Introduction
Worldwide, patients with end-stage bladder disease
are treated with cystoplasty using their own gastrointestinal
segments; however, serious complications are attributable to the
absorptive, mucus-secreting epithelial lining in the urinary
tracts. Practical and functional tissue engineering of the bladder
represents an ideal substitute and a number of studies have focused
on this process in previous years (1,2).
However, simple accumulation of the cells and matrix does not
generate satisfactory results (3)
and organic combinations of these cells and the scaffolds
[extracellular matrix (ECM)] remain to be identified.
It is well documented that appropriate mechanical
stimulation is critical for gene expression and cell proliferation,
differentiation, migration, function optimization and production of
structurally suitable ECM components (4–9).
Human bladder smooth muscle cells (HBSMCs) are constantly subjected
to mechanical stimuli, including hydrodynamic pressure and stretch,
during filling and voiding cycles. Our previous studies indicated
that HBSMC proliferation is stimulated by hydrodynamic pressure
(10,11). However, to the best of our
knowledge, stretch, which is considerably more important than
hydrostatic pressure in physiological conditions, is far from well
explored and the mechanisms by which HBSMCs perceive exterior
mechanical stimulation remain poorly defined. It is well known that
all cellular life recognizes and responds to stimuli from the
extracellular environment. Environmental sensing at the cellular
level relies on signal transduction involving the binding of
extracellular signaling molecules and ligands to cell surface
receptors that trigger events inside the cell (12). Elucidation of the interactions
between physiological cyclic stretch and these signal transduction
pathways may be beneficial or even fundamental to functional tissue
engineering of the urinary bladder, and have important implications
for the development of interventions for cell remodeling diseases,
including incontinence, overactive bladder and bladder outlet
obstruction (BOO) (13–15). The PI3K pathway is one of the most
common physiological and pathological pathways, and is involved in
a number of processes, including cell proliferation, metabolism,
survival and tumorigenesis. AKT and SGK1 are related downstream
effectors of the PI3K cascade, sharing similar downstream targets
and 45–55% homology in their catalytic domains (16–18).
For a specific type of cell, which of the ‘two sisters’ is
responsible for the proliferation remains controversial (19–24).
Cell proliferation requires an increase in the expression and
function of potassium (K+) channels. Blockade of
K+ channels inhibits the proliferation of a number of
cell types (23,25,26).
The Kv1.3 channel represents a novel target for vascular diseases
due to its important relationship with the proliferation of
vascular smooth muscle cells (27). However, the regulation of HBSMC
proliferation by cyclic stretch and the mechanism of this process
remains undefined.
Based on these previous studies (10), the aim of the present study was to
explore the correlation between appropriate cyclic stretch and
HBSMC proliferation, and furthermore, to identify changes in PI3K,
SGK1, AKT and Kv1.3 expression and activity during regulated
proliferation induced by cyclic stretch. The results indicate that
HBSMC proliferative activity is upregulated by physiological cyclic
stretch and when the stretch was applied by 5% elongation and 0.1
Hz, HBSMCs were demonstrated to exhibit maximum proliferative
activity. Expression of PI3K, SGK1 and Kv1.3 was observed to be
significantly increased. By contrast, AKT expression was unchanged.
Increased proliferative activity was eliminated following blockade
of SGK1 and Kv1.3. In addition, increased Kv1.3 expression was
downregulated following blockade of SGK1, indicating that the
PI3K-SGK1-Kv1.3 pathway is involved, at least in part, in HBSMC
proliferation induced by cyclic stretch.
Materials and methods
Cell culture and identification
HBSMCs (ScienCell, Carlsbad, CA, USA; cat. no. 4310)
were grown and maintained at 37°C, in a 5% CO2/95% air
gas mixture and humidified atmosphere in a cell incubator.
Dulbecco’s modified Eagle’s medium (DMEM; low glucose) was
supplemented with 10% fetal bovine serum (both Hyclone
Laboratories, Inc., Logan, UT, USA), penicillin (100 U/ml) and
streptomycin (100 μg/ml). All experiments were performed on cells
between passages 3 and 7 with normal morphology and good activity,
which were confirmed by immunocytochemistry (data not shown).
