasis (27,28). Loss of expression of the epithelial
marker, E-cadherin, is the hallmark of EMT, resulting in reduced
epithelial integrity and polarized function. The downregulated
expression of E-cadherin is common in HCC and has been found to be
associated with hypermethylation of the E-cadherin promoter
(29), HBV infection (30) and the aberrant activity of several
transcription factors, including Snail and Twist (31). Previously, KLF8 was found to
directly bind and repress the promoter of E-cadherin, markedly
inducing EMT and enhancing motility and invasiveness in breast
cancer cells (11). In the present
study, KLF8 expression levels were found to negatively correlate
with E-cadherin expression in HCC tissues. KLF8 was highly
expressed in portal vein cancer emboli, while E-cadherin expression
was low (data not shown). The negative correlation between KLF8 and
E-cadherin indicated that E-cadherin may be regulated by KLF8 in
HCC cells and the loss of E-cadherin expression may be caused, in
part, by KLF8 overexpression. Notably, MMP-9 degrades E-cadherin
into soluble E-cadherin, thus suppressing E-cadherin activity and
inducing EMT (32). These
observations indicate that a loss of E-cadherin expression and
activity may be a result of the KLF8-mediated repression of
E-cadherin and induction of MMP-9.
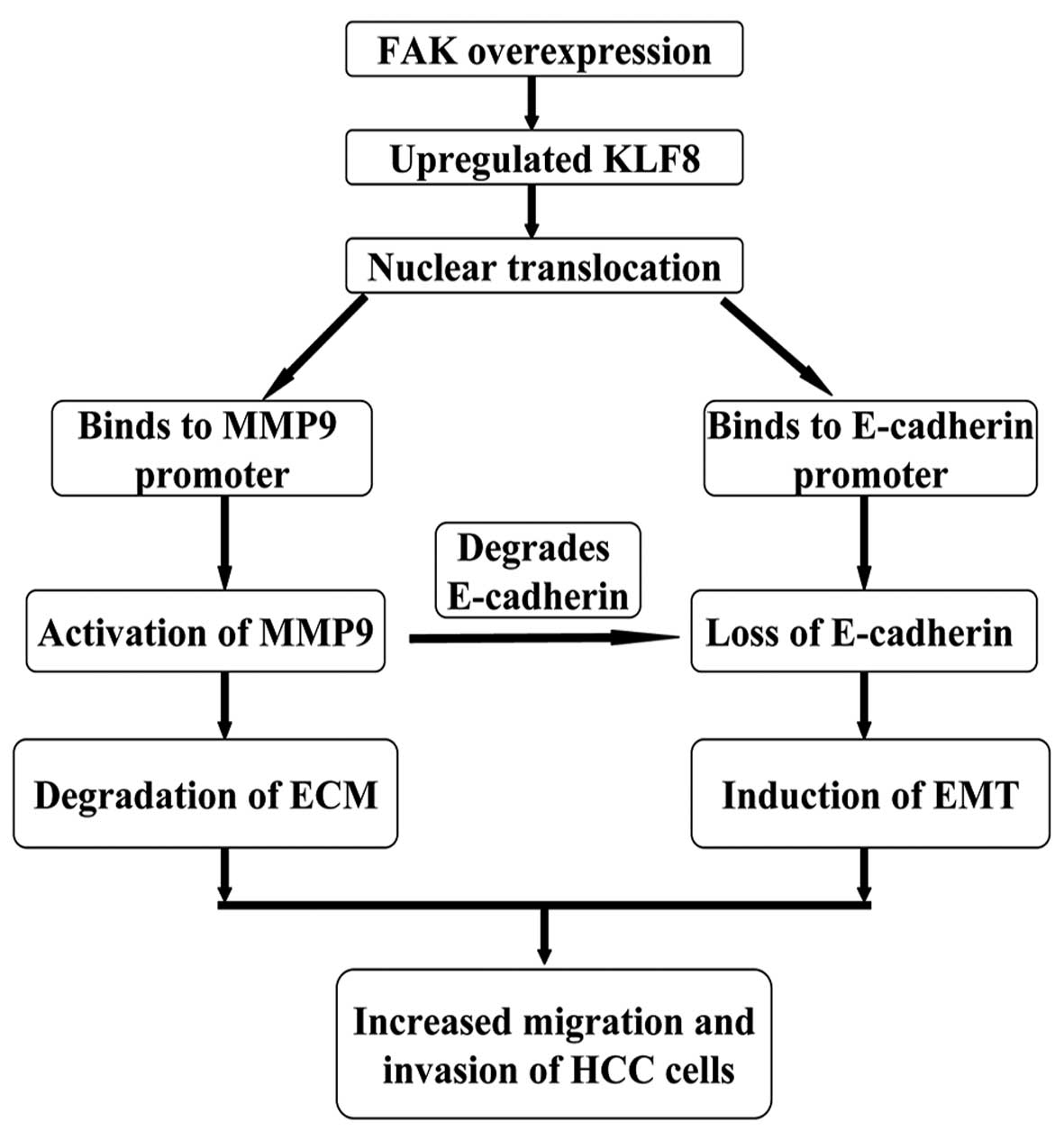
Results of the present study demonstrate the
importance of the FAK-KLF8-MMP-9/E-cadherin signaling axis during
HCC progression (Fig. 6).
