Introduction
Bladder cancer is a common malignant tumor in
humans; however, the molecular mechanism underlying its growth and
invasion remains unclear. MicroRNAs (miRNAs) are small, endogenous
and non-coding RNAs that inhibit gene expression via interaction
with target sites in the 3′-untranslational region (UTR) of mRNA
(1). Emerging data has shown that
miRNAs are important in the regulation of various biological
processes, including the development of several types of human
cancer (2). miRNA-430 (miR-430)
has mainly been investigated in zebrafish and has been shown to be
associated with early embryo development (3–5).
However, whether miR-430 is involved with the development of
malignant tumors has not been reported.
CXCR7 is 7-transmembrane chemokine receptor of the
stroma-derived factor-1α (SDF-1α). Its expression has been reported
to be enhanced during tumor development, indicating that CXCR7 is
an attractive therapeutic target for cancer (6). Yates et al observed that CXCR7
expression was elevated in bladder cancer tissue and was associated
with high-grade tumors and metastasis (7). In addition, CXCR7 has been
demonstrated to be associated with proliferation, migration and
invasion of bladder cancer through several signaling pathways,
including ERK, Stat3 and AKT signaling (7,8).
The present study firstly revealed an miR-430
expression pattern in normal bladder, adjacent tissue and bladder
cancer tissue. Furthermore, the overexpression of miR-430 in human
bladder cancer 5637 cells significantly inhibited cell
proliferation, migration and colony formation efficiency. These
findings were contrary to those obtained following the
overexpression of CXCR7, which was validated to be a direct target
of miR-430 in this study. Further analysis showed that cell
proliferation- and migration-related genes, including ERK,
phosphorylated-ERK (p-ERK), matrix metalloproteinase-2 (MMP-2) and
MMP-9, were significantly downregulated in miR-430 overexpressed
5637 cells, while markedly upregulated in CXCR7 overexpressed 5637
cells. Our study reveals a novel role and a potential regulatory
mechanism of miR-430 in bladder cancer cells.
Materials and methods
Cell culture
The human bladder carcinoma 5637 cell line was
cultured in RPMI-1640 medium with 10% FBS and 1%
penicillin/streptomycin at 37°C with 5% CO2. The study
was approved by the ethics committee of Second Xiangya Hospital of
Central South University, Changsha, China.
RNA extraction and quantitative real-time
PCR (qRT-PCR) analysis
TRIzol reagent (Invitrogen Life Technologies,
Carlsbad, CA, USA) was used to extract RNA from tissues or the 5637
cell line. The RNA integrity was then assessed. TaqMan qRT-PCR
miRNA assay (Applied Biosystems, Carlsbad, CA, USA) was performed
to determine the miR-430 expression level. The relative expression
of miR-430 was normalized to U6 endogenous control. Each
measurement was performed in triplicate. For CXCR7 expression, 1 μg
total RNA was reverse transcribed into cDNA using SuperScript™ III
First-Strand Synthesis SuperMix (Invitrogen Life Technologies).
SYBR Green qRCR Mix (Toyobo, Osaka, Japan) was then used to perform
qRT-PCR with the following CXCR7 primers: forward
5′-TGGGTGGTCAGTCTCGT-3′ and reverse 5′-CCGGCAGTAGGTCTCAT-3′.
β-actin was used as a control and its primers were as follows:
forward 5′-AGGGGCCGGACTCGTCATACT-3′ and reverse
5′-GGCGGCACCACCATGTACCCT-3′.
Western blotting
Cells were solubilized in cold RIPA lysis buffer and
then separated with 10% SDS-PAGE. Proteins were then transferred
onto a polyvinylidene fluoride (PVDF) membrane. After blocking in
5% nonfat dried milk in PBS for 3 h, the membrane was then
incubated overnight with specific antibodies for CXCR7, ERK, p-ERK,
MMP-2, MMP-9 and β-actin (Abcam, Cambridge, MA, USA). After
incubation with the specific secondary antibody, respectively
(Abcam), immune complexes were detected using the enhanced
chemiluminescence (ECL) method. The results were visualized by
autoradiography using preflashed Kodak XAR film (Kodak, Tokyo,
Japan).
Dual luciferase reporter assays
A normal and a mutated 3′-UTR of CXCR7 was subcloned
to construct reporter vectors with miRNA-binding sites, which were
then inserted into the multiple cloning sites downstream of the
luciferase gene in the psiCHECK™-2 luciferase miRNA expression
reporter vector. For the luciferase assay, a dual-luciferase
reporter assay system (E1910, Promega Corporation, Madison, WI,
USA) was used. According to the manufacturer’s instructions, 20,000
cells were cultured in 24-well plates at 37°C with 5%
CO2 for 24 h. When cells reached 70–80% confluence, the
cells were cotransfected with psiCHECK™-2-CXCR7-3′-UTR or
psiCHECK™-2-mut CXCR7-3′-UTR vector using Lipofectamine 2000.
miR-430 or miR-430 inhibitor was added, respectively (Table I). The cells were then incubated
with transfection reagent/DNA complex for 5 h, and then refreshed
with fresh medium containing 10% FBS. A total of 48 h after
transfection, firefly and renilla luciferase activities were
evaluated using the dual-luciferase reporter assay system (Promega
Corporation) and renilla luciferase activity was normalized to
firefly luciferase activity.
 | Table IDetailed information for dual
luciferase reporter assays. |
Table I
Detailed information for dual
luciferase reporter assays.
| Sample | microRNA (nM) | Plasmid (μg) | Inhibitor (nM) |
|---|
| CXCR7 | - | 0.5 | - |
| Mut CXCR7 | - | 0.5 | - |
| NC + CXCR7 | 50 | 0.5 | - |
| NC + Mut CXCR7 | 50 | 0.5 | - |
| NC inhibitor +
CXCR7 | - | 0.5 | 100 |
| NC inhibitor + Mut
CXCR7 | - | 0.5 | 100 |
| miR-430 + CXCR7 | 50 | 0.5 | - |
| miR-430 + Mut
CXCR7 | 50 | 0.5 | - |
| miR-430 inhibitor +
CXCR7 | - | 0.5 | 100 |
| miR-430 inhibitor +
Mut CXCR7 | - | 0.5 | 100 |
Plasmid construction and
transfection
The plasmids of miR-SCR, miR-430 and CXCR7 were
constructed by Aijia Bio (Changsha, Hunan, China). Retroviral
supernatants were prepared using Eco-Phoenix packaging cells. The
5673 cells were transfected using 20 mg/ml polybrene for 48 h.