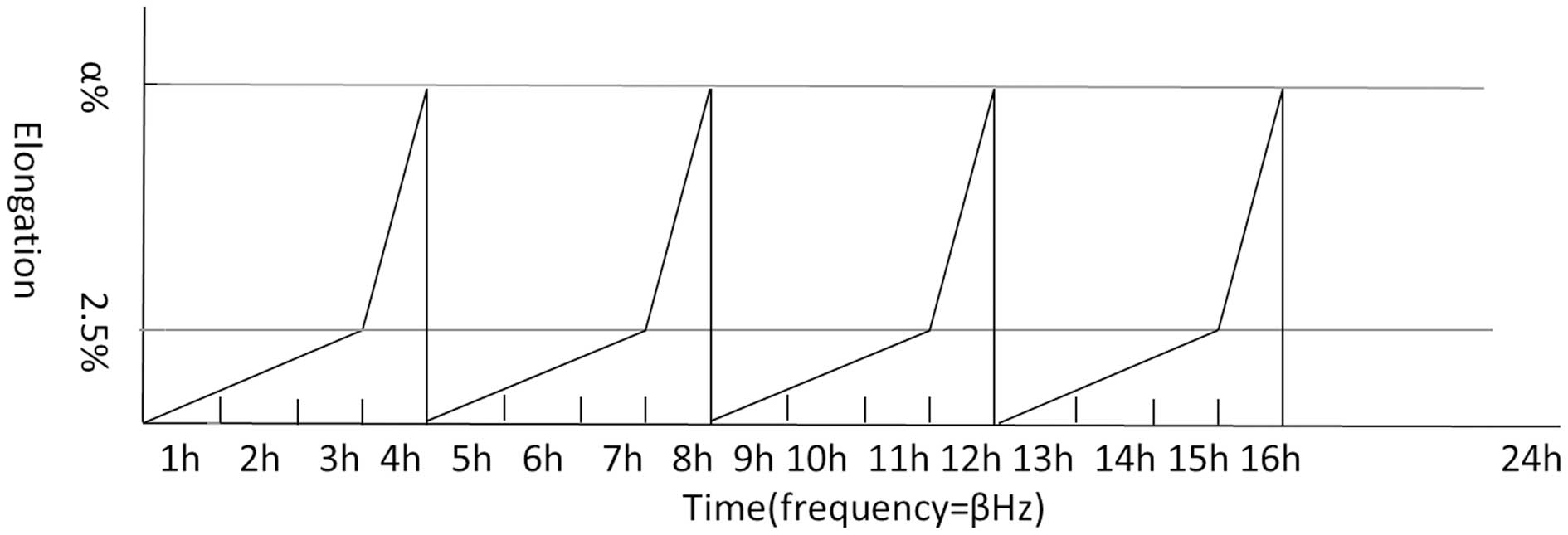
Application of cyclic stretch
HBSMCs were seeded onto a silicone membrane for 24 h
and then subjected to cyclic stretch. Stretch was applied based on
the physiological bladder cycles using the Bose BioDynamic (Bose
Corporation, Eden Prairie, MN, USA). In the first 3 h, elongation
of stretch rose gradually from 0 to 2.5% and in the next 1 h,
stretch rose from 2.5 to 5, 10 or 15%, or was maintained at 2.5%.
Following a rapid decrease, this 4-h stretch cycle was repeated 4
times. Next, the silicone membrane was maintained in a relaxed
state for 8 h (simulation of bladder cycles during night time).
After applied elongations were divided into groups, each group was
subjected to 0.05, 0.1, 0.2, 0.5 and 1 Hz with a sine wave stretch
pattern by a 1:1 stretch/relaxation ratio (see Table I).
 | Table IAbsorbance values of BrdU
incorporation. |
Table I
Absorbance values of BrdU
incorporation.