Overexpression of FAK in HCC cells leads to the upregulation of
KLF8 expression and increased KLF8 nuclear translocation. KLF8
binds to the promoters of MMP-9 and E-cadherin, inducing marked
increases in MMP-9 activity and a loss of E-cadherin expression. In
this manner, HCC cells gain the ability to degrade the
extracellular matrix and undergo EMT.
Acknowledgements
The present study was supported by a grant from the
National Natural Science Foundation of China (no. 81272645).
References
|
1
|
Farazi PA and DePinho RA: Hepatocellular
carcinoma pathogenesis: from genes to environment. Nat Rev Cancer.
6:674–687. 2006. View
Article : Google Scholar : PubMed/NCBI
|
|
2
|
Eguchi S, Kanematsu T, Arii S, et al:
Recurrence-free survival more than 10 years after liver resection
for hepatocellular carcinoma. Br J Surg. 98:552–557.
2011.PubMed/NCBI
|
|
3
|
El-Serag HB, Marrero JA, Rudolph L and
Reddy KR: Diagnosis and treatment of hepatocellular carcinoma.
Gastroenterology. 134:1752–1763. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Tang ZY, Ye SL, Liu YK, et al: A decade’s
studies on metastasis of hepatocellular carcinoma. J Cancer Res
Clin Oncol. 130:187–196. 2004.
|
|
5
|
McLean GW, Carragher NO, Avizienyte E,
Evans J, Brunton VG and Frame MC: The role of focal-adhesion kinase
in cancer. A new therapeutic opportunity. Nat Rev Cancer.
5:505–515. 2005. View
Article : Google Scholar : PubMed/NCBI
|
|
6
|
Watermann DO, Gabriel B, Jager M, et al:
Specific induction of pp125 focal adhesion kinase in human breast
cancer. Br J Cancer. 93:694–698. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Miyazaki T, Kato H, Nakajima M, et al: FAK
overexpression is correlated with tumour invasiveness and lymph
node metastasis in oesophageal squamous cell carcinoma. Br J
Cancer. 89:140–145. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Rovin JD, Frierson HF Jr, Ledinh W,
Parsons JT and Adams RB: Expression of focal adhesion kinase in
normal and pathologic human prostate tissues. Prostate. 53:124–132.
2002. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Aronsohn MS, Brown HM, Hauptman G and
Kornberg LJ: Expression of focal adhesion kinase and phosphorylated
focal adhesion kinase in squamous cell carcinoma of the larynx.
Laryngoscope. 113:1944–1948. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Itoh S, Maeda T, Shimada M, et al: Role of
expression of focal adhesion kinase in progression of
hepatocellular carcinoma. Clin Cancer Res. 10:2812–2817. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Wang XH, Zheng MZ, Liu G, et al:
Krüppel-like factor 8 induces epithelial to mesenchymal transition
and epithelial cell invasion. Cancer Res. 67:7184–7193. 2007.
|
|
12
|
Fu WJ, Li JC, Wu XY, et al: Small
interference RNA targeting Krüppel-like factor 8 inhibits the renal
carcinoma 786–0 cells growth in vitro and in vivo. J Cancer Res
Clin Oncol. 136:1255–1265. 2010.
|
|
13
|
Wei H, Wang X, Gan B, et al: Sumoylation
delimits KLF8 transcriptional activity associated with the cell
cycle regulation. J Biol Chem. 281:16664–16671. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Liu L, Liu N, Xu M, et al:
Lentivirus-delivered Krüppel-like factor 8 small interfering RNA
inhibits gastric cancer cell growth in vitro and in vivo. Tumour
Biol. 33:53–61. 2012.
|
|
15
|
Li JC, Yang XR, Sun HX, et al:
Up-regulation of Krüppel-like factor 8 promotes tumor invasion and
indicates poor prognosis for hepatocellular carcinoma.