MTT assay
Human bladder cancer 5673 cells were plated at a
density of 5,000 cells per well. After adding MTT (Promega
Corporation) to the medium at a final concentration of 0.5 μg/ml,
5673 cells were then incubated at 37°C with 5% CO2 for 3
h. The medium was then removed and 100 μl DMSO was added. The plate
was gently rotated on an orbital shaker for 10 min to completely
dissolve the precipitation. The microplate reader (Bio-Rad,
Hercules, CA, USA) was used to determine the absorbance at 570
nm.
Flow cytometric analysis
Flow cytometry was performed in order to determine
the cell cycle in all groups. In total, 200,000 cells were washed
twice with Dulbecco’s modified phosphate-buffered saline and
resuspended in 70% ethanol. After fixation overnight at −20°C, the
cells were pelleted, washed twice in 1X PBS with 3% BSA, and
pelleted. Subsequently, the cells were resuspended and incubated
for 30 min at room temperature in propidium iodide (PI) staining
buffer containing 3% BSA, 40 μg/ml PI and 0.2 mg/ml RNase in 1X
PBS. DNA content analysis was performed using flow cytometry
(FACSCalibur, Beckman Coulter, Miami, FL, USA).
Transwell assay
For all groups, migration was measured in 24-well
transwell chambers (Chemicon, Temecula, CA, USA). After 24-h
incubation at 37°C with 5% CO2, the migrated cells were
stained and counted.
Colony formation assay
A colony formation assay was performed in order to
determine the colony formation efficiency of the 5673 cells. In
total, 200 cells were added to each well of a 6-well plate, which
was then incubated at 37°C for 14 days. The cells were subsequently
gently washed and stained with crystal violet. Viable colonies
containing ≥50 cells were counted.
Statistical analysis
Data are shown as the mean values ± standard
deviation (SD) of at least triplicate determinations. Statistical
analysis was performed using SPSS 17.0 statistical software. The
statistical significance of the differences was determined by
ANOVA. P<0.05 was considered to indicate a statistically
significant difference, while P<0.01 was considered to indicate
a markedly significant difference.
Results
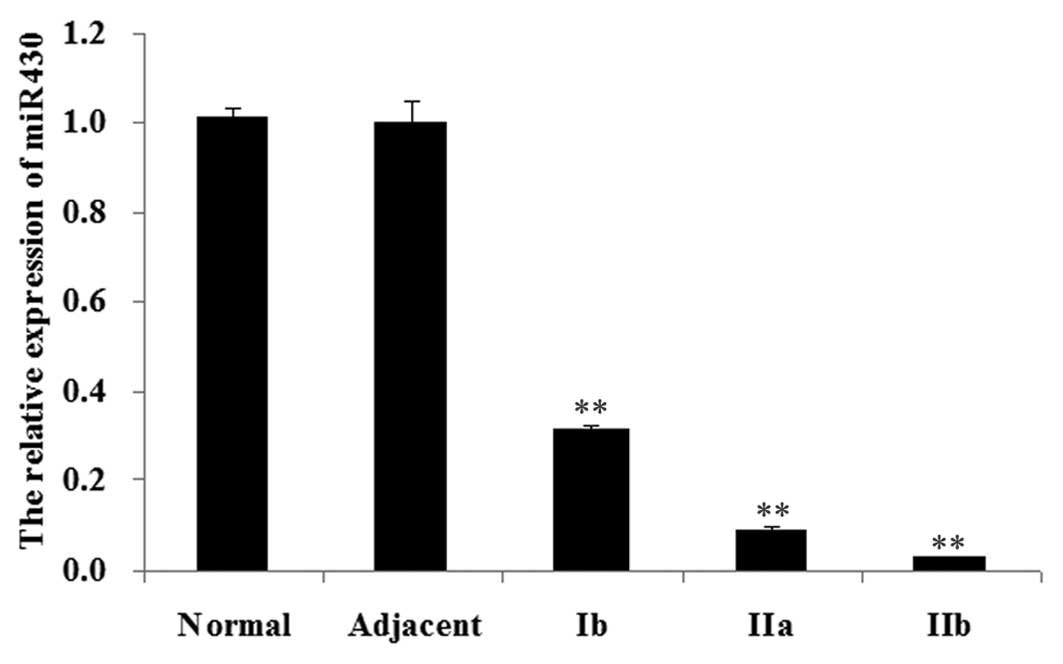
Expression of miR-430 in normal bladder,
adjacent tissue and bladder cancer tissue
We firstly determined the expression of miR-430 in
different tissues, including normal bladder tissue, adjacent tissue
and bladder cancer tissue of different stages, including Ib, IIa
and IIb. As shown in Fig. 1, the
miR-430 expression levels in bladder cancer tissue were
significantly decreased compared with those in normal tissue and
adjacent tissue (P<0.01). Furthermore, among bladder cancer
tissue of different stages, the miR-430 expression level in stage
Ib was the highest, whereas the miR-430 expression level in stage
IIb was the lowest.