| Frequency, Hz | 0% (control) | 2.5% | 5% | 10% | 15% |
|---|
| 0.05 | 0.826±0.019a | 0.965±0.035 | 1.264±0.029 | 1.281±0.053 | 1.235±0.026 |
| 0.10 | | 1.161±0.033 | 1.460±0.015b | 1.330±0.028 | 1.226±0.045 |
| 0.20 | | 1.112±0.023 | 1.213±0.020 | 1.204±0.017 | 1.178±0.036 |
| 0.50 | | 1.095±0.019 | 1.240±0.052 | 1.018±0.025 | 1.014±0.041 |
| 1.00 | | 1.048±0.031 | 1.074±0.074 | 0.974±0.028 | 0.981±0.016 |
Proliferation studies
Bromodeoxyuridine (BrdU) incorporation (Roche
Diagnostics GmbH, Mannheim, Germany) was employed as a direct
parameter of DNA synthesis to quantify cell proliferation according
to the manufacturer’s instructions. Briefly, HBSMCs from each group
were harvested and suspended at a concentration of 4×105
cells/ml in DMEM. The cell suspension was transferred into a
96-well plate, with 200 μl in each well. BrdU labeling reagent
(final concentration, 10 μM) was added and the cells were
reincubated for 3 h. Following centrifugation, culture medium was
removed and cells were fixed by FixDenat. Next, anti-BrdU antibody
(1:100) was added to bind the BrdU incorporated in newly
synthesized cellular DNA. Proliferation was quantified by measuring
the absorbance value at a wavelength of 450 nm using an uQuant
ELISA microplate reader (BioTek Instruments, Inc., Winooski, VT,
USA).
RNA expression profile
Total RNA was extracted using TRIzol and cDNA was
synthesized with SuperScript II (both Takara Bio, Inc., Shiga,
Japan) according to the manufacturer’s instructions, at 37°C for 15
min and 85°C for 5 sec. PI3K, SGK1, AKT and Kv1.3 mRNA were
quantified by real-time PCR using the GAPDH housekeeping gene as an
internal control. Real-time PCR was performed using the SYBR Premix
Ex Taq reagent (Takara Bio, Inc.) and the Bio-Rad iQ5 machine
(Hercules, CA, USA). The PCR conditions were programmed as 94°C for
3 min and 40 cycles of 94°C for 5 sec, 54°C for 30 sec and 72°C for
20 sec. PCR product quality was monitored using post-PCR melt curve
analysis. The following primer sequences were used: GAPDH forward,
5′-GCTTCGCTCTCTGCTCCT-3′ and reverse, 5′-CGCCCAATACGACCAAAT-3′;
PI3K forward, 5′-TGGCCTTAGCTCTTAGCCAAACAC-3′ and reverse,
5′-ATTGGAACACGGCCTTTGACA-3′; SGK1 forward, 5′-CTATGCTGCTGAAATAGC-3′
and reverse, 5′-GTCCGAAGTCAGTAAGG-3′; AKT forward,
5′-TCGGCAAGGTGATCCTGGTGAA-3′ and reverse,
5′-AGGCGGTCGTGGGTCTGGAAAG-3′; Kv1.3 forward,
5′-AGTATATGGTGATCGAAGAGG-3′ and reverse,
5′-AGTGAATATCTTCTTGATGTT-3′.
Western blot analysis
Expression of AKT/p-AKT, SGK1/p-SGK1 and Kv1.3 in
HBSMCs was analyzed by western blot analysis, using GAPDH as an
internal control. Briefly, total cells on the silicone membrane
(with or without stretch) were harvested and then stored at −70°C.
Protein extracts were obtained from HBSMC samples treated with cell
lysis buffer [50 mM Tris-HCl (pH 8.0), 150 mM NaCl, 100 μg/ml PMSF
and 1% Triton X-100] for 30 min on ice. Following removal of cell
debris by centrifugation (4°C, 12,000 × g, 5 min), the lysate
sample was boiled for 5 min in sample buffer, separated by 10%
SDS-PAGE and transferred onto a nitrocellulose membrane. The
membrane was incubated with specific primary antibodies at 4°C
overnight, followed by secondary anti-rabbit IgG (Jackson
Immunoresearch Inc., West Grove, PA, USA) for 1 h. Reactive protein
was detected by exposure on BioMax MR-1 film (Kodak, Rochester, NY,
USA).
Statistical analysis
BrdU and RT-PCR assays were performed at least in
triplicate, yielding similar results. Data are presented as the
mean ± SD. Statistical significance was analyzed by one-way ANOVA
test. P<0.05 was considered to indicate a statistically
significant difference.
Results
Cyclic stretch increases
proliferation
To investigate the proliferative activity of HBSMCs
with or without stretch, the BrdU assay was performed. The
correlation between absorbance values and proliferative activities
was investigated in each group with a peak at 30 min following the
addition of the anti-BrdU antibody. As demonstrated in Table I, proliferative activity was
enhanced in each group compared with the control (elongation = 0).