Gastroenterology. 139:2146–2157. 2010.
|
|
16
|
Zhao J, Bian ZC, Yee K, Chen BP, Chien S
and Guan JL: Identification of transcription factor KLF8 as a
downstream target of focal adhesion kinase in its regulation of
cyclin D1 and cell cycle progression. Mol Cell. 11:1503–1515. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Wang X, Urvalek AM, Liu J and Zhao J:
Activation of KLF8 transcription by focal adhesion kinase in human
ovarian epithelial and cancer cells. J Biol Chem. 283:13934–13942.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Wang X, Lu H, Urvalek AM, et al: KLF8
promotes human breast cancer cell invasion and metastasis by
transcriptional activation of MMP-9. Oncogene. 30:1901–1911. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Tu K, Zheng X, Zan X, Han S, Yao Y and Liu
Q: Evaluation of Fbxw7 expression and its correlation with the
expression of c-Myc, cyclin E and p53 in human hepatocellular
carcinoma. Hepatol Res. 42:904–910. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Pantel K and Brakenhoff RH: Dissecting the
metastatic cascade. Nat Rev Cancer. 4:448–456. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Huber MA, Kraut N and Beug H: Molecular
requirements for epithelial-mesenchymal transition during tumor
progression. Curr Opin Cell Biol. 17:548–558. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Fujii T, Koshikawa K, Nomoto S, et al:
Focal adhesion kinase is overexpressed in hepatocellular carcinoma
and can be served as an independent prognostic factor. J Hepatol.
41:104–111. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Peck-Radosavljevic M: Back to basics:
Staging and prognosis in HCC for medical oncologist. J Hepatol.
56:488–489. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Chen JS, Wang Q, Fu XH, et al: Involvement
of PI3K/PTEN/AKT/mTOR pathway in invasion and metastasis in
hepatocellular carcinoma: association with MMP-9. Hepatol Res.
39:177–186. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Chen R, Cui J, Xu C, et al: The
significance of MMP-9 over MMP-2 in HCC invasiveness and recurrence
of hepatocellular carcinoma after curative resection. Ann Surg
Oncol. 19(Suppl 3): S375–S384. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Yang J and Weinberg RA:
Epithelial-mesenchymal transition: at the crossroads of development
and tumor metastasis. Dev Cell. 14:818–829. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
van Zijl F, Zulehner G, Petz M, et al:
Epithelial-mesenchymal transition in hepatocellular carcinoma.
Future Oncol. 5:1169–1179. 2009.
|
|
28
|
Lee TK, Poon RT, Yuen AP, et al: Twist
overexpression correlates with hepatocellular carcinoma metastasis
through induction of epithelial-mesenchymal transition. Clin Cancer
Res. 12:5369–5376. 2006. View Article : Google Scholar
|
|
29
|
Lee S, Lee HJ, Kim JH, Lee HS, Jang JJ and
Kang GH: Aberrant CpG island hypermethylation along multistep
hepatocarcinogenesis. Am J Pathol. 163:1371–1378. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Liu J, Lian Z, Han S, et al:
Downregulation of E-cadherin by hepatitis B virus X antigen in
hepatocellullar carcinoma. Oncogene. 25:1008–1017. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Yang MH, Chen CL, Chau GY, et al:
Comprehensive analysis of the independent effect of twist and snail
in promoting metastasis of hepatocellular carcinoma. Hepatology.
50:1464–1474. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Zuo JH, Zhu W, Li MY, et al: Activation of
EGFR promotes squamous carcinoma SCC10A cell migration and invasion
via inducing EMT-like phenotype change and MMP-9-mediated
degradation of E-cadherin. J Cell Biochem. 112:2508–2517. 2011.
View Article : Google Scholar : PubMed/NCBI
|