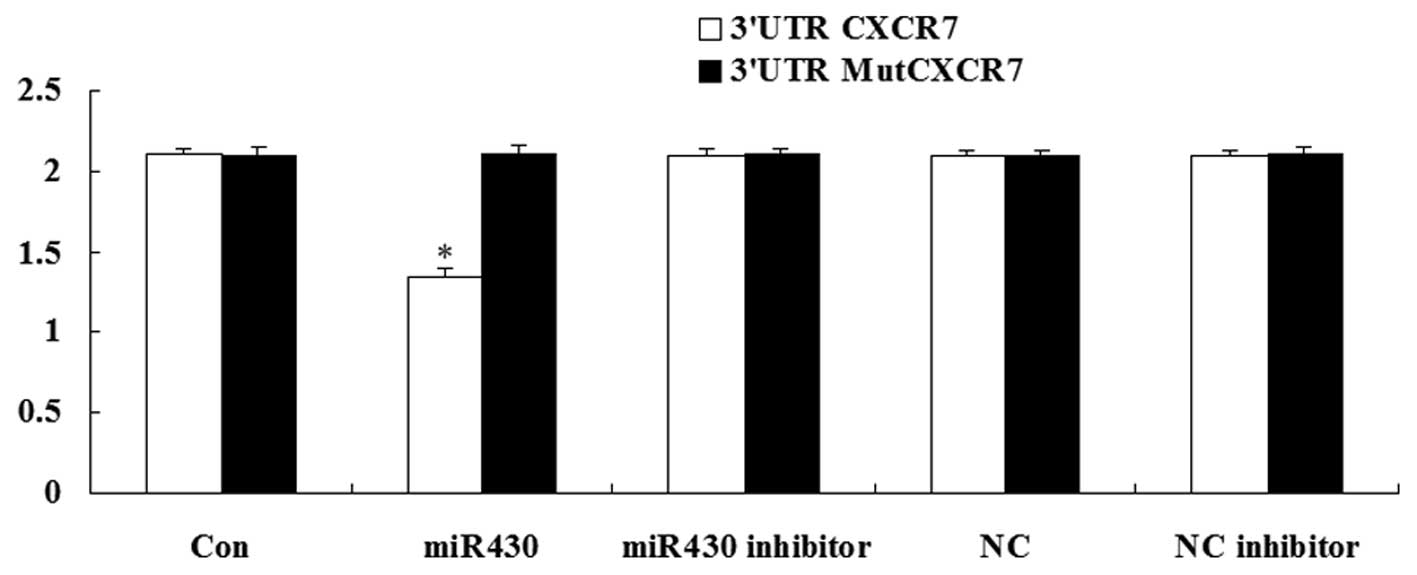
CXCR7 is the direct target of
miR-430
A luciferase assay was performed to detect whether
CXCR7 was the direct target of miR-430. The result demonstrated
that the renilla/firefly value of luciferase was significantly
decreased only following transfection with the 3′-UTR of the CXCR7
gene in miR-430 treatment cells. However, in other groups, the
renilla/firefly value of luciferase showed no difference compared
with the control (Fig. 2). These
data suggest that CXCR7 was the direct target of miR-430.
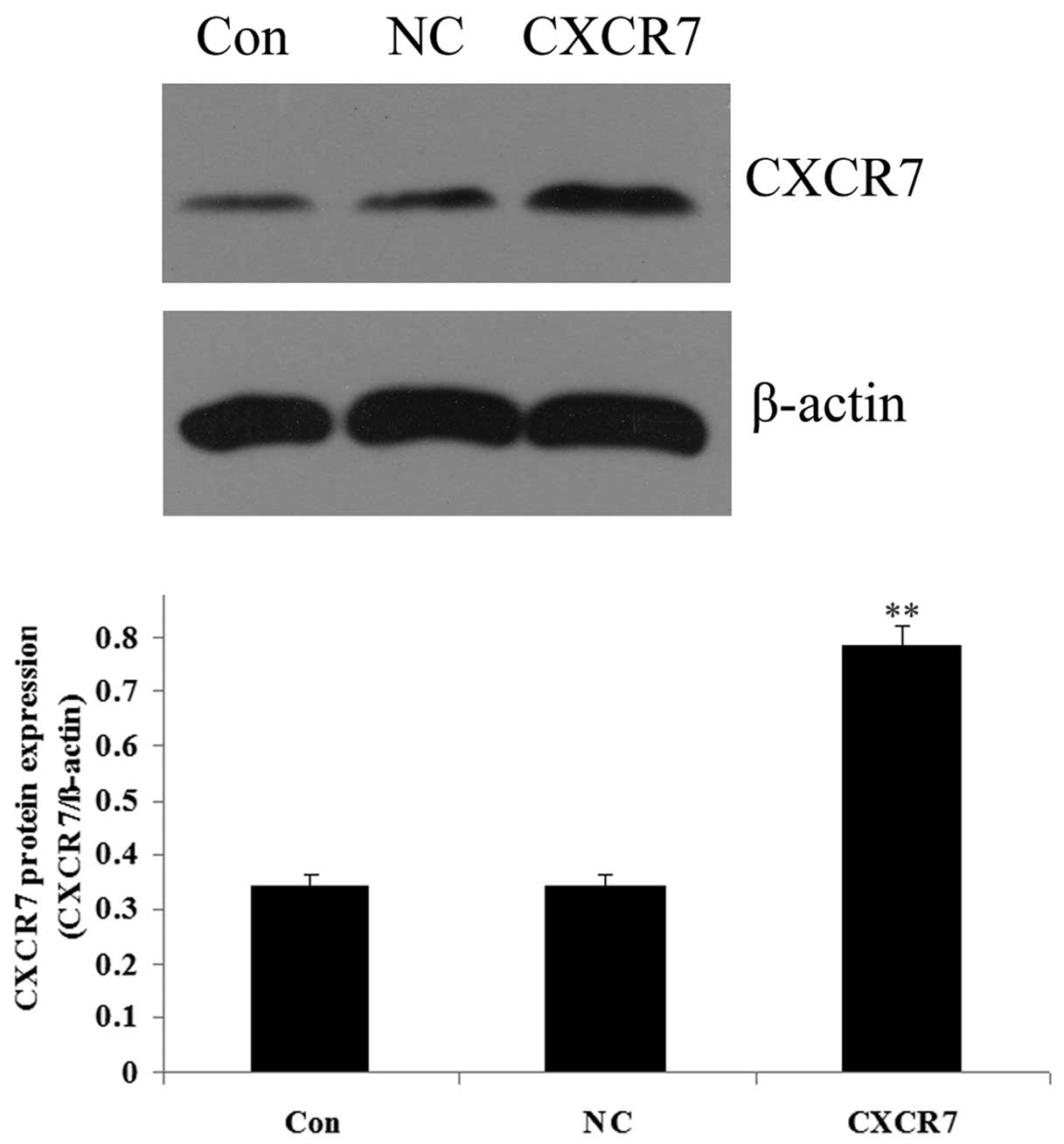
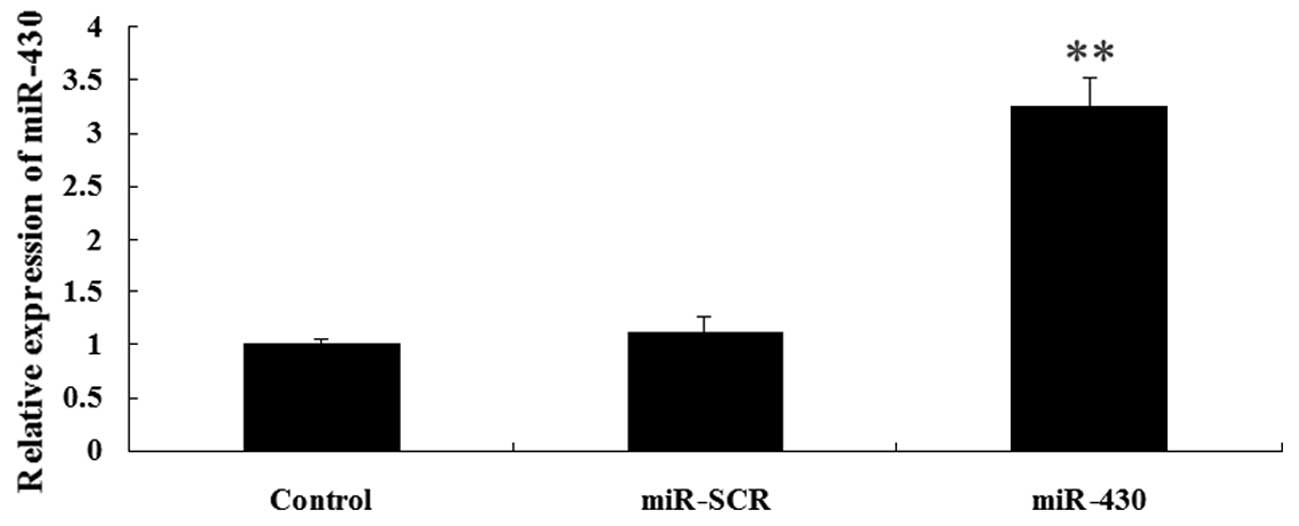
Expression of CXCR7 and miR-430 following
transfection
As demonstrated in Fig.
3, the results from western blotting showed that the protein
level of CXCR7 was significantly increased in the 5637 cells
following transfection with the CXCR7 overexpression plasmid
compared with the controls (P<0.01). The expression of miR-430
in 5637 cells was upregulated following transfection with miR-430
lentivirus vector compared with the controls (P<0.01; Fig. 4). These data indicated that we
successfully constructed the overexpression plasmids of CXCR7 and
miR-430, which could then be used in the subsequent
experiments.
Effect of miR-430 and CXCR7
overexpression on the proliferation of 5637 cells
Since the miR-430 expression level was lower in
bladder cancer tissue and CXCR7 was shown to be a direct target of
miR-430, we studied the effects of transfection with miR-430 and
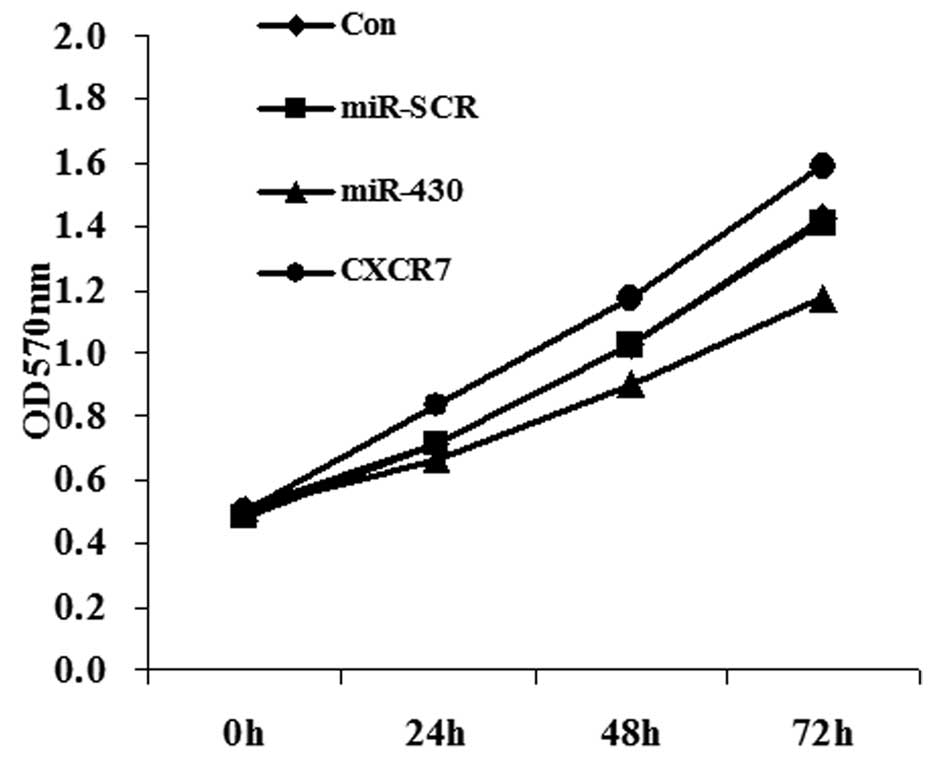
CXCR7 on 5637 cell proliferation. MTT data showed that miR-430
significantly inhibited cell proliferation while CXCR7 promoted
proliferation of 5367 cells, compared with the controls (Fig. 5).
Effect of miR-430 and CXCR7
overexpression on the cell cycle of 5637 cells
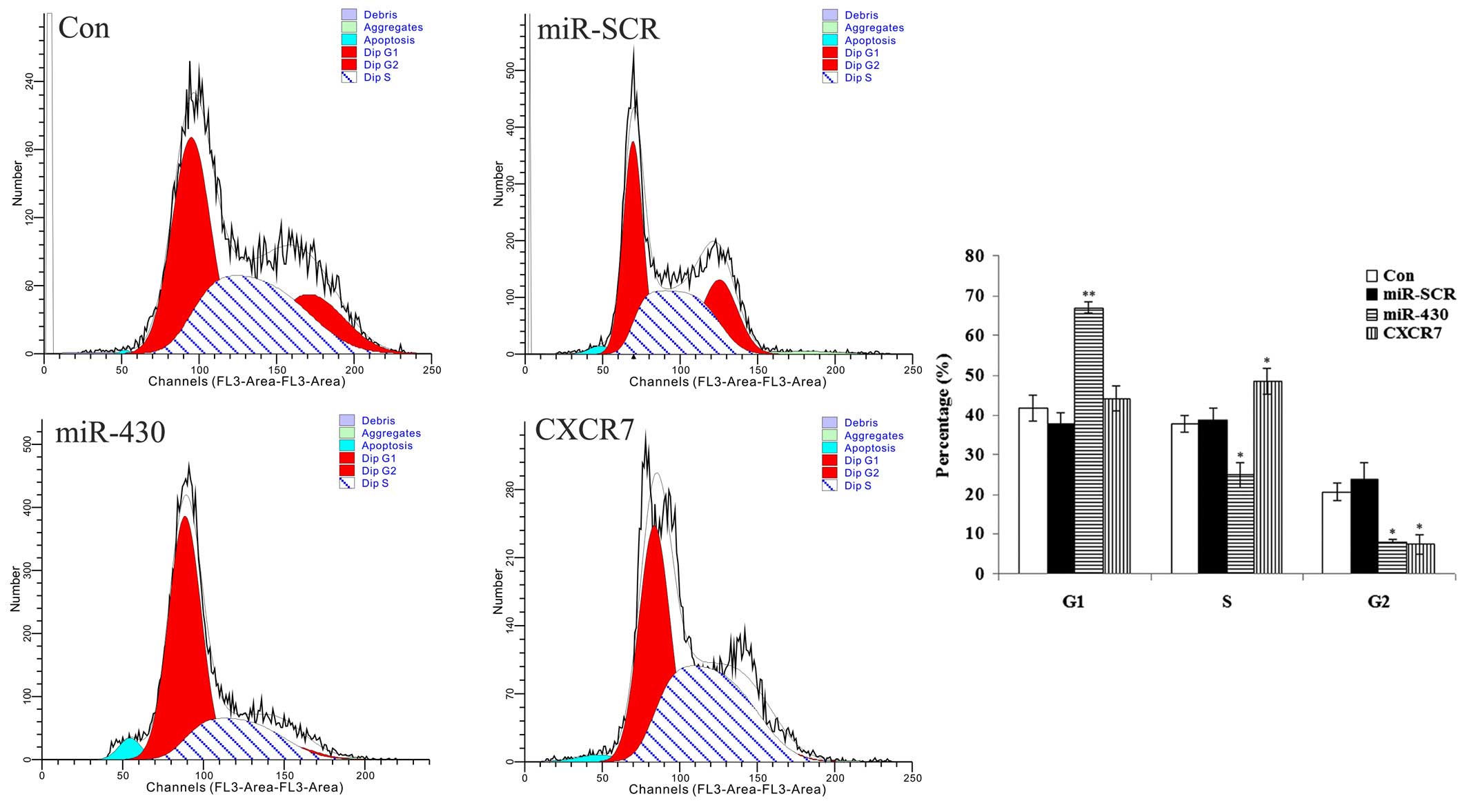
As shown in Fig. 6,
the 5637 cells transfected with various plasmids showed differences
in the cell cycle. The data demonstrated that the majority of the
cells transfected with miR-430 lentiviral vector were in the G1
phase, and the percentage in the S phase was lowest among all
groups, indicating that the cell cycle was blocked at the G1 phase.
For those cells transfected with the CXCR7 overexpression plasmid,
the data showed that the majority of the cells were in the G1 and S
phases, and only a few cells were in the G2 phase, indicating that
cell division was active. These results suggested that miR-430
suppressed the mitosis of 5637 cells. By contrast, CXCR7 promoted
the mitosis of 5637 cells.
Effect of miR-430 and CXCR7
overexpression on the migration of 5637 cells
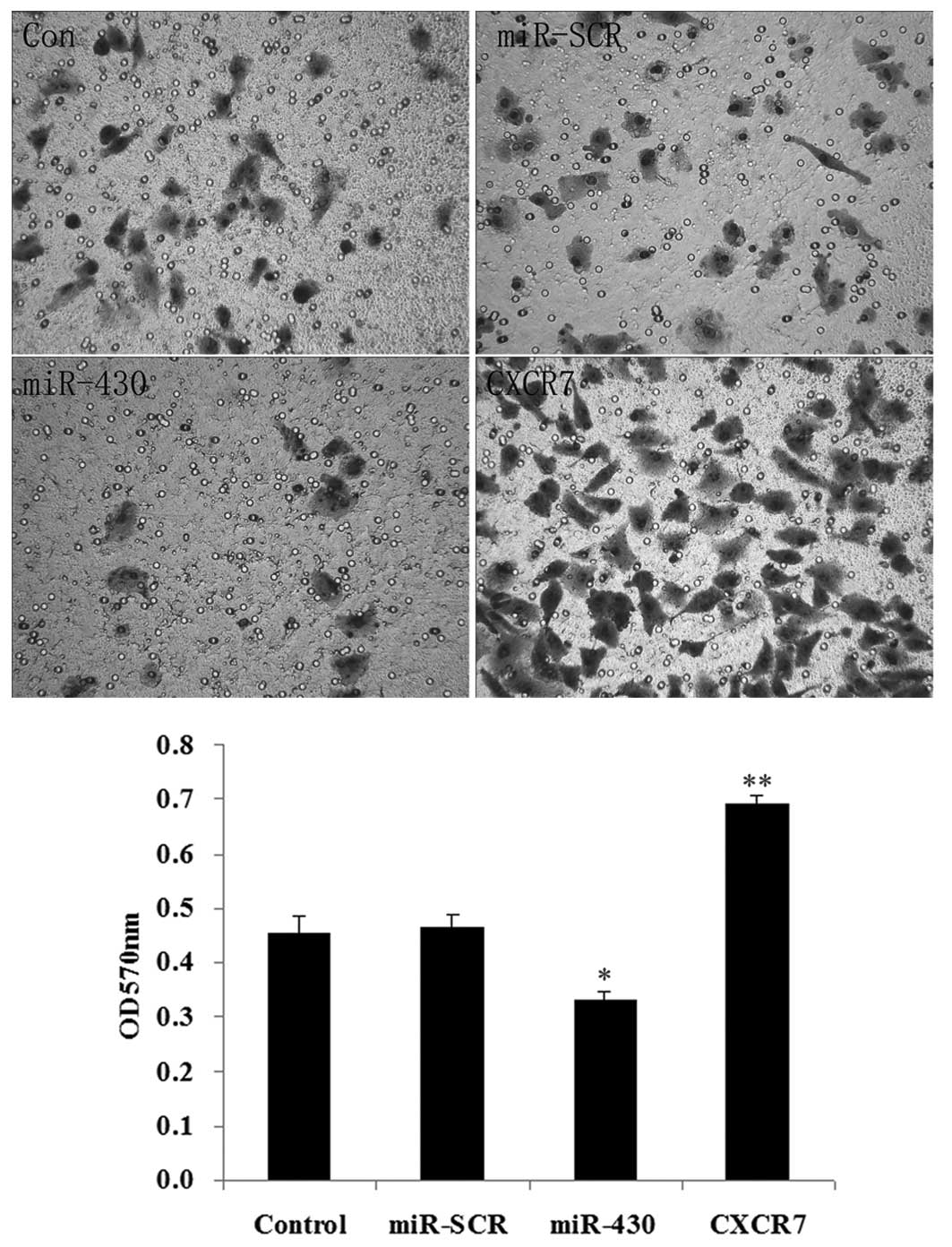
As shown in Fig. 7,
the overexpression of miR-430 had a negative effect on cell
migration (P<0.05), while the overexpression of CXCR7
significantly promoted cell migration (P<0.01).
Effect of miR-430 and CXCR7
overexpression on colony formation efficiency of 5637 cells
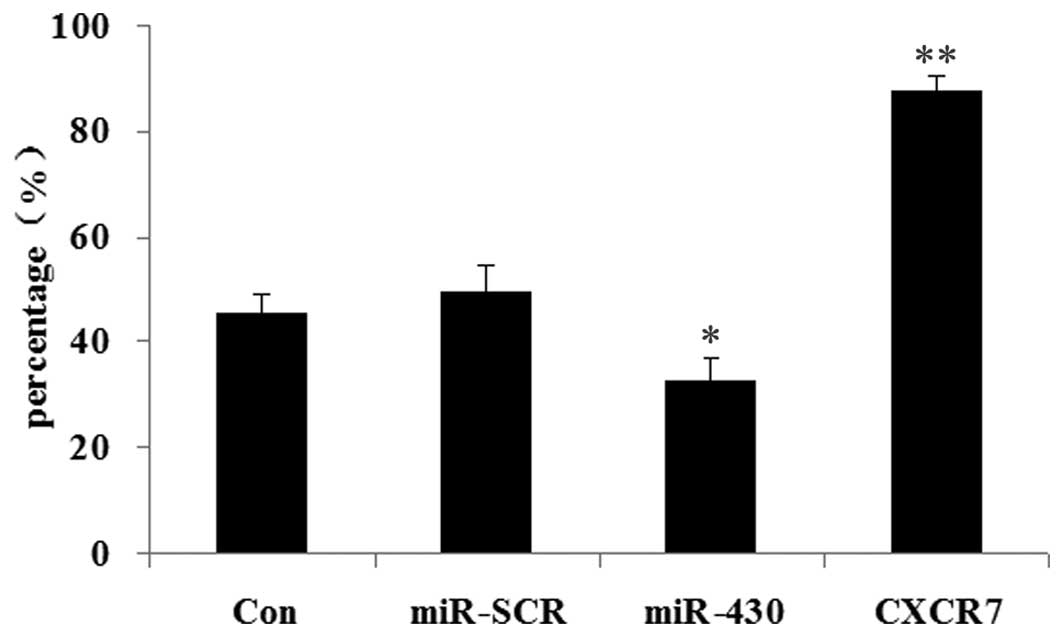
The effects of CXCR7 and miR-430 on colony formation
efficiency were then examined in 5637 cells. As shown in Fig. 8, when compared with the control
groups, those 5637 cells overexpressing miR-430 showed the lowest
colony formation efficiency (P<0.05). However, the 5637 cells
transfected with CXCR7 overexpression plasmid demonstrated the
highest colony formation efficiency (P<0.01).
Effect of miR-430 and CXCR7
overexpression on the protein expression of proliferation- and
migration-related genes
The protein expression of certain important
proliferation- and migration-related genes in the 5637 cells were
examined following transfection with different vectors. As shown in
Fig. 9, western blotting results
demonstrated that the expression of ERK, p-ERK, MMP-2 and MMP-9 was
decreased in cells transfected with miR-430 lentiviral vectors,
while it was upregulated in the cells transfected with CXCR7
overexpression plasmid compared with the control groups. These data
suggested that miR-430 inhibited cell proliferation and migration
via the suppression of the protein expression of ERK as well as its
phosphorylation level, and MMP-2 and MMP-9. By contrast, CXCR7
enhanced cell proliferation and migration through upregulating the
protein expression levels of the above genes.
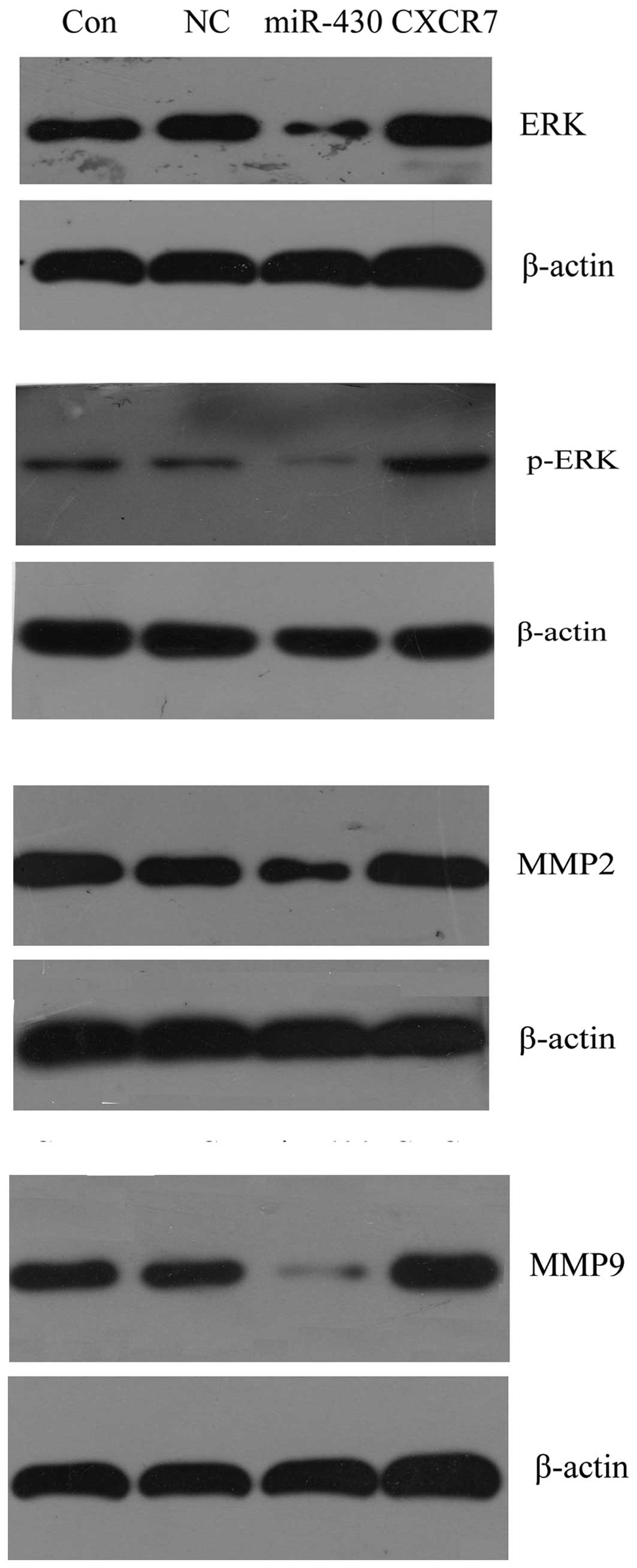 | Figure 9Western blotting was used to determine
the effect of miR-430 and CXCR7 overexpression on proliferation-
and migration-related gene expression in 5637 cells. Protein
expression of ERK, p-ERK, MMP-2 and MMP-9 were examined, and
β-actin was used as an internal reference. Con, normal 5637 cells;
NC, 5637 cells transfected with miR-SCR lentiviral vectors;
miR-430, 5637 cells transfected with miR-430 lentiviral vectors;
CXCR7, 5637 cells transfected with CXCR7 lentiviral vectors; miRNA,
microRNA; miR-430, miRNA-430; p-ERK, phosphorylated-ERK; MMP,
matrix metalloproteinase. |
Discussion
miRNAs inhibit gene expression mainly by interacting
with a specific site in the 3′-UTR of target mRNA (9). Several studies have demonstrated that
miRNAs are involved in various biological functions, including
tumorigenesis (10,11). The expression of various miRNAs was
markedly suppressed in human malignant tumors, thus they may
function as tumor suppressors (12,13).
miR-430 was previously demonstrated to be expressed at the onset of
zygotic transcription and involved in the regulation of brain
morphogenesis in zebrafish (14).
It was then shown to directly regulate several hundred target mRNAs
(15). In fact, studies on miR-430
were mainly focused in zebrafish and no previous study has revealed
its role in the development of human bladder cancer. To the best of
our knowledge, in the present study we report for the first time
that the expression of miRNA-430 was significantly decreased in
bladder cancer tissue, compared with normal bladder tissue and
adjacent tissue. Furthermore, in bladder cancer tissue, the
expression level of miR-430 was shown to be associated with
high-grade tumors.
CXCR7 is a specific receptor of SDF-1α (also known
as chemokine CXCL12), involved in the activation of the SDF-1α
pathway (6). Recently, increasing
evidence has reported that the activation of the SDF-1α pathway is
a potential mechanism of tumor resistance to conventional therapies
and biological agents (16). In
addition, CXCR7 is highly expressed on certain cancer cells,
including bladder cancer cells, and is involved in the regulation,
proliferation, migration and invasion of bladder cancer through
several signaling pathways, including ERK, Stat3 and AKT signaling
(7,8). In the present study, we identified
that CXCR7 is the direct target of miR-430. In fact, CXCR7 has been
reported to be the direct target of miR-430 in zebrafish; however,
to the best of our knowledge, this has not been reported in human
cells. Furthermore, according to this study and other studies
discussed previously, we hypothesize that the loss of the
endogenous CXCR7 inhibitor, miRNA-430, may contribute to the
development of bladder cancer through promoting the aberrant
expression of CXCR7. Thus, this study highlights the potential
essential role of miR-430 in the regulation of the development of
human bladder cancer.
Based on these findings, we further investigated the
roles of miR-430 and CXCR7 in human bladder 5637 cells. MTT data
demonstrated that miR-430 overexpression markedly decreased cell
proliferation while CXCR7 significantly promoted cell proliferation
in 5637 cells, which also supported our hypothesis in vitro.
In addition, the majority of the cells overexpressing miR-430 were
in the G1 phase, and the percentage of these cells in the S phase
was the lowest, indicating that the cell cycle was blocked. By
contrast, the majority of the cells overexpressing CXCR7 were in
the G1 and S phases, and only a few cells were in the G2 phase,
indicating that the division of these cells was in an active state.
In addition, the results of the cell migration assay and colony
formation assay suggested that miR-430 inhibited the cell migration
and colony formation efficiency of 5637 cells, while CXCR7 enhanced
them. All these in vitro cell experiments supported the
theory that miR-430 has a negative effect on proliferation, mitosis
and migration in bladder cancer cells, partly via inhibiting the
expression of CXCR7.
Molecular experiments were then conducted to
investigate the mechanism of miR-430 and CXCR7 in bladder cancer
5637 cells. To investigate the molecular regulatory pathway,
western blotting was performed to determine the proliferation- and
migration-relative protein expression of ERK, p-ERK, MMP-2 and
MMP-9 following the overexpression of miR-430 or CXCR7 in 5637
cells. miR-430 overexpression significantly decreased the protein
expression of ERK, p-ERK, MMP-2 and MMP-9; while CXCR7
overexpression markedly promoted ERK, p-ERK, MMP-2 and MMP-9
protein expression. Invasion and metastasis are complex biological
processes. Activation of MMP-2 and MMP-9 has been shown to be
important in the invasion and metastasis of cancer cells (17,18).
In malignant tumors, MMP-2 and MMP-9 have been shown to enhance
angiogenesis, an important biological process during the invasion
and metastasis of cancer cells (19,20).
In fact, the ERK signaling pathway is involved in the regulation of
MMP-2 and MMP-9 through several mechanisms, including at the
post-transcriptional, protein or cell surface localization level
(17,21,22).
As a result, our study showed that when overexpressed in 5637
cells, miR-430 suppresses this signaling pathway partly via
deregulating CXCR7. These findings revealed a novel regulatory
mechanism of miR-430 in human bladder cancer cells.
In conclusion, our study revealed a novel regulatory
pattern in bladder cancer, involving the correlation between
miR-430 and CXCR7. Using a luciferase assay, CXCR7 was confirmed to
be the direct target of miR-430. In vitro experiments at the
cellular and molecular levels further demonstrated that miR-430
deregulates proliferation and metastasis in bladder cancer cells,
partly via suppressing the expression of CXCR7. As a result,
miR-430 and CXCR7 may become promising molecular targets for the
treatment of human bladder cancer.
References
|
1
|
Ambros V: The functions of animal
microRNAs. Nature. 431:350–355. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Garzon R, Marcucci G and Croce CM:
Targeting microRNAs in cancer: rationale, strategies and
challenges. Nat Rev Drug Discov. 9:775–789. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Wei C, Salichos L, Wittgrove CM, Rokas A
and Patton JG: Transcriptome-wide analysis of small RNA expression
in early zebrafish development. RNA. 18:915–929. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Tani S, Kusakabe R, Naruse K, Sakamoto H
and Inoue K: Genomic organization and embryonic expression of
miR-430 in medaka (Oryzias latipes): insights into the
post-transcriptional gene regulation in early development. Gene.
449:41–49. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Mishima Y, Giraldez AJ, Takeda Y, et al:
Differential regulation of germline mRNAs in soma and germ cells by
zebrafish miR-430. Curr Biol. 16:2135–2142. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Sánchez-Martín L, Sánchez-Mateos P and
Cabañas C: CXCR7 impact on CXCL12 biology and disease. Trends Mol
Med. 19:12–22. 2012.PubMed/NCBI
|
|
7
|
Yates TJ, Knapp J, Gosalbez M, et al:
C-X-C chemokine receptor 7: a functionally associated molecular
marker for bladder cancer. Cancer. 119:61–71. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Hao M, Zheng J, Hou K, et al: Role of
chemokine receptor CXCR7 in bladder cancer progression. Biochem
Pharmacol. 84:204–214. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Zhao Y, Tang L, Nie W, Wang Z and Guan X:
Functional variants at the miRNA binding sites of the E2F1 gene and
its mRNA expression. Oncol Lett. 5:398–402. 2013.PubMed/NCBI
|
|
10
|
Liang YJ, Wang QY, Zhou CX, et al: MiR-124
targets slug to regulate epithelial-to-mesenchymal transition and
metastasis of breast cancer. Carcinogenesis. Jan 12–2012.(Epub
ahead of print).
|
|
11
|
Zabaleta J: MicroRNA: A bridge from H.
pylori infection to gastritis and gastric cancer development.
Front Genet. 3:2942012.PubMed/NCBI
|
|
12
|
Qiu MT, Hu JW, Ding XX, et al: Hsa-miR-499
rs3746444 polymorphism contributes to cancer risk: a meta-analysis
of 12 studies. PLoS One. 7:e508872012. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Kobayashi N, Uemura H, Nagahama K, et al:
Identification of miR-30d as a novel prognostic maker of prostate
cancer. Oncotarget. 3:1455–1471. 2012.PubMed/NCBI
|
|
14
|
Giraldez AJ, Cinalli RM, Glasner ME, et
al: MicroRNAs regulate brain morphogenesis in zebrafish. Science.
308:833–838. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Giraldez AJ, Mishima Y, Rihel J, et al:
Zebrafish MiR-430 promotes deadenylation and clearance of maternal
mRNAs. Science. 312:75–79. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Duda DG, Kozin SV, Kirkpatrick ND, Xu L,
Fukumura D and Jain RK: CXCL12 (SDF1alpha)-CXCR4/CXCR7 pathway
inhibition: an emerging sensitizer for anticancer therapies? Clin
Cancer Res. 17:2074–2080. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Bauvois B: New facets of matrix
metalloproteinases MMP-2 and MMP-9 as cell surface transducers:
outside-in signaling and relationship to tumor progression. Biochim
Biophys Acta. 1825:29–36. 2012.PubMed/NCBI
|
|
18
|
Groblewska M, Siewko M, Mroczko B and
Szmitkowski M: The role of matrix metalloproteinases (MMPs) and
their inhibitors (TIMPs) in the development of esophageal cancer.
Folia Histochem Cytobiol. 50:12–19. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Siefert SA and Sarkar R: Matrix
metalloproteinases in vascular physiology and disease. Vascular.
20:210–216. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Grange C, Tapparo M, Collino F, et al:
Microvesicles released from human renal cancer stem cells stimulate
angiogenesis and formation of lung premetastatic niche. Cancer Res.
71:5346–5356. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Rietz A and Spiers J: The relationship
between the MMP system, adrenoceptors and phosphoprotein
phosphatases. Br J Pharmacol. 166:1225–1243. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Mannello F and Medda V: Nuclear
localization of matrix metalloproteinases. Prog Histochem Cytochem.
47:27–58. 2012. View Article : Google Scholar
|