Introduction
Several studies have proposed that the regeneration
and growth of deer antlers may be used as a model for biomedical
research (1,2). Deer antlers are bony organs, which
are cast and regenerated each year. The antler growth center is
located in its tip and the antler consists of three layers. These
layers are distoproximally the reserve mesenchyme, precartilage and
cartilage. Antler growth is achieved through the activity of cells
residing in the proliferation zone, particularly in the reserve
cartilage layer. Insulin-like growth factor-1 (IGF-1) is a
multifunction cell proliferation regulation factor, named due to
its structural similarity to insulin (3). Numerous studies have observed that
the biological activation of IGF-1 is varied, playing an active
role in cell growth, embryonic differentiation, metabolism and the
adjustment of the nervous and endocrine systems (4–6).
IGF-1 has a positive effect on controlling cell metabolism,
regulating cell division and differentiation, inhibiting cell
apoptosis and various cell function adjustments (7).
The proliferation of deer antler cells may be
regulated by IGF-1, which has a major effect on promoting the
formation of cartilage (8). As
IGF-1 has a growth-promoting role in cartilage formation and the
majority of the antler growth center is composed of cartilage,
Suttie et al(8) suggested
that IGF-I may be the antler growth stimulus (AGS). IGF-1 receptors
are located in growing antler tissues (9). Results of a study by Sadighi et
al(10) support the theory
that IGF-1 may be the AGS. IGF-1 was found to increase the
proliferation of mesenchymal and cartilaginous cells derived from
the antler proliferation zone in a dose-dependent manner. The
development of deer antlers and the concentration of IGF-1 have a
dependent relationship, such that regions containing high levels of
IGF-1 grow rapidly (11). The
content of IGF-1 gradually decreases from the top to the bottom of
the antler; the cartilage layer has the highest level of IGF-1 and
the mesenchyme layer has the lowest level of IGF-1. This is
consistent with the theory that cartilage is the fastest growing
area of the deer antler (12).
However, the underlying mechanisms regulating the rapid
proliferation of velvet antler remain unclear.
MicroRNAs (miRNAs) are a class of endogenous
non-coding RNAs formed of 18–24 nucleotides, whose function is to
downregulate or inhibit the translation of mRNA and to control gene
expression through binding to the 3′-untranslated region (3′UTR) of
mRNA (13). Since miRNA was first
located in Caenorhabditis elegans in 1993, it has been
studied in various biological processes (14). With the development of research, a
large number of miRNAs have been identified and are known to widely
exist in numerous plants and animals (15). According to the MiRBase database,
>9,000 miRNAs have been found in eukaryotes, including 1,000
miRNAs in humans (16).
Furthermore, information in the MiRBase database (www.MiRBase.org) suggests that a single miRNA may
regulate various target genes, different miRNAs are capable of
regulating the same target genes and ~30% of protein-coding genes
may be regulated by miRNAs (17).
Since the diversity and universality of miRNAs have been revealed,
certain scholars have hypothesized that miRNA molecules may be the
core component of the gene regulation network (18). Numerous studies investigating miRNA
have revealed a deeper gene expression regulation system in
organisms, and highlighted the important role of RNA in network
control systems (19). However,
whether miRNA is deregulated during the proliferation of deer
antler cells and its role in progression remains unclear.
In the present study, we investigated whether IGF-1
is regulated by miRNAs. We used miRNA GeneChip®
(Affymetrix, Santa Clara, CA, USA) and an miRNA target-prediction
tool to identify miRNAs that may target IGF-1. MicroRNA-1 (miR-1)
was identified as participating in the growth of antler tissues and
the restoration of miR-1 expression in deer antler cell lines
reduced cell proliferation. Furthermore, we found that the effects
of miR-1 were achieved by binding directly to the 3′UTR of IGF-1,
thus inhibiting the expression of IGF-1. Our results suggest that
miR-1 is crucial in the proliferation of deer antler cells and has
potential applications with regard to antler regeneration and
growth.
Materials and methods
Cell culture
Top tissue samples from deer antlers were obtained
from a three-year-old male Cervus nippon (Changchun, China).
Mesenchyme and cartilage tissues were isolated under a dissecting
microscope. The tissues were blended and digested by collagenase I
and hyaluronic acid (Sigma-Aldrich, St. Louis, MO, USA) for 1.5 h
at 37°C, respectively. Subsequently, the tissues were digested
further by adding collagenase-II (Sigma-Aldrich) for 3 h at 37°C.
Cartilage cells were cultured in DMEM (HyClone, Logan, UT, USA)
with 20% fetal bovine serum (FBS), 200 U/ml penicillin and 100 U/ml
streptomycin (Tianjin Hao Yang Biological Technology Co., Ltd.
Tianjin, China) at 37°C with 5% CO2. The study was
approved by the ethics committee of the Institute of Life Sciences,
Jilin Agriculture University, Changchun, China.
miRNA gene chip preparation
After culturing mesenchyme cells and cartilage cells
for 72 h, they were centrifuged and washed with PBS, respectively.
miRNA gene chips were synthesized by RiboBio Co., Ltd. (Guangzhou,
China). Data were analyzed using miRNA QC Tool 1 software (Beijing
Boao Biological Co., Ltd., Beijing, China) in order to detect
differentially expressed miRNAs in the top tissue of the deer
antler.
Transfection
Cartilage cells were seeded into each well of
96-well or 6-well plates, respectively. Cells were incubated
overnight and subsequently transfected with negative scramble
control (NC) RNA or an miR-1 mimic using X-tremeGENE HP DNA
transfection reagent (Roche, Mannheim, Germany) at a final
concentration of 50 nM.
Quantitative polymerase chain reaction
(qPCR)
Following transfection with miR-1 for 24 and 48 h,
total RNA, including miRNA, was isolated as described previously
(12) and subsequently reverse
transcribed to cDNA with a stem-loop RT primer for miR-1 and Oligo
d(T)18 for the β-actin gene. The stem-loop RT primer for
miR-1 analysis was 5′-ATGACTGGCCTCCTCAGATCAGTTTGGCCACTG
ACTGATCTGAAGGCCAGTCATATACATACTTCTTTA CATTCCA-3′ and the qPCR
primers were 5′-GCATCTCCA GCCTCCTCAGAT-3′ and 5′-GCGCTGGAATGTAA
AGAAGTATGTAT-3′. The PCR primers for the β-actin internal control
were 5′-TGACCCTTAAGTACC CCATCGA-3′ and
5′-TTGTAGAAGGTGTGGTGCCAGAT-3′. Relative expression levels were
calculated using the 2−ΔΔCt method.
Cell proliferation assays
Deer antler cartilage cells, seeded into each well
of 96-well plates with complete medium, were transfected with an