Introduction
Papillary thyroid cancer (PTC) accounts for 80–85%
of thyroid cancer cases (1). The
clinical prognosis for the majority of cases is satisfactory;
however, 14% of cases demonstrate relatively early recurrence and
others present with severe invasion, multiple lymph node metastasis
and occasionally, distant metastasis (2). Currently, extremely few biological
markers that are useful for the diagnostic and prognostic analysis
of PTC have been identified.
Forkhead box E1 [FOXE1, formerly known as thyroid
transcription factor 2 (TTF2)] belongs to a large family of
transcription factors characterized by a distinct forkhead domain
and is an important thyroid-specific factor. Numerous members of
the forkhead family are potent transcriptional activators in the
adult thyroid and are important in cell growth and differentiation
(3,4), including FOXE1 which is important in
the development and differentiation of thyroid follicular cells
(5,6). In addition, a growing body of data
have indicated that FOXE1 is important in the initiation of
specific tumors, including pancreatic cancer, cutaneous squamous
cell carcinoma and thyroid neoplasms (7–9).
Furthermore, previous studies demonstrated that FOXE1 transcripts
are detectable in PTC (10), and a
recent genome-wide association study showed that a common variant
on 9q22.33 led to an increased risk of papillary and follicular
thyroid carcinoma (11). Notably,
the FOXE1 gene is located on human chromosome 9q22.
To the best of our knowledge, the expression of the
FOXE1 gene and its correlation with the clinicopathological
parameters of PTC patients has not been previously reported. Thus,
in the present study, we investigated FOXE1 expression, and then
analyzed the correlation between FOXE1 expression and the
clinicopathological parameters in PTC.
Materials and methods
Tumor specimens and patient
information
PTC and adjacent non-tumor thyroid tissue specimens
were collected immediately following surgical removal and stored at
−80°C until use. A total of 30 pairs of fresh-frozen PTC tissues
and adjacent non-tumor tissues were used for the analysis of FOXE1
gene expression by quantitative real-time PCR (qPCR) and western
blotting. An additional 81 paraffin-embedded tissue blocks of PTC
were obtained from the Department of Pathology, Affiliated Sixth
People’s Hospital, Shanghai Jiaotong University (Shanghai, China)
between 2010 and 2012, and were randomly selected for IHC
analysis.
The average age of the patients from which the 81
PTC cases were derived was 49.6 years (range, 25–73 years) and
included 27 males and 54 females. The study protocol and consent
form were approved by the Institutional Ethics Committee of the
Affiliated Sixth People’s Hospital, Shanghai Jiaotong University.
All patients were informed of the aims of the study and provided
written informed consent.
RNA isolation and qPCR assay
Total RNA was extracted from PTC and adjacent
non-tumor tissue specimens using TRIzol reagent (Invitrogen Life
Technologies, Carlsbad, CA, USA) according to the manufacturer’s
instructions. First strand cDNA was then synthesized using the
Reverse Transcription Reagent kit (Takara Bio, Inc., Dalian,
China). The qPCR assay was conducted in a 10-μl reaction mixture
according to the SYBR-Green PCR kit (Takara Bio, Inc.) and analyzed
in a 96-well plate using the Applied Biosystems 7500 Real Time PCR
system (Applied Biosystems, Foster City, CA, USA). The primer
sequences used in this study are shown in Table I. The PCR conditions were as
follows: 95°C for 15 sec, followed by 40 cycles at 95°C for 5 sec
and 60°C for 34 sec. β-actin was used as an internal control.
Relative values of transcripts were calculated using the formula:
2−ΔΔCt. Experiments were performed in triplicate.
 | Table IPCR primers and conditions. |
Table I
PCR primers and conditions.
| Gene | Primer sequence | Temperature (°C) | Product size
(bp) |
|---|
| FOXE1 |
F-GCTGGTTTTCCCTGTCTCTG | 60 | 100 |
|
R-AGATGGGGGAGACTGAAGGT | 60 | |
| β-actin |
F-TTGTTACAGGAAGTCCCTTGCC | 60 | 101 |
|
R-ATGCTATCACCTCCCCTGTGTG | 61 | |
Western blotting
To evaluate the level of FOXE1 protein expression,
tissue lysates were prepared from fresh-frozen PTC and adjacent
non-tumor tissue specimens by centrifugation at 12,000 × g for 20
min at 4°C. The concentration of the protein lysate was then
determined using BCA reagent (Beyotime, Shanghai, China). For each
sample, a volume equivalent to 20-μg protein lysate was separated
by SDS-PAGE followed by transfer onto a nitrocellulose membrane
(Bio-Rad, Hercules, CA, USA). The membrane was blocked with 5%
non-fat milk for 1 h and then incubated with rabbit anti-FOXE1
monoclonal antibody (1:1000; Abcam, Cambridge, MA, USA) overnight
at 4°C. After washing in Tris-buffered saline with Tween-20 to
remove excess primary antibody, the blots were incubated for 1 h
with a specific secondary antibody (goat anti-rabbit IgG, 1:5,000;
Santa Cruz Biotechnology, Inc., CA, USA). Antibody binding was
detected using the enhanced chemiluminescent reagents (Pierce
Biotechnology, Inc., Rockford, IL, USA) and measured using Kodak
Scientific Imaging Systems (New Haven, CT, USA). A goat-specific
monoclonal GAPDH antibody (1:5,000; Bioworld Technology,
Minneapolis, MN, USA) was used as a control.
IHC analysis
In total, 81 paraffin-embedded tissue blocks of PTC
were retrieved for IHC analysis. The blocks were cut into 4-μm
sections, deparaffinized with xylene and rehydrated in a graded
ethanol series. Antigen retrieval was performed by boiling tissue
sections in EDTA solution (1:50) for 20 min. The sections were
incubated with the primary antibody, rabbit anti-FOXE1 monoclonal
antibody (1:250; Abcam) overnight at 4°C. The slides were then
incubated with a biotinylated secondary antibody (goat anti-rabbit
IgG) at 37°C for 30 min. The slides were then stained with
hematoxylin and eosin (H&E) for 2 min, dehydrated, mounted and
imaged using the Microscope ScanScope (Olympus, Tokyo, Japan).
Immunohistological scores and
clinicopathological parameters
Two pathologists blinded to the identity of the
specimens, examined the percentage of positively stained cells in
contrast to the total section area (TSA = 100%). They also assessed
the intensity of the immunostained slides and scored them as
previously described (12). Based
on the percentage of positive cells, the level of staining was
defined as follows: 0%, negative (−); 1–33%, weak (+); 34–66%,
moderate (++) and 67–100%, strong (+++). The intensity of the
immunoreactions was scored as follows: 0, negative (−); 1, weak
(+); 2, moderate (++) and 3, strong (+++). The total scores in the
tumor and non-tumor regions were determined as the sum of the
products of the positivity and intensity grades. A total score of
>2.0 was considered to represent a strong positive score.
Clinicopathological parameters, including age,
gender, tumor size, extra-capsular invasion, multifocality, lymph
node metastasis, distant metastasis and tumor stage (TNM
classification) were analyzed (13,14).
Correlations between the IHC results and the clinicopathological
parameters were evaluated.
Statistical analysis
The differences between the FOXE1 expression levels
of PTC and adjacent non-tumor tissue specimens were determined
statistically using the Mann-Whitney U test or the independent
samples t-test. Correlations between FOXE1 expression and the
clinicopathological parameters of PTC patients were analyzed using
the Pearson’s Chi-squared test. Statistical analyses were performed
using SPSS software (version 17.0; SPSS, Inc., Chicago, IL, USA).
P<0.05 was considered to indicate a statistically significant
result.
Results
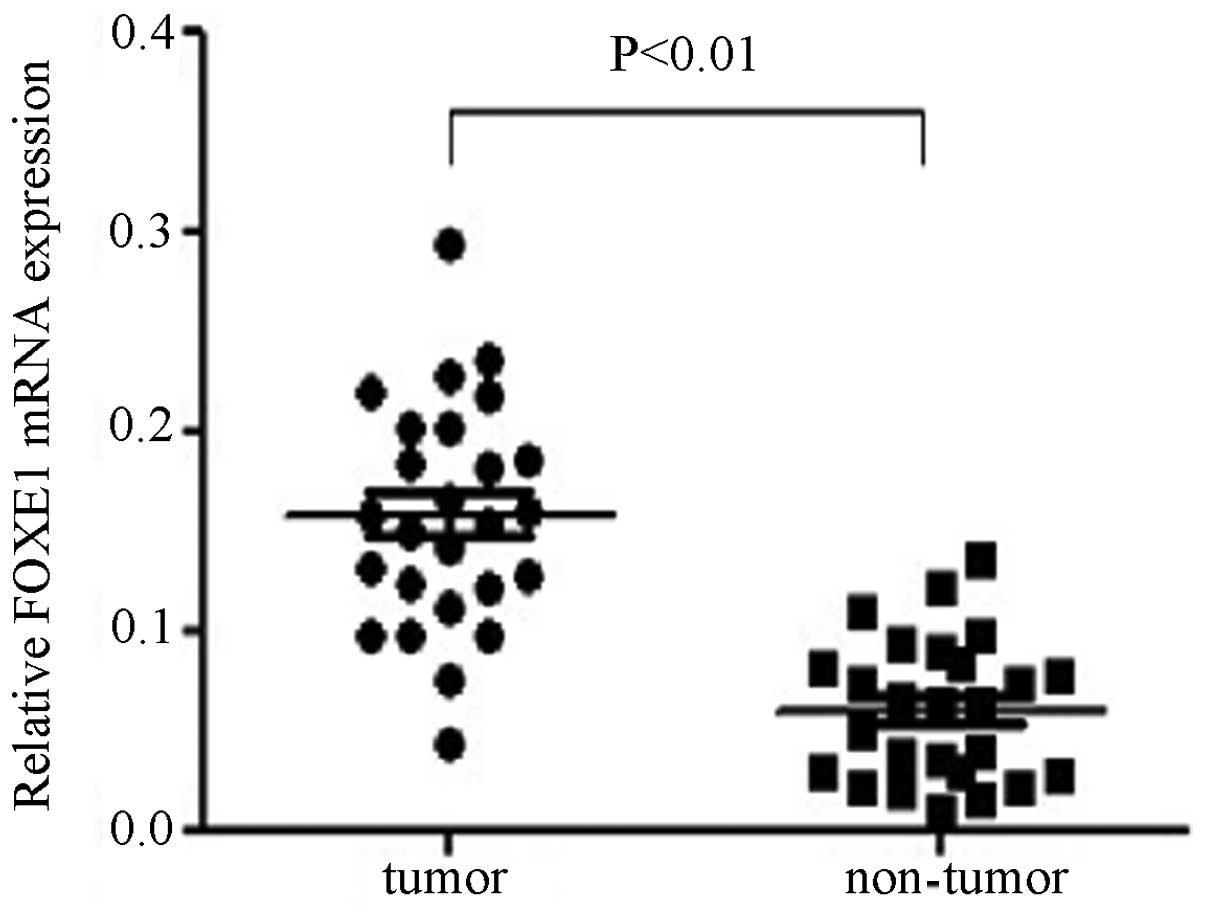
FOXE1 mRNA expression in PTC
Out of the 30 pairs of specimens investigated by
qPCR, 26 paired results were analyzed. The average mRNA expression
levels of FOXE1 were significantly higher in PTC tissues compared
with adjacent non-tumor thyroid tissues (0.1575±0.0566 versus
0.0598±0.0350, P<0.01), equating to an ~2.6-fold increase in the
expression of FOXE1 in PTC tissues (Fig. 1). The results also demonstrated
that 65% (17 out of 26) of PTC tissues expressed higher levels of
FOXE1 compared with the matched adjacent non-tumor thyroid
tissues.
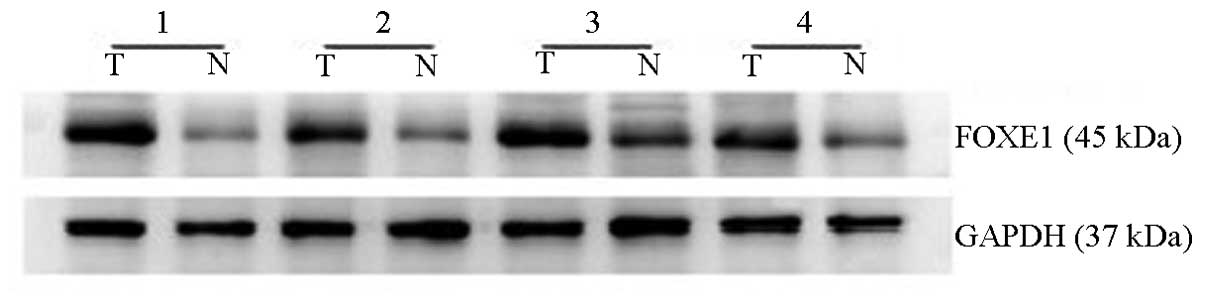
FOXE1 protein expression levels in PTC
versus non-tumor thyroid tissues
FOXE1 protein expression levels were also analyzed
by western blotting. FOXE1 protein levels in PTC tissues were
significantly higher compared with the matched adjacent non-tumor
thyroid tissues. FOXE1 was more markedly expressed in 58% (15 out
of 26) of PTC tissues compared with the paired non-tumor tissues
(Fig. 2). Thus, these results are
consistent with the data obtained by the qPCR assay.
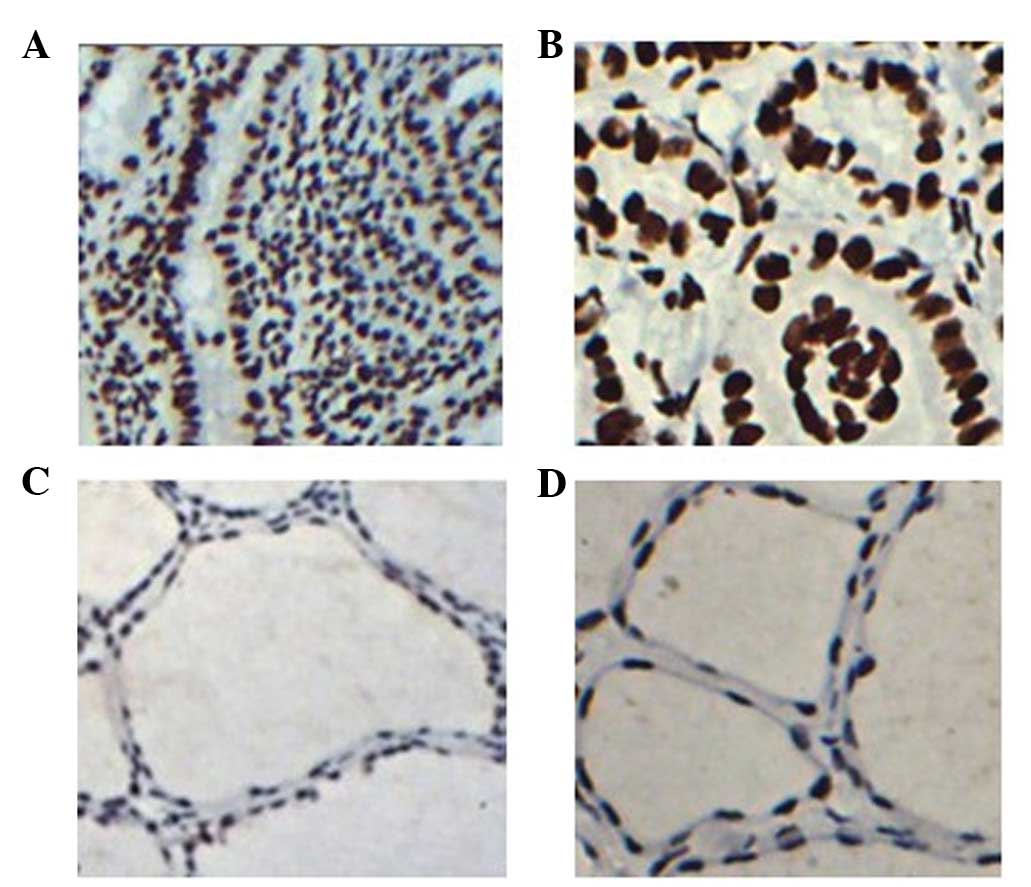
FOXE1 protein staining indices by
IHC
IHC staining was performed to analyze FOXE1
expression in paraffin-embedded tissue samples of PTC. The results
demonstrated that FOXE1 staining was widely expressed in all tumor
regions of the 81 PTC tissue sections (100%); however, it was
present in only 31 out of 81 samples in the non-tumor regions
(38.3%). A higher degree of FOXE1 staining was evident in the tumor
regions compared with the corresponding non-tumor tissues (Fig. 3). Additionally, FOXE1 staining was
predominantly present in cell nuclei. However, in non-tumor thyroid
follicular epithelial cells, FOXE1 staining was negative or
exhibited a weak level of staining. Thus, FOXE1 expression is
significantly elevated at the RNA and protein levels in PTC.
Correlation between FOXE1 expression and
the clinicopathological parameters of PTC
To examine the clinical significance of FOXE1 in
PTC, samples were divided into the high and low expression groups,
according to the mean value of the FOXE1 staining scores in the PTC
sections (Table II). We then
examined the correlation between FOXE1 and the clinicopathological
parameters of PTC. Our data demonstrated that FOXE1 expression in
PTC significantly correlated with the extra-capsular invasion of
tumor cells, lymph node metastasis and tumor stage (P=0.048,
P=0.036 and P=0.009, respectively). No significant correlation was
identified between FOXE1 expression and other clinical parameters,
including age, gender, tumor size, multifocality and
thyroid-stimulating hormone (TSH) level. However, we demonstrated
that FOXE1 expression was significantly higher in stages III/IV
compared with stages I/II of PTC (P<0.01). Taken together, these
data suggest that FOXE1 is useful as a prognostic index for
PTC.
 | Table IICorrelation analysis of FOXE1
expression and the clinicopathological parameters of PTC
patients. |
Table II
Correlation analysis of FOXE1
expression and the clinicopathological parameters of PTC
patients.
| | FOXE1 expression | |
|---|
| |
| |
|---|
| Parameter | Number (%) | High (n=33) | Low (n=48) | P-value |
|---|
| Gender | | | | 0.752 |
| Male | 23 (28.4) | 10 | 13 | |
| Female | 58 (71.6) | 23 | 35 | |
| Age (years) | | | | 0.346 |
| <45 | 33 (40.7) | 11 | 21 | |
| ≥45 | 48 (59.3) | 22 | 27 | |
| Tumor size (cm) | | | | 0.912 |
| ≤ 2 | 57 (70.4) | 23 | 34 | |
| >2 | 24 (29.6) | 10 | 14 | |
| Multifocality | | | | 0.055 |
| No | 54 (66.7) | 18 | 36 | |
| Yes | 27 (33.3) | 15 | 12 | |
| Extracapsular
invasion | | | | 0.048a |
| No | 68 (84.0) | 24 | 44 | |
| Yes | 13 (16.0) | 9 | 4 | |
| Lymph node
metastasis | | | | 0.036a |
| No | 21 (25.9) | 4 | 17 | |
| Yes | 60 (74.1) | 29 | 31 | |
| Distant
metastasis | | | | 1.0 |
| No | 77 (95.1) | 31 | 46 | |
| Yes | 4 (4.9) | 2 | 2 | |
| Tumor stage
(TNM) | | | | 0.009a |
| I/II | 55 (67.9) | 17 | 38 | |
| III/IV | 26 (32.1) | 16 | 10 | |
| TPO-Ab | | | | 0.624 |
| Positive | 22 (27.2) | 8 | 14 | |
| Negative | 59 (72.8) | 25 | 34 | |
| TSH level | | | | 0.093 |
| Positive | 15 (18.5) | 9 | 6 | |
| Negative | 66 (81.5) | 24 | 42 | |
Discussion
Transcription factors are a group of proteins that
promote and regulate gene expression as well as the cell
proliferation and differentiation.
A number of transcription factors belonging to the
FOX family are key regulators of diverse cell functions, including
oncogenesis (15), and are
important in the development, invasion and metastasis of carcinoma,
including FOXC1 in breast cancer (16), and FOXA1 and FOXM1 in anaplastic
thyroid carcinoma (17,18). However, few studies have examined
the expression and function of the FOXE1 gene in PTC. In the
present study, the mRNA and protein expression levels of the FOXE1
gene were investigated in Chinese patients with PTC. Out of the 26
pairs of specimens investigated by qPCR, the expression levels of
FOXE1 were significantly higher in PTC tissues compared with
adjacent non-tumor thyroid tissues (P<0.01). Similarly, data
obtained from western blotting and IHC analysis were consistent
with FOXE1 mRNA expression. The correlation between FOXE1 gene
expression and clinical prognosis were then evaluated. Our data
demonstrate that increased levels of FOXE1 expression in PTC are
significantly associated with the extra-capsular invasion of tumor
cells, lymph node metastasis and tumor stage, suggesting that the
expression of FOXE1 is correlated with the invasion and metastasis
of PTC.
Examination of the clinicopathological parameters
revealed that a higher FOXE1 expression was significantly
associated with advanced tumor stages and poor clinical prognosis.
This hypothesis is in line with several other transcription factors
of the forkhead family, including FOXA1 and FOXM1 in anaplastic
thyroid carcinoma (17,18). Taken together, these results
suggest that the upregulation of FOXE1 may be an important event
during PTC progression.
The increased expression of the thyroid-specific
transcription factor, FOXE1, in PTC likely reflects the
hyperactivity of the thyroid during this disease. Additionally, we
demonstrated that several thyroid papillary microcarcinomas also
expressed FOXE1. These observations suggest that the expression of
FOXE1 occurs during the early stages of PTC development. Therefore,
it is possible that the FOXE1 gene may function as an activator in
PTC tumorigenesis. These results are consistent with a recently
published study, which revealed that FOXE1 expression is associated
with genetic susceptibility to PTC and forms a high-risk factor for
the development of PTC (19).
In addition, there is still some debate with regard
to the precise role of FOXE1 in tumorigenesis. It has been
demonstrated that FOXE1 is able to function as a transcriptional
repressor in cutaneous squamous cell carcinoma (9), whereas, other indirect evidence has
demonstrated that FOXE1 acts as a transcriptional activator
(3). Our data indicated that FOXE1
expression is elevated in PTC samples, suggesting that FOXE1 gene
expression may be associated with a high risk of developing
PTC.
Notably, our results have demonstrated that FOXE1
expression is upregulated at the mRNA and protein levels in PTC
tissues. Although the sample size in this study was small and the
findings cannot be generalized to the broader population, we
hypothesize that FOXE1 may function as a positive regulator in
thyroid carcinogenesis. However, it remains unclear whether the
activation of certain pathways, including the Sonic Hedgehog (SHh)
pathway, in thyroid cancer contribute to FOXE1 transcriptional
activity and subsequently lead to an increased expression of FOXE1
(20).
By contrast, the TSH signaling cascade, which is
present in thyroid follicular cells is important in the cell
differentiation of the thyroid gland. The pathway is initiated by
TSH binding to its transmembrane receptor, leading to the release
of adenosine cyclophosphate (cAMP) and protein kinase A (PKA),
which subsequently activate thyroid-specific transcription factors,
including PAX-8, TTF1 and FOXE1. In the present study, based on the
preoperatively evaluated expression levels of TSH, we
retrospectively investigated whether TSH expression correlated with
FOXE1, and thus may result in higher expression levels of FOXE1 in
certain PTC cases. However, although a positive correlation was
observed, the correlation between TSH levels and FOXE1 expression
was not statistically significant. The precise role and molecular
mechanism responsible for the potential tumor activator role of
FOXE1 in PTC requires additional investigation, and may have
potential clinical implications for the clinical prognosis and
therapy of PTC.
To the best of our knowledge, this is the first
study to report the mRNA and protein expression levels of FOXE1 in
PTC and analyze its clinical significance. In conclusion, we have
demonstrated that the FOXE1 gene exhibits significant differential
expression levels between PTC tissues and adjacent non-tumor
thyroid tissues. Our results suggest that FOXE1 may be important in
the development of PTC and may be a candidate prognostic biomarker
and a new therapeutic target.
References
|
1
|
Davies L and Welch HG: Increasing
incidence of thyroid cancer in the United States, 1973–2002. JAMA.
295:2164–2167. 2006.
|
|
2
|
Hay ID, Thompson GB, Grant CS, et al:
Papillary thyroid carcinoma managed at the Mayo Clinic during six
decades (1940–1999): temporal trends in initial therapy and
long-term outcome in 2444 consecutively treated patients. World J
Surg. 26:879–885. 2002.PubMed/NCBI
|
|
3
|
Zannini M, Avantaggiato V, Biffali E, et
al: TTF-2, a new forkhead protein, shows a temporal expression in
the developing thyroid which is consistent with a role in
controlling the onset of differentiation. EMBO J. 16:3185–3197.
1997. View Article : Google Scholar
|
|
4
|
Katoh M and Katoh M: Human FOX gene family
(Review). Int J Oncol. 25:1495–1500. 2004.PubMed/NCBI
|
|
5
|
Dathan N, Parlato R, Rosica A, De Felice M
and Di Lauro R: Distribution of the titf2/foxe1 gene product is
consistent with an important role in the development of foregut
endoderm, palate and hair. Dev Dyn. 224:450–456. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
De Felice M, Ovitt C, Biffali E, et al: A
mouse model for hereditary thyroid dysgenesis and cleft palate. Nat
Genet. 19:395–398. 1998.PubMed/NCBI
|
|
7
|
Brune K, Hong SM, Li A, et al: Genetic and
epigenetic alterations of familial pancreatic cancers. Cancer
Epidemiol Biomarkers Prev. 17:3536–3542. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Weisenberger DJ, Trinh BN, Campan M, et
al: DNA methylation analysis by digital bisulfite genomic
sequencing and digital MethyLight. Nucleic Acids Res. 36:4689–4698.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Venza I, Visalli M, Tripodo B, Lentini M,
Teti D and Venza M: Investigation into FOXE1 genetic variations in
cutaneous squamous cell carcinoma. Br J Dermatol. 162:681–683.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Sequeira MJ, Morgan JM, Fuhrer D, Wheeler
MH, Jasani B and Ludgate M: Thyroid transcription factor-2 gene
expression in benign and malignant thyroid lesions. Thyroid.
11:995–1001. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Gudmundsson J, Sulem P, Gudbjartsson DF,
et al: Common variants on 9q22.33 and 14q13.3 predispose to thyroid
cancer in European populations. Nat Genet. 41:460–464. 2009.
View Article : Google Scholar
|
|
12
|
Nam KH, Noh TW, Chung SH, et al:
Expression of the membrane mucins MUC4 and MUC15, potential markers
of malignancy and prognosis, in papillary thyroid carcinorma.
Thyroid. 21:745–750. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Shaha AR: TNM classification of thyroid
carcinoma. World J Surg. 31:879–887. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Cooper DS, Doherty GM, Haugen BR, et al;
American Thyroid Association (ATA) Guidelines Taskforce on Thyroid
Nodules and Differentiated Thyroid Cancer. Revised American Thyroid
Association management guidelines for patients with thyroid nodules
and differentiated thyroid cancer. Thyroid. 19:1167–1214. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Hannenhalli S and Kaestner KH: The
evolution of Fox genes and their role in development and disease.
Nat Rev Genet. 10:233–240. 2009. View
Article : Google Scholar : PubMed/NCBI
|
|
16
|
Sizemore ST and Keri RA: The forkhead box
transcription factor FOXC1 promotes breast cancer invasion by
inducing matrix metalloprotease 7 (MMP7) expression. J Biol Chem.
287:24631–24640. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Nucera C, Eeckhoute J, Finn S, et al:
FOXA1 is a potential oncogene in anaplastic thyroid carcinoma. Clin
Cancer Res. 15:3680–3689. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Bellelli R, Castellone MD, Garcia-Rostan
G, et al: FOXM1 is a molecular determinant of the mitogenic and
invasive phenotype of anaplastic thyroid carcinoma. Endocr Relat
Cancer. 19:695–710. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Matsuse M, Takahashi M, Mitsutake N, et
al: The FOXE1 and NKX2-1 loci are associated with susceptibility to
papillary thyroid carcinoma in the Japanese population. J Med
Genet. 48:645–648. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Xu X, Ding H, Rao G, et al: Activation of
the Sonic Hedgehog pathway in thyroid neoplasms and its potential
role in tumor cell proliferation. Endocr Relat Cancer. 19:167–179.
2012. View Article : Google Scholar : PubMed/NCBI
|