At a specific frequency (e.g., 0.1 Hz), proliferative activity was
increased from 2.5 to 5% elongation; however, gradually decreased
following 5%, indicating 5% elongation is the optimal magnitude of
stretch for HBSMC proliferation (Fig.
1A). Similarly, when cyclic stretch was performed at a specific
elongation (e.g., 5%), maximum proliferative activity was
identified at 0.1 Hz, indicating that 0.1 Hz is an ideal parameter
of cyclic stretch (Fig. 1B).
Following the examination of proliferative activity induced by
cyclic stretch, the simulated optimal physiological stretch (5%
elongation, 0.1 Hz) was established. All subsequent stretches were
performed based on these results.
Activation of the PI3K-SGK1-Kv1.3 pathway
by cyclic stretch
To determine the possible mechanism of proliferative
activity in response to the physiological stretch applied, the
expression levels of proteins involved in the PI3K pathway,
including AKT and SGK1 (the two main related downstream targets
which regulate cell survival and proliferation) were assessed.
Significant upregulation of PI3K (3.75±0.56-fold, P<0.05) and
SGK1 (11.47±1.09-fold, P<0.05); however, not AKT
(1.17±0.14-fold, P>0.05), was observed compared with the
non-stretch group. In addition, mRNA expression of Kv1.3, which is
responsible for proliferation, was increased by 3.05±0.30-fold
(P<0.05; Fig. 2A). mRNA
expression levels were verified with western blot analysis. In
response to cyclic stretch, SGK1, p-SGK1 and Kv1.3 protein levels
were significantly increased; however, AKT gene expression and
activation did not respond to stretch stimulation (Fig. 2B). These results indicate that the
PI3K-SGK1-Kv1.3 pathway, but not the PI3K-AKT pathway, is involved
in stretch-induced proliferation of HBSMCs.
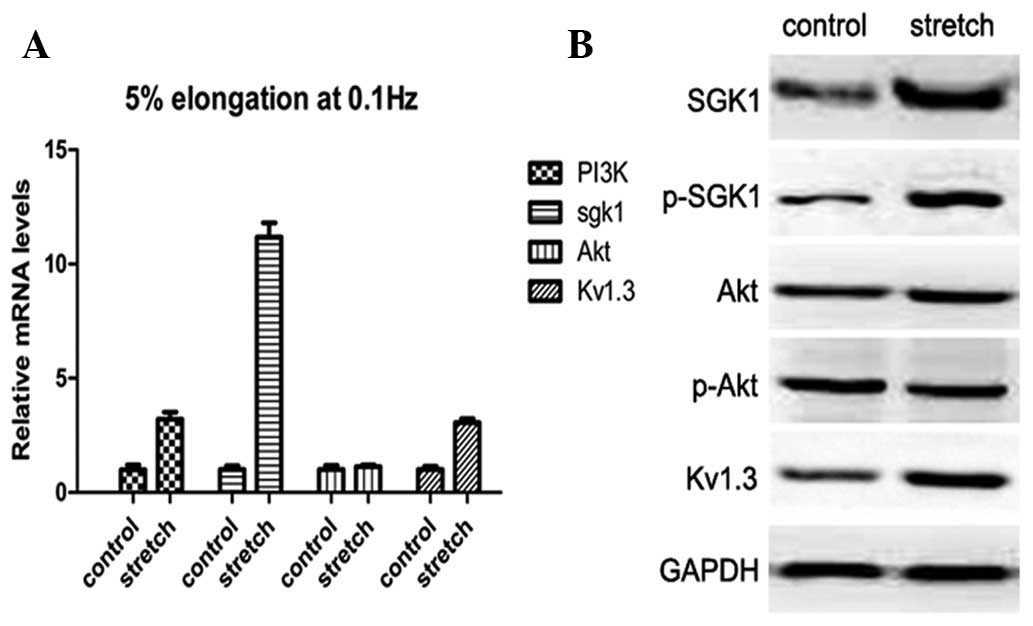 | Figure 2Expression of PI3K, SGK1, p-SGK1, AKT,
p-AKT and Kv1.3 in the control and optimal stretch groups. (A)
Real-time PCR of relative mRNA expression of PI3K, SGK1, AKT and
Kv1.3 in HBSMCs with or without stretch. Expression of PI3K, SGK1
and Kv1.3; however, not AKT, was significantly increased compared
with the corresponding controls. (B) Western blot analysis revealed
the relative protein expression of SGK1, p-SGK1, AKT, p-AKT and
Kv1.3. GAPDH was used as an internal control. Consistent with
real-time PCR, expression of SGK1, p-SGK1 and Kv1.3; however, not
AKT, was markedly elevated by stretch stimuli. HBSMCs, human
bladder smooth muscle cells. |
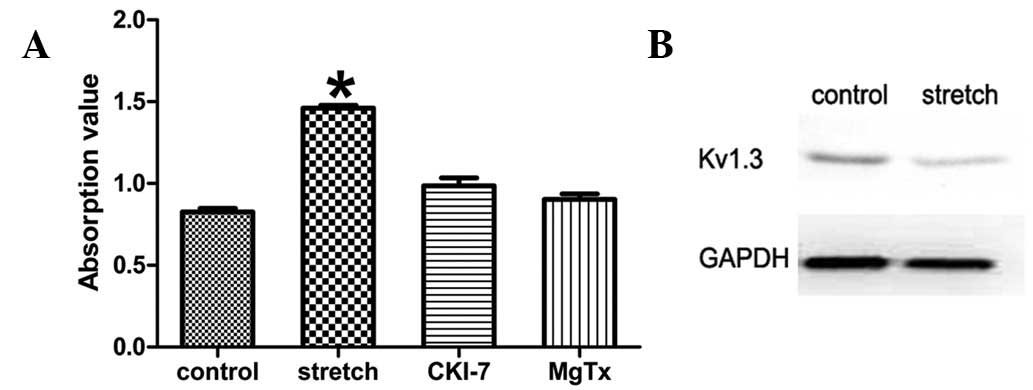
Inhibitors of SGK-1 and Kv1.3 eliminate
the increase in proliferative activity
The role of the PI3K-SGK1-Kv1.3 pathway in
stretch-induced HBSMC proliferation was further confirmed using
SGK-1 and Kv1.3 inhibitors. Cyclic stretch was applied following
exposure of the cells to the SKG1 inhibitor, CKI-7 dihydrochloride
(Sigma-Aldrich, St. Louis, MO, USA), at a final concentration of
100 μM. Proliferative activity was significantly suppressed
compared with the stretch group without SGK1 inhibitor (0.986±0.042
vs. 1.460±0.015, P<0.05; Fig.
3A). In addition, Kv1.3 protein expression levels were analyzed
by western blot analysis and no differences between the stretch and
non-stretch group was identified (Fig.
3B). Similarly, when the high-affinity blocker of Kv1.3,
margatoxin (Sigma-Aldrich), was used at a final concentration of 10
nM, the increased proliferative activity was largely eradicated
compared with the same stretch group; however, without the Kv1.3
inhibitor (0.902±0.030 vs. 1.460±0.015, P<0.05; Fig. 3A). These results indicate that
Kv1.3 is a downstream target of SGK1 which, in turn, is responsible
for HBSMC proliferation induced by physiological cyclic
stretch.
Discussion
Due to the unique properties and functions of the
human bladder compared with the gastrointestinal segments, the
generation of functional bladders using tissue engineering is
important for organ regeneration and replacement. However, there
are a number of technical limitations associated with bladder
tissue engineering (28). HBSMCs
are fundamental for bladder tissue engineering, but HBSMCs are an
inadequate source and show dysfunctional contractility in the
tissue engineered urinary bladder without appropriate external
stimuli. Mechanical stimuli, including stretch and hydrostatic and
hydrodynamic pressure, are crucial for the maturity of these
mechanically sensitive cells. Stretch is considerably more
important than hydrostatic pressure and other mechanical stimuli
for bladder tissue engineering (9).
Although previous studies have attempted to apply
stretch to SMCs, the majority of these stretches were not designed
based on physiological conditions (29). The proliferative effects varied
with different strains in magnitude and frequency, thus making
normative application of stretch imperative. With the two key
parameters (elongation and frequency) defined, in the present
study, optimal simulated physiological cyclic stretch for HBSMC
proliferation was established. When cyclic stretch was applied at
5% elongation and 0.1 Hz, a significantly augmented proliferative
activity was detected (1.460±0.015, P<0.05) compared with the
other groups. To the best of our knowledge, this study is the first
to report the optimal stretch parameters for HBSMC proliferation.
Identification of the optimal proliferation stretch model is
beneficial to the solution of cell source inadequacy and is also
important for the elucidation of the pathophysiological mechanisms
involved in diseases associated with HBSMC overproliferation.
A vast number of kinases in the cell constitute a
complicated but specific network in which kinases activate their
own pathways and interact with other pathways. Located downstream
from PI3K, SGK1 and AKT are considered to be extremely similar
kinase proteins (19). As proteins
implicated as mechanotransduction mediators of HBSMC proliferation,
the expression of PI3K, AKT, SGK1 and Kv1.3 was investigated at the
transcriptional and translational levels. Results of RT-PCR
revealed increased mRNA expression of PI3K, SGK1 and Kv1.3, but not
AKT, consistent with the results of western blot analysis.
Initially, we hypothesized that PI3K-SGK1-Kv1.3 was responsible for
stretch-induced proliferation of HBSMCs. Inhibitors of SGK1 and
Kv1.3 largely eliminated the proliferative activity and confirmed
the role of this pathway. These results indicated that SGK1, but
not AKT, is a mediator of mechanical signaling events in HBSMC
proliferation. These observations are consistent with the results
of a previous study in which SGK1 predisposed vasculature smooth
muscle to an increased proliferative response to mechanical stimuli
and this proliferative response was markedly suppressed by SGK1
knockout (24). In addition, in
the present study, Kv1.3 was identified to be involved in the
stretch-induced proliferation process downstream of SGK1. As a
dominant intracellular secondary messenger, increased
concentrations of cytoplasmic Ca2+ are involved in the
regulation of cell proliferation (30). K+ channels have been
hypothesized to be important for the maintenance of cell membrane
potential, which, in turn, is required for correct function of the
Ca2+ release-activated Ca2+ channel (31). The latter channel, which is highly
sensitive to membrane potential, mediates Ca2+ entry
upon stimulation of cells, a prerequisite for triggering cell
proliferation (32,33).
PI3K-AKT is a critical signaling pathway in the
survival and proliferation of a number of cells (21,34)
and is relatively well understood. However, SGK1, a ‘sister’ of
AKT, has always been neglected. Functional analysis of
gene-targeted mice lacking SGK1 provided insight into the
functional significance of SGK1-dependent regulation of
physiological functions. Knockout of SGK1 led to no severe
phenotypes, indicating that SGK1 is not required for survival
(35). Results indicated that SGK1
is a stimuli-responsive kinase that may mediate mechanical
stretch-induced proliferation of HBSMCs, leading to bladder
formation. Therefore, a hypothetical theory is raised: of the two
‘sisters’, AKT is implicated with basal proliferative activity and
by contrast, SGK1 is responsible for the stimuli-induced activity.
However, further confirmation and efforts must be performed to
clearly illustrate this assumption.
In contrast to previous studies, several innovations
were employed in this study: i) Cells were obtained from humans
instead of animals; ii) physiological cyclic stretch was applied to
stimulate the real bladder environment; iii) different elongations
and frequencies were well studied, improved representative results
were obtained and an optimized stretch model was established. Based
on these results, we are likely to be able to provide qualified
seed cells to bladder engineering techniques. In addition,
observations indicate that the PI3K-SGK1-Kv1.3 pathway, but not
PI3K-AKT, is the signal transduction pathway involved in
stretch-induced proliferation and represents a promising pathway
for novel targeted therapies for specific urinary bladder diseases
caused by excessive mechanical forces, including BOO. The use of
drugs or inhibitors to inhibit SGK1 or Kv1.3 may provide promising
methods to interrupt this pathological process. In particular, in
the case of SGK1, which is not required for individual survival
(35), ‘block’ treatment is likely
be extremely effective, without severe complications.
Acknowledgements
This study was supported by grants from the National
Natural Science Foundation of China (no. 30872593 and 31170907),
the Technology Support Program of Science and Technology Department
of Sichuan Province (no. 2010SZ0163) and the Ph.D. Programs
Foundation of Ministry of Education of China (no.
20110181110028).
References
|
1
|
Fraser M, Thomas DF, Pitt E, Harnden P,
Trejdosiewicz LK and Southgate J: A surgical model of composite
cystoplasty with cultured urothelial cells: a controlled study of
gross outcome and urothelial phenotype. BJU Int. 93:609–616. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Southgate J, Gross W, Eardley I, Thomas DF
and Trejdosiewicz LK: Bladder reconstruction - from cells to
materials. Proc Inst Mech Eng H. 217:311–316. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Zhang Y, Frimberger D, Cheng EY, Lin HK
and Kropp BP: Challenges in a larger bladder replacement with
cell-seeded and unseeded small intestinal submucosa grafts in a
subtotal cystectomy model. BJU Int. 98:1100–1105. 2006. View Article : Google Scholar
|
|
4
|
Lee DY, Yeh CR, Chang SF, Lee PL, Chien S,
Cheng CK and Chiu JJ: Integrin-mediated expression of bone
formation-related genes in osteoblast-like cells in response to
fluid shear stress: roles of extracellular matrix, Shc and
mitogen-activated protein kinase. J Bone Miner Res. 23:1140–1149.
2008. View Article : Google Scholar
|
|
5
|
Liu D, Genetos DC, Shao Y, Geist DJ, Li J,
Ke HZ, Turner CH and Duncan RL: Activation of extracellular-signal
regulated kinase (ERK1/2) by fluid shear is Ca(2+)- and
ATP-dependent in MC3T3-E1 osteoblasts. Bone. 42:644–652. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Lee AA, Graham DA, Dela CS, Ratcliffe A
and Karlon WJ: Fluid shear stress-induced alignment of cultured
vascular smooth muscle cells. J Biomech Eng. 124:37–43. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Wei X, Li DB, Xu F, Wang Y, Zhu YC, Li H
and Wang KJ: A novel bioreactor to simulate urinary bladder
mechanical properties and compliance for bladder functional tissue
engineering. Chin Med J (Engl). 124:568–573. 2011.PubMed/NCBI
|
|
8
|
Ramachandran A, Gong EM, Pelton K, Ranpura
SA, Mulone M, Seth A, Gomez P 3rd and Adam RM: FosB regulates
stretch-induced expression of extracellular matrix proteins in
smooth muscle. Am J Pathol. 179:2977–2989. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Farhat WA and Yeger H: Does mechanical
stimulation have any role in urinary bladder tissue engineering?
World J Urol. 26:301–305. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Chen L, Wei TQ, Wang Y, Zhang J, Li H and
Wang KJ: Simulated bladder pressure stimulates human bladder smooth
muscle cell proliferation via the PI3K/SGK1 signaling pathway. J
Urol. 188:661–667. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Wu T, Chen L, Wei TQ, Wang Y, Xu F and
Wang K: Effect of cyclic hydrodynamic pressure-induced
proliferation of human bladder smooth muscle through Ras-related C3
botulinum toxin substrate 1, mitogen-activated protein kinase
kinase 1/2 and extracellular regulated protein kinases 1/2. Int J
Urol. 19:867–874. 2012. View Article : Google Scholar
|
|
12
|
Brivanlou AH and Darnell JJ: Signal
transduction and the control of gene expression. Science.
295:813–818. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Elbadawi A: Pathology and pathophysiology
of detrusor in incontinence. Urol Clin North Am. 22:499–512.
1995.PubMed/NCBI
|
|
14
|
el-Feky H, Mangoud AM, Aly MA, Eissa MH,
Abdel-Wahab RM, Kamhawy M, Ghobish A, Sabry AH, el Zayyat EA and
Morsy TA: Detrusor morphology and pathology in relation to bladder
outflow obstruction in bilharzial patients. J Egypt Soc Parasitol.
21:699–706. 1991.PubMed/NCBI
|
|
15
|
Cerruto MA, Asimakopoulos AD, Artibani W,
Del Popolo G, La Martina M, Carone R and Finazzi-Agrò E: Insight
into new potential targets for the treatment of overactive bladder
and detrusor overactivity. Urol Int. 89:1–8. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Webster MK, Goya L, Ge Y, Maiyar AC and
Firestone GL: Characterization of sgk, a novel member of the
serine/threonine protein kinase gene family which is
transcriptionally induced by glucocorticoids and serum. Mol Cell
Biol. 13:2031–2040. 1993.PubMed/NCBI
|
|
17
|
Park J, Leong ML, Buse P, Maiyar AC,
Firestone GL and Hemmings BA: Serum and glucocorticoid-inducible
kinase (SGK) is a target of the PI 3-kinase-stimulated signaling
pathway. EMBO J. 18:3024–3033. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Kobayashi T and Cohen P: Activation of
serum- and glucocorticoid-regulated protein kinase by agonists that
activate phosphatidylinositide 3-kinase is mediated by
3-phosphoinositide-dependent protein kinase-1 (PDK1) and PDK2.
Biochem J. 339:319–328. 1999. View Article : Google Scholar
|
|
19
|
Lang F, Böhmer C, Palmada M, Seebohm G,
Strutz-Seebohm N and Vallon V: (Patho)physiological significance of
the serum- and glucocorticoid-inducible kinase isoforms. Physiol
Rev. 86:1151–1178. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Liu G, Hitomi H, Hosomi N, Lei B, Nakano
D, Deguchi K, Mori H, Masaki T, Ma H, Griendling KK and Nishiyama
A: Mechanical stretch augments insulin-induced vascular smooth
muscle cell proliferation by insulin-like growth factor-1 receptor.
Exp Cell Res. 317:2420–2428. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Stover J and Nagatomi J: Cyclic pressure
stimulates DNA synthesis through the PI3K/AKT signaling pathway in
rat bladder smooth muscle cells. Ann Biomed Eng. 35:1585–1594.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Sakoda H, Gotoh Y, Katagiri H, Kurokawa M,
Ono H, Onishi Y, Anai M, Ogihara T, Fujishiro M, Fukushima Y, Abe
M, Shojima N, Kikuchi M, Oka Y, Hirai H and Asano T: Differing
roles of AKT and serum- and glucocorticoid-regulated kinase in
glucose metabolism, DNA synthesis and oncogenic activity. J Biol
Chem. 278:25802–25807. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Gamper N, Fillon S, Huber SM, Feng Y,
Kobayashi T, Cohen P and Lang F: IGF-1 up-regulates K+
channels via PI3-kinase, PDK1 and SGK1. Pflugers Arch. 443:625–634.
2002.PubMed/NCBI
|
|
24
|
Cheng J, Wang Y, Ma Y, Chan BT, Yang M,
Liang A, Zhang L, Li H and Du J: The mechanical stress-activated
serum-, glucocorticoid-regulated kinase 1 contributes to neointima
formation in vein grafts. Circ Res. 107:1265–1274. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Wonderlin WF and Strobl JS: Potassium
channels, proliferation and G1 progression. J Membr Biol.
154:91–107. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Pardo LA: Voltage-gated potassium channels
in cell proliferation. Physiology (Bethesda). 19:285–292. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Jackson WF: KV1.3: a new therapeutic
target to control vascular smooth muscle cell proliferation.
Arterioscler Thromb Vasc Biol. 30:1073–1074. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Atala A: Tissue engineering of human
bladder. Br Med Bull. 97:81–104. 2011. View Article : Google Scholar
|
|
29
|
Lang F, Bohmer C, Palmada M, Seebohm G,
Strutz-Seebohm N and Vallon V: (Patho)physiological significance of
the serum- and glucocorticoid-inducible kinase isoforms. Physiol
Rev. 86:1151–1178. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Kahl CR and Means AR: Regulation of cell
cycle progression by calcium/calmodulin-dependent pathways. Endocr
Rev. 24:719–736. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Parekh AB and Penner R: Store depletion
and calcium influx. Physiol Rev. 77:901–930. 1997.PubMed/NCBI
|
|
32
|
Berridge MJ, Lipp P and Bootman MD: The
versatility and universality of calcium signalling. Nat Rev Mol
Cell Biol. 1:11–21. 2000. View
Article : Google Scholar : PubMed/NCBI
|
|
33
|
Means AR: Calcium, calmodulin and cell
cycle regulation. FEBS Lett. 347:1–4. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Allard D, Figg N, Bennett MR and
Littlewood TD: AKT regulates the survival of vascular smooth muscle
cells via inhibition of FoxO3a and GSK3. J Biol Chem.
283:19739–19747. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Wu W, Chaudhuri S, Brickley DR, Pang D,
Karrison T and Conzen SD: Microarray analysis reveals
glucocorticoid-regulated survival genes that are associated with
inhibition of apoptosis in breast epithelial cells. Cancer Res.
64:1757–1764. 2004. View Article : Google Scholar : PubMed/NCBI
|