Introduction
Asthma is a major cause of chronic morbidity and
mortality worldwide. The prevalence of asthma has increased
considerably over the past two decades, particularly in children
(1). Chronic inflammatory
disorders and consequent airway hyperreactivity are critical
processes in asthma. The inflammatory response and mucus plugs in
the airway cause airflow obstruction, and consequently induce the
onset of asthma (2). The
development of asthma is associated with the production of
immunoglobulin E (IgE) and Th2 cytokines, including interleukin
(IL)-4, -5 and -13 (3). In a
previous study, IL-5 deficiency was found to eliminate airway
hyperreactivity in mice with asthma (4). The disequilibrium between Thl and
Th2, usually demonstrated by Th2 cell hyperfunction, is considered
to be one of the most important immunological pathogeneses of
bronchial asthma. Th2 cell-derived cytokines and chemokines,
including IL-4, -5, -6, -9 and -13 and granulocyte-macrophage
colony-stimulating factor, promote the recruitment and activation
of eosinophils (EOS), the production of IgE, and thus cause chronic
inflammation and higher reactivity of airways (3). IL-5 functions as an important
cytokine in the effector phase of asthma, and inhibition of the
expression or function of IL-5 has been hypothesized to represent a
promising mechanism for asthma treatment (5,6).
GATA binding protein 3 (GATA-3) is a zinc finger transcription
factor, which enhances IL-5 expression by binding to a specific
motif of the IL-5 promoter in Th2 cells (7–9).
Changes in GATA-3 expression and function are commonly accompanied
by altered levels of IL-5, indicating that GATA-3 may represent an
important agent for the regulation of IL-5 expression (10).
Suplatast tosilate (IPD),
(2-(4-(3-ethoxy-2-hydroxypropoxy)phenylcarbamoyl)ethyl)dimethylsulfonium
p-toluenesulfonate, is a novel and unique compound that suppresses
cytokine production from Th2 cells (11). The compound has been reported to
inhibit IL-5 and IL-4 synthesis and EOS inflammation, reduce IgE
antibody titer, attenuate labrocyte degranulation and therefore
improve the inflammatory response and hyperreactivity of airways
(11–13). IPD has also been found to induce a
marked effect through its metabolic product,
4-(3-ethoxy-2-hydroxypropoxy) acrylanilide (M-1) (14,15).
In addition, IPD has been revealed to intercept histoleucocyte to
dendritic cell differentiation, and thus also dendritic cell
maturity and function, and induces Th1 reaction reinforcement in
patients with asthma (16). Its
effective target may be associated with a chloride channel located
on the blood EOS surface (17).
Clinical analysis has confirmed that IPD reduces asthma (including
hormone-dependent and cough variant asthma) symptom score, improves
lung function and upregulates β2 receptor levels resulting in a
smaller dose of β2-agonist being applied, which was named ‘saving
hormone’ (18).
To date, the specific molecular mechanisms of IPD
action in asthma treatment remain unclear. The current study aimed
to observe the effect of IPD on asthma and determine the mechanisms
associated with the GATA-3/IL-5 signaling pathway in rat models by
intraperitoneal injection with ovalbumin (OVA). Airway
resistance, the percentage of EOS in peripheral blood and
expression of GATA-3 and IL-5 were observed. To ensure the results
of this study were accurate, the classical anti-asthma
glucocorticoid drug, budesonide (BUD), was used as a
comparison.
Materials and methods
Animals and drugs
Four-week-old healthy male Sprague-Dawley rats of a
specific pathogen-free grade, weighing 200±20 g, were purchased
from the Animal Department of Hunan Agricultural University
(Changsha, China). IPD was synthesized and supplied by Beijing
Sinocro PharmaScience Co., Ltd. (Beijing, China). BUN was supplied
by AstraZeneca (London, UK). IL-5 and GATA-3 antibodies were
purchased from Cell Signaling Technology, Inc. (Danvers, MA, USA).
OVA was provided by Sigma-Aldrich (St. Louis, MO, USA). An
enzyme-linked immunosorbent assay (ELISA) kit was obtained from
Wuhan Boster Biological Technology, Ltd. (Wuhan, China). The study
was approved by the ethics committee of Hunan Provincial People’s
Hospital, The First Affiliated Hospital of Hunan Normal University
(Changsha, China).
Experimental group and protocol
Rats were divided randomly into 5 groups: control,
OVA, BUD, continuous intervention with IPD (C-IPD) and late
intervention with IPD (L-IPD).
Sensitization was performed as described previously,
with certain modifications (19).
All the groups, with the exception of control group rats, were
sensitized by intraperitoneal injection with 100 mg OVA, 100 mg
aluminum hydroxide and the 5×109 inactive pertussis
bacillus, on days 1 and 8. Control rats were administered
physiological normal saline (NS) instead of OVA. In the C-IPD
group, the rats were intragastrically administered IPD (50
mg/kg/day; dissolved in NS) from day 1, while rats in the remaining
groups were treated with the same doses of NS, once per day for 2
weeks.
Following 2 weeks, sensitized rats were treated as
follows: control rats were administered 2 ml NS by pump atomization
for 10 min; OVA rats received 2 ml 2% OVA by pump atomization for
10 min to maintain asthma; BUD rats were administered 0.64 ml/kg
BUD solution, followed by 2 ml 2% OVA by pump atomization 1 h
later; C-IPD and L-IPD rats received 50 mg/kg IPD intragastrically,
followed by 2 ml 2% OVA by pump atomization 1 h later. All the
treatments were performed once per day for 7 days.
Measurement of airway resistance
Following the indicated treatments, airway
resistance was measured by recording respiratory pressure curves
using whole body plethysmography (Buxco Research Systems,
Wilmington, NC, USA), as described previously (20). Rats were anesthetized by
intraperitoneal injection of 10% chloral hydrate solution (4 ml/kg
body weight). The anesthetized rats were fixed in a supine position
on a wooden fixation plate. The trachea was exposed by centrally
cutting the skin at the neck and endotracheal intubation was
performed. The rats were put into a sealed container to monitor the
airway pressure and baseline resistance was recorded for 5 min.
After an inhalation aerosol delivery system was embedded, rats were
exposed to NS or various concentrations of histamine
dihydrochloride (0.32–2.56 mg/ml) for 20 sec and airway resistance
values were recorded. Prior to recording the next pressure curve,
resistance values were allowed to return The data displayed in
whole body plethysmography were collected and transformed into
airway resistance values.
Determination of the percentage of EOS in
peripheral blood
Following the indicated treatments, rats of all
groups were stimulated with 2.56 mg/ml histamine dihydrochloride.
Whole peripheral blood was collected via the rat vena caudalis and
EOS percentage in the blood was investigated at day 1
(pre-experiment) and day 21 (post-experiment), respectively.
Subsequently, 100 μl whole peripheral blood was dropped on a slide
and dispersed uniformly. Giemsa staining solution (50 μl) was added
to the slide and the same dose of balanced solution was added.
After the blood on the slide was dry, 100 leucocytes were observed
under an optical microscope and the number of EOS was recorded.
Hematoxylin and eosin (H&E)
staining
Lung tissue was removed and fixed with 4%
paraformaldehyde. Resin-embedded specimens were cut into 2-μm
slices, mounted onto slides and stained with H&E, as described
previously (21). The stained
slices were examined under a light microscope (BX-41; Olympus,
Tokyo, Japan). Images of the intrapulmonary airway epithelium
(large airway) were recorded from 5 consecutive high-power
fields.
Western blot analysis for IL-5 and GATA-3
expression
Lung tissue homogenates were dissociated in 1 ml
RIPA buffer for 30 min and then centrifuged at 12,000 × g for 15
min at 4°C. Following quantification with a BCA protein assay kit
(KangChen Bio-tech Inc., Shanghai, China), 10 μg total protein from
each sample was mixed with loading buffer. Samples were separated
by 10% SDS-PAGE and transferred onto PVDF membranes. Membranes were
blocked with 5% fat-free milk in TBS-T for 1 h at room temperature,
followed by incubation with primary antibodies against IL-5 or
GATA-3 for 12 h at 4°C. Next, the membrane was incubated for 1 h at
room temperature with specific horseradish peroxidase-conjugated
secondary antibodies. Signals were detected using an enhanced
chemiluminescence system according to the manufacturer’s
instructions (Applygen Technologies, Peking, China). Protein
expression was quantified by scanning the X-ray films and analyzing
with ImageJ 1.47d software (22).
Reverse transcription polymerase chain
reaction (RT-PCR)
Following the indicated treatments, lung tissue was
collected from the rats. Total RNA was extracted with TRIzol
reagent and then reverse-transcribed into cDNA. IL-5 mRNA splicing
was indirectly assessed by PCR. Primers spanning the splice site
were designed as follows: forward, TGCTTCTGTGCT TGAACGTTCTAAC and
reverse, TTCTCTTTTTGTCCGT CAATGTATTTC. The primers for GAPDH were
designed as follows: forward, CAAGGTCCATGACAACTTTG and reverse,
GTCCACCCTGTTGCTGTAG. PCR products were electrophoretically resolved
on 2% agarose gel and observed under UV illumination.
Detection of IL-5 in bronchoalveolar
lavage fluid (BALF) with ELISA
Phosphate-buffered saline (0.5 ml) was injected into
the trachea via a cannula and this procedure was repeated 3 times.
BALF was collected and stored at −80°C until use. The concentration
of IL-5 in BALF and specific concentration of standard IL-5
provided by the kit was determined by ELISA according to the
manufacturer’s instructions (R&D Systems, Minneapolis, MN,
USA). The optical density was determined using a microplate reader
(EL340; BioTek Instruments, Inc., Winooski, VT, USA) at 450 nm.
Statistical analysis
All data are presented as the mean ± SD.
Statistically significant differences between groups were analyzed
by one-way analysis of variance with SPSS 16.0 (SPSS Inc., Chicago,
IL, USA). P<0.05 was considered to indicate a statistically
significant difference.
Results
IPD inhibits airway hyperreactivity in
asthmatic rats
As demonstrated in Table I, following the indicated
treatments, rats were exposed to histamine dihydrochloride at
increasing concentrations via an embedded inhalation aerosol
delivery system. Airway resistance of the OVA-injected rats was
found to be significantly augmented compared with the control rats.
Under the same challenge concentrations, airway resistance levels
were markedly decreased by the administration of BUD, a widely used
anti-asthmatic agent. Notably, treatment with C-IPD or L-IPD
markedly alleviated OVA-induced airway resistance elevation. No
statistical significance was observed between airway resistance
levels in C-IPD and L-IPD groups (P>0.05).
 | Table IEffect of various treatments on airway
resistance in histamine dihydrochloride stimulated-rats |
Table I
Effect of various treatments on airway
resistance in histamine dihydrochloride stimulated-rats
| | | Histamine
dihydrochloride (mg/ml) |
|---|
| | |
|
|---|
| Group | n | Saline | 0.32 | 0.64 | 1.28 | 2.56 |
|---|
| Control | 10 | 1 | 1.09±0.03 | 1.35±0.21 | 1.90±0.10 | 2.07±0.12 |
| OVA | 10 | 1 | 1.62±0.17a | 2.24±0.24a | 3.04±0.23a | 4.27±0.42a |
| BUD | 10 | 1 | 1.23±0.12c | 1.71±0.30c | 2.10±0.16c | 2.93±0.38c |
| C-IPD | 9 | 1 | 1.26±0.10c | 1.85±0.31c | 2.16±0.18c | 2.97±0.39c |
| L-IPD | 10 | 1 | 1.34±0.17b | 1.97±0.22b | 2.27±0.16c | 3.20±0.30c |
Rats were administered with the indicated treatments
and stimulated by various concentrations of histamine
dihydrochloride prior to recording airway resistance levels.
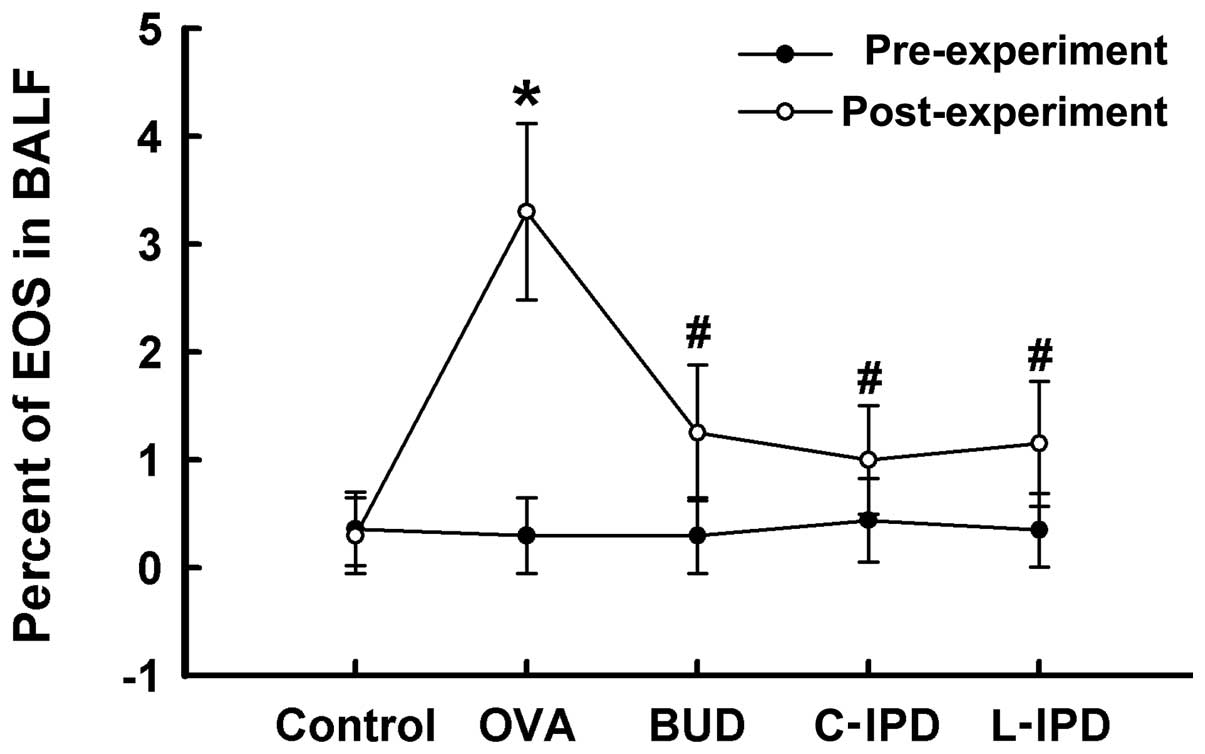
IPD decreases the percentage of EOS in
peripheral blood
Prior to sensitization by OVA, the percentage of EOS
in peripheral blood among the groups was not found to be
statistically significant (P>0.05). However, following
sensitization, the percentage of EOS in peripheral blood was
markedly increased compared with that in the control group
(P<0.01). C-IPD and L-IPD were found to markedly reduce the
percentage of EOS caused by OVA exposure, which was similar to the
effect of BUD (Fig. 1).
IPD improves morphological changes of
lung tissue in asthmatic rats
As sensitization by OVA markedly enhanced levels of
airway resistance, we determined whether airway hyperreactivity is
due to changes in the structure of lung tissue using H&E
staining. As demonstrated in Fig.
2, lung tissue samples obtained from rats intraperitoneally
injected with OVA demonstrated marked thickening of the epithelium,
goblet cell hyperplasia and submucosal cell infiltration (Fig. 2B). Similar to the effect of
intervention with BUD on the injured lung tissue (Fig. 2C), intervention with C-IPD
(Fig. 2D) or L-IPD (Fig. 2E) markedly reduced these structural
changes.
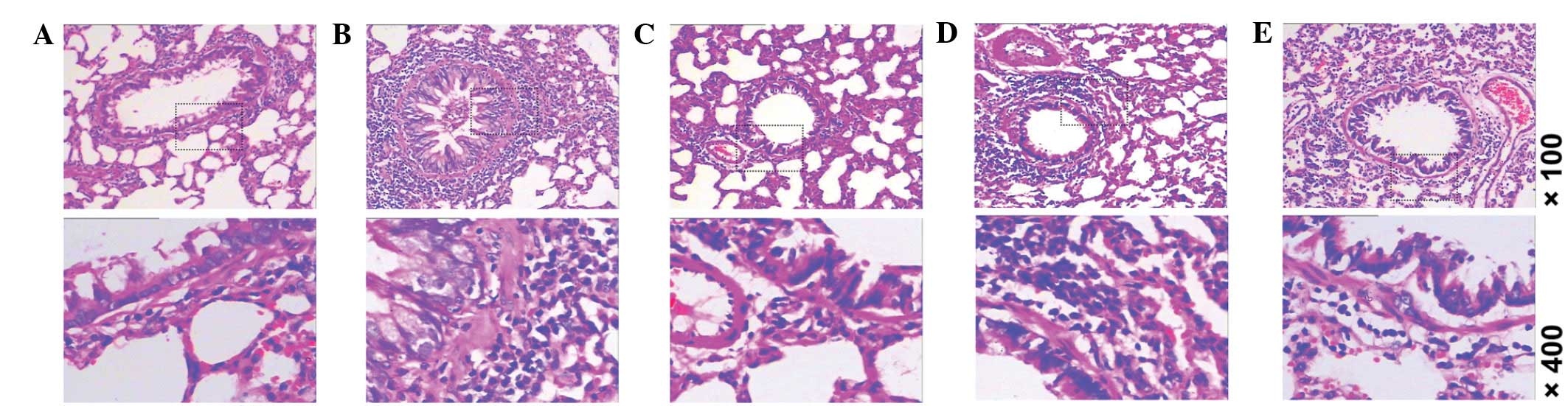 | Figure 2Morphological changes in lung tissue,
as detected by H&E staining. (A) Control, (B) OVA, (C) BUD, (D)
C-IPD and (E) L-IPD (top row magnification, ×100; bottom row,
×400). H&E, hematoxylin and eosin; IPD, suplatast tosilate;
OVA, ovalbumin; BUD, budesonide; C, continuous; L, late-stage. |
IL-5 is involved in the action of IPD in
ameliorating OVA-induced airway hyperreactivity
IL-5 expression in lung tissue at the transcription
and translation level was detected and results indicated that,
following sensitization by OVA, IL-5 mRNA (Fig. 3A and B) and protein (Fig. 3C and D) levels were markedly
enhanced in the lung tissue of OVA group rats. Increased expression
of mRNA and protein was significantly attenuated by the
administration of C-IPD or L-IPD, as well as BUD.
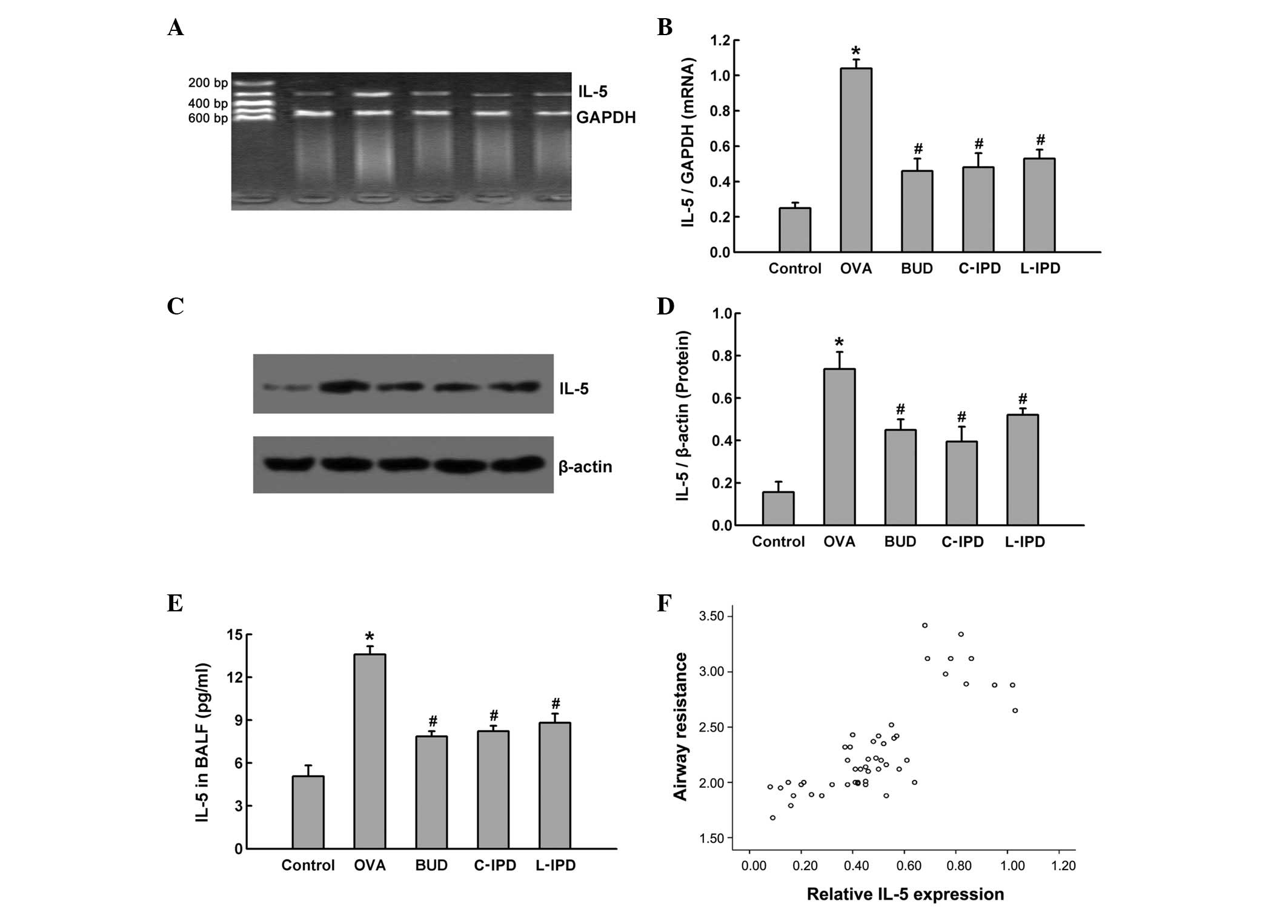 | Figure 3Role of IL-5 in the anti-asthma
effects of IPD. Rats were divided into 5 groups: control, OVA, BUD,
C-IPD and L-IPD. Following treatment, lung tissue was removed to
extract RNA and protein. IL-5 mRNA levels were detected by (A)
RT-PCR and (B) quantified. IL-5 protein levels were measured by (C)
western blot analysis and (D) analyzed by densitometry. (E) IL-5
levels were detected in BALF by ELISA. (F) Correlation analysis
between the expression of IL-5 in lung tissue and airway resistance
was performed. Data are presented as the mean ± SD, n=10.
*P<0.01 vs. control; #P<0.05 vs. OVA.
IPD, suplatast tosilate; OVA, ovalbumin; BUD, budesonide; C,
continuous; L, late-stage; IL, interleukin; BALF, bronchoalveolar
lavage fluid. |
Next, BALF samples were analyzed for IL-5 levels in
BALF using ELISA. Following sensitization, IL-5 levels in the BALF
of OVA group rats were markedly increased and this elevation was
found to be partially blocked by C-IPD, L-IPD and BUD (Fig. 3E). These results indicated that OVA
not only induced IL-5 expression, but also enhanced its secretion,
and treatment with IPD suppressed IL-5 expression and
secretion.
In addition, the association between increased
levels of IL-5 and airway hyperreactivity was investigated by
correlation analysis between IL-5 protein expression in lung tissue
and airway resistance. The correlation coefficient was 0.909
(Fig. 3F), indicating that airway
hyperreactivity may be associated with increased IL-5 levels, and
the inhibition of IL-5 by IPD ameliorates the OVA-induced airway
hyperreactivity.
GATA-3 protein regulates IL-5 expression
in the OVA-induced asthma model
To determine which transcription factors regulate
IL-5 expression, GATA-3 expression was analyzed at the protein
level. Following sensitization by OVA, GATA-3 expression was
markedly increased, and this was found to be reduced by
intervention with C-IPD, L-IPD or BUD (Fig. 4A and B). The correlation between
GATA-3 expression in the lungs and airway hyperreactivity or IL-5
expression was determined and the correlation coefficients were
0.775 (Fig. 4C) and 0.828
(Fig. 4D), respectively. These
results indicated that increased airway resistance and IL-5
expression may be mediated by increased GATA-3 expression, and
inhibition of GATA-3 expression by C-IPD, L-IPD or BUD ameliorates
airway hyperreactivity and IL-5 expression.
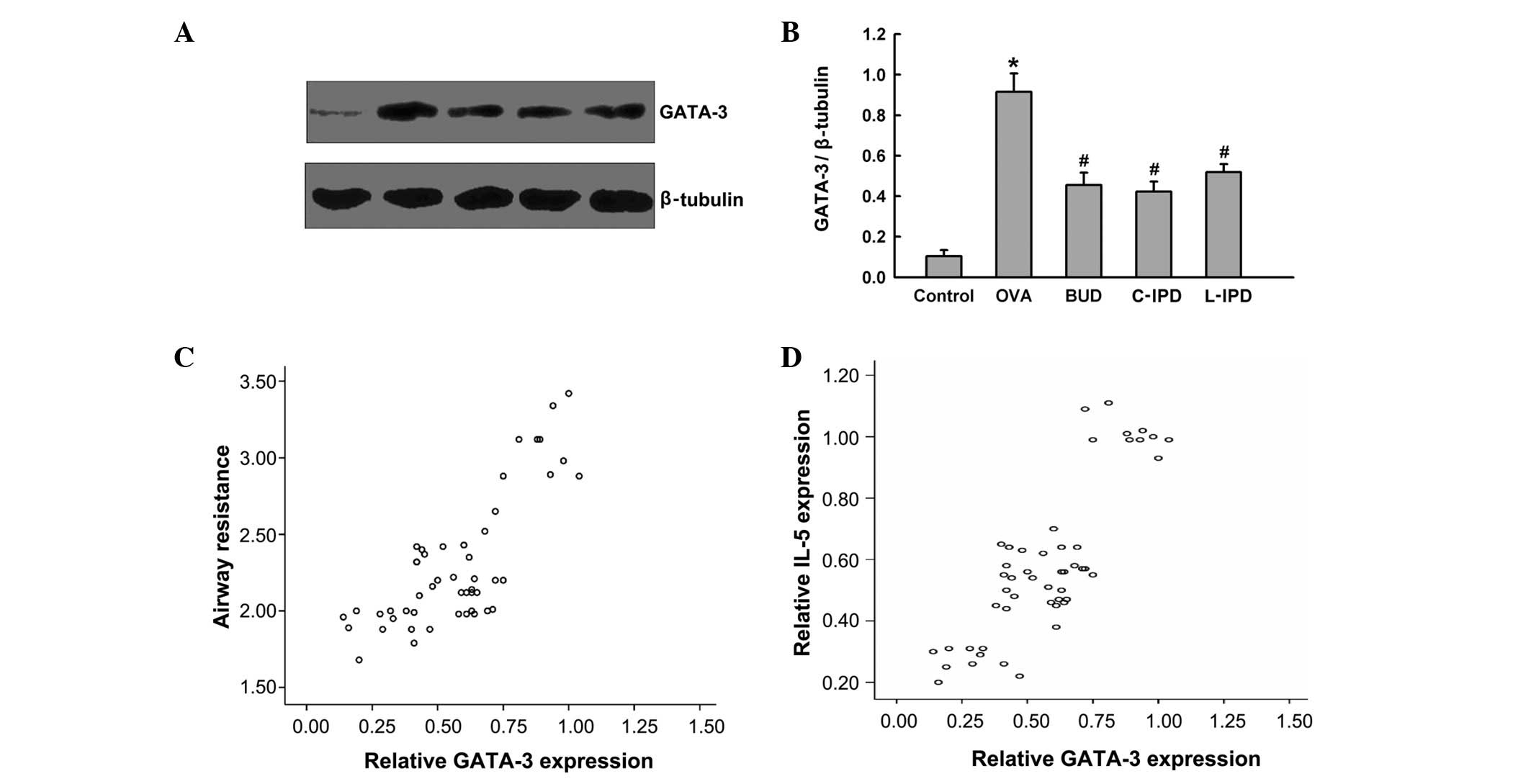 | Figure 4Role of GATA-3 in the anti-asthma
effects of IPD. Rats were divided into 5 groups: control, OVA, BUD,
C-IPD and L-IPD. Following treatment, nuclear proteins from lung
tissues were extracted and (A) GATA-3 expression was determined by
western blot analysis and (B) analyzed by densitometry. The
correlation between GATA-3 expression and (C) airway resistance or
(D) IL-5 expression was determined. Data are presented as the mean
± SD, n=10. *P<0.01 vs. control;
#P<0.05 vs. OVA. GATA-3, GATA binding protein 3; IPD,
suplatast tosilate; OVA, ovalbumin; BUD, budesonide; C, continuous;
L, late-stage; IL, interleukin. |
Discussion
Bronchial asthma is one of the most common allergic
disorders in humans and in recent years, disease incidence and
mortality rates have increased rapidly. Therefore, understanding
the pathogenesis of bronchial asthma is extremely important for the
development of methods to prevent and treat this disease. Studies
in animals are useful for the identification of etiological
factors, exploration of disease mechanisms and evaluation of
therapeutic effects (23,24). In the current study, Sprague-Dawley
rats were treated according to a previous study by Matsumoto et
al(19) to establish a model
of asthma. OVA was injected intraperitoneally as an anaphylactogen,
combined with aluminum hydroxide and inactive Bacillus
pertussis (an immune adjuvant) at days 1 and 8 to sensitize the
rats (25). Following
sensitization, severe symptoms of asthma were observed in rats of
the OVA group only. In addition, airway resistance was markedly
increased compared with levels prior to sensitization, indicating
hyperreactivity in the airways. The percentage of EOS in peripheral
blood was also increased. Pathological analysis of lung tissue was
performed, revealing increased levels of IL-5 and GATA-3,
indicative of a successful model of asthma.
Previous studies have demonstrated that the blockade
of Th2 cytokines may represent a major therapeutic target in
asthma. Inhibition of Th2 cytokines using monoclonal antibodies
against IL-5 and IL-3 and an IL-4 receptor antagonist was
demonstrated to improve asthma symptoms (26,27).
In addition, IPD has been reported to suppress Th2 cytokines,
including IL-5 and IL-4, and inhibit EOS inflammation, resulting in
a decrease in IgE antibody titer (20). Results of the current study
revealed that C-IPD and L-IPD treatment attenuated airway
resistance, similar to the classical anti-asthma drug, BUD,
indicating that hyperreactivity of the airway was ameliorated. In
addition, the systemic inflammatory response was analyzed by
calculating the percentage of EOS in the peripheral blood, and
C-IPD and L-IPD treatments were found to reduce the percentage of
EOS. H&E staining also revealed that IPD inhibited labrocyte
degranulation and mediators of inflammation secretion. Previously,
Sano et al(12) reported
that IPD represses Th2 cytokine-induced allergic inflammation.
Results of the present study were consistent with previous studies
(13–15). In addition, IPD has been
administered in clinical studies and was found to improve the
clinical symptoms and lung function of patients. Early IPD
treatment was also revealed to prevent food allergies or
sensitization dermatitis (28).
Consistent with these observations, results of the current study
indicated that the late administration of IPD has the same effect
on asthma as continuous intervention with IPD.
Since IPD was found to suppress the airway
inflammatory response and hyperreactivity associated with Th2
cytokines, and as IL-5 is one of the most important Th2 cytokines,
the effect of IL-5 inhibition on IPD-induced decreases in airway
resistance and the percentage of EOS in peripheral blood was
investigated by analysis of IL-5 mRNA and protein expression in the
lung tissue. The results demonstrated that, following sensitization
with OVA, IL-5 mRNA and protein levels were markedly elevated. In
addition, IL-5 levels in BALF were analyzed by ELISA, and IL-5
release levels were also observed to be enhanced. Notably,
treatment with continuous or late-stage IPD blocked IL-5 expression
and its release induced by OVA. To confirm that IPD-induced IL-5
downregulation is associated with ameliorated OVA-induced
hyperreactivity of the airway, a correlation analysis was performed
between IL-5 expression in the lung tissue and airway resistance.
The results revealed that airway resistance positively correlated
with IL-5 expression, indicating that IPD-induced attenuation of
airway hyperreactivity was due to inhibition of IL-5 expression. In
a previous study, IL-5 expression was markedly enhanced during
inflammation in atopic asthma (29). Till et al(30) also found that IL-5 and EOS levels
were increased in the peripheral blood and BALF of asthma patients
and there was a positive correlation between the two parameters. In
addition, Shi et al(31)
reported that, following the inhalation of IL-5, the percentage of
EOS and airway hyperreactivity were significantly increased in
asthma patients. These observations and the present study indicate
that increased IL-5 levels mediate the inflammatory response in
asthma patients and the inhibition of IL-5 biosynthesis and release
represents an important mechanism by which IPD treats asthma. To
ensure the results of this study were accurate, rats were exposed
to BUD by pump atomization and BUD was also found to reduce IL-5
expression and release, similar to treatment with IPD.
GATA-3 is an important transcription factor that
regulates the expression of a number of genes at the transcription
level in Th2 cells (32–34). A GATA-3 binding site was previously
identified in the promotor region of the IL-5 gene (35) and therefore, we hypothesized that
OVA-induced asthma and the upregulation of IL-5 in rats is
associated with GATA-3. To examine this hypothesis, the expression
of GATA-3 was analyzed in the lung tissue and treatment with OVA
was found to markedly enhance GATA-3 protein expression in rats.
Notably, increased GATA-3 levels were found to positively correlate
with airway resistance and IL-5 expression. The results indicated
that the upregulation of IL-5 and hyperreactivity may be triggered
by GATA-3. However, following continuous or late-stage treatment
with IPD, GATA-3 protein expression in the lung tissue was observed
to be markedly reduced. Therefore, the inhibition of GATA-3
expression appears to function as an upstream mechanism by which
IPD targets asthma and represses IL-5 expression.
In conclusion, the present study demonstrated that
IPD treatment alleviated OVA-induced asthma in rats by inhibition
of the GATA-3/IL-5 signaling pathway. These results provide novel
insights into the mechanisms underlying the anti-asthmatic action
of IPD and represent preliminary evidence for the clinical
application of IPD.
Acknowledgements
The present study was supported by a grant from the
Science and Technology Planning Project of the Health Department of
Hunan province (no. B2009077).
References
|
1
|
Rabinovitch N, Silveira L, Gelfand EW and
Strand M: The response of children with asthma to ambient
particulate is modified by tobacco smoke exposure. Am J Respir Crit
Care Med. 184:1350–1357. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Meng W and Busija DW: Comparative effects
of angiotensin-(1–7) and angiotensin II on piglet pial arterioles.
Stroke. 24:2041–2044. 1993.
|
|
3
|
Umetsu DT: Revising the immunological
theories of asthma and allergy. Lancet. 365:98–100. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Foster PS, Hogan SP, Ramsay AJ, et al:
Interleukin 5 deficiency abolishes eosinophilia, airways
hyperreactivity and lung damage in a mouse asthma model. J Exp Med.
183:195–201. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Karras JG, McGraw K, McKay RA, et al:
Inhibition of antigen-induced eosinophilia and late phase airway
hyperresponsiveness by an IL-5 antisense oligonucleotide in mouse
models of asthma. J Immunol. 164:5409–5415. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Tan GH, Su JM, Wang CC, et al: A
recombinant DNA plasmid encoding the human interleukin-5 breaks
immunological tolerance and inhibits airway inflammation in a
murine model of asthma. Int Arch Allergy Immunol. 145:313–323.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Zheng W and Flavell RA: The transcription
factor GATA-3 is necessary and sufficient for Th2 cytokine gene
expression in CD4 T cells. Cell. 89:587–596. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Yamashita M, Ukai-Tadenuma M, Miyamoto T,
et al: Essential role of GATA3 for the maintenance of type 2 helper
T (Th2) cytokine production and chromatin remodeling at the Th2
cytokine gene loci. J Biol Chem. 279:26983–26990. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Zhu J, Yamane H, Cote-Sierra J, et al:
GATA-3 promotes Th2 responses through three different mechanisms:
induction of Th2 cytokine production, selective growth of Th2 cells
and inhibition of Th1 cell-specific factors. Cell Res. 16:3–10.
2006. View Article : Google Scholar
|
|
10
|
Pai SY, Truitt ML and Ho IC: GATA-3
deficiency abrogates the development and maintenance of T helper
type 2 cells. Proc Natl Acad Sci USA. 101:1993–1998. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Tamaoki J, Takeyama K, Aoshiba K, et al: A
TH2 cytokine inhibitor for airway inflammation in mild asthma. J
Allergy Clin Immunol. 111:197–198. 2003.PubMed/NCBI
|
|
12
|
Sano Y, Suzuki N, Yamada H, et al: Effects
of suplatast tosilate on allergic eosinophilic airway inflammation
in patients with mild asthma. J Allergy Clin Immunol. 111:958–966.
2003. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Hoshino M, Fujita Y, Saji J, et al: Effect
of suplatast tosilate on goblet cell metaplasia in patients with
asthma. Allergy. 60:1394–1400. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Ding L, Zhou X, Yang L, et al: Liquid
chromatography/electrospray ionization mass spectrometry method for
the determination of the active metabolite M-1 of suplatast
tosilate in human plasma. Biomed Chromatogr. 21:1297–1302. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Satoh T, Sasaki G, Wu MH, et al: Suplatast
tosilate inhibits eosinophil production and recruitment into the
skin in murine contact sensitivity. Clin Immunol. 108:257–262.
2003. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Tanaka A, Minoguchi K, Samson KT, et al:
Inhibitory effects of suplatast tosilate on the differentiation and
function of monocyte-derived dendritic cells from patients with
asthma. Clin Exp Allergy. 37:1083–1089. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Agrawal DK, Cheng G, Kim MJ and Kiniwa M:
Interaction of suplatast tosilate (IPD) with chloride channels in
human blood eosinophils: a potential mechanism underlying its
anti-allergic and anti-asthmatic effects. Clin Exp Allergy.
38:305–312. 2008.PubMed/NCBI
|
|
18
|
Tamaoki J, Kondo M, Sakai N, et al: Effect
of suplatast tosilate, a Th2 cytokine inhibitor, on
steroid-dependent asthma: a double-blind randomised study. Tokyo
Joshi-Idai Asthma Research Group. Lancet. 356:273–278. 2000.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Matsumoto K, Hayakawa H, Ide K, et al:
Effects of suplatast tosilate on cytokine profile of
bronchoalveolar cells in allergic inflammation of the lung.
Respirology. 7:201–207. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Zhao GD, Yokoyama A, Kohno N, et al:
Effect of suplatast tosilate (IPD-1151T) on a mouse model of
asthma: inhibition of eosinophilic inflammation and bronchial
hyperresponsiveness. Int Arch Allergy Immunol. 121:116–122. 2000.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Moore RG, Granai CO, Gajewski W, et al:
Pathologic evaluation of inguinal sentinel lymph nodes in vulvar
cancer patients: a comparison of immunohistochemical staining
versus ultrastaging with hematoxylin and eosin staining. Gynecol
Oncol. 91:378–382. 2003. View Article : Google Scholar
|
|
22
|
Yang C, Yang Z, Zhang M, et al: Hydrogen
sulfide protects against chemical hypoxia-induced cytotoxicity and
inflammation in HaCaT cells through inhibition of ROS/NF-κB/COX-2
pathway. PLoS ONE. 6:e219712011.PubMed/NCBI
|
|
23
|
Temelkovski J, Hogan SP, Shepherd DP, et
al: An improved murine model of asthma: selective airway
inflammation, epithelial lesions and increased methacholine
responsiveness following chronic exposure to aerosolised allergen.
Thorax. 53:849–856. 1998. View Article : Google Scholar
|
|
24
|
Karol MH: Animal models of occupational
asthma. Eur Respir J. 7:555–568. 1994. View Article : Google Scholar
|
|
25
|
Vanacker NJ, Palmans E, Pauwels RA and
Kips JC: Dose-related effect of inhaled fluticasone on
allergen-induced airway changes in rats. Eur Respir J. 20:873–879.
2002. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Maneechotesuwan K, Yao X, Ito K, et al:
Suppression of GATA-3 nuclear import and phosphorylation: a novel
mechanism of corticosteroid action in allergic disease. PLoS Med.
6:e10000762009. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Tamauchi H, Terashima M, Ito M, et al:
Evidence of GATA-3-dependent Th2 commitment during the in vivo
immune response. Int Immunol. 16:179–187. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Yoshihara S, Ono M, Yamada Y, et al: Early
intervention with suplatast tosilate for prophylaxis of pediatric
atopic asthma: a pilot study. Pediatr Allergy Immunol. 20:486–492.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Humbert M, Corrigan CJ, Kimmitt P, et al:
Relationship between IL-4 and IL-5 mRNA expression and disease
severity in atopic asthma. Am J Respir Crit Care Med. 156:704–708.
1997. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Till S, Dickason R, Huston D, et al: IL-5
secretion by allergen-stimulated CD4+ T cells in primary
culture: relationship to expression of allergic disease. J Allergy
Clin Immunol. 99:563–569. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Shi HZ, Xiao CQ, Zhong D, et al: Effect of
inhaled interleukin-5 on airway hyperreactivity and eosinophilia in
asthmatics. Am J Respir Crit Care Med. 157:204–209. 1998.
View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Karlen S, Mordvinov VA and Sanderson CJ:
How is expression of the interleukin-5 gene regulated? Immunol Cell
Biol. 74:218–223. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Barnes PJ and Adcock IM: Transcription
factors and asthma. Eur Respir J. 12:221–234. 1998. View Article : Google Scholar
|
|
34
|
Yamashita N, Tashimo H, Ishida H, et al:
Involvement of GATA-3-dependent Th2 lymphocyte activation in airway
hyperresponsiveness. Am J Physiol Lung Cell Mol Physiol.
290:L1045–L1051. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Szabo SJ, Kim ST, Costa GL, et al: A novel
transcription factor, T-bet, directs Th1 lineage commitment. Cell.
100:655–669. 2000. View Article : Google Scholar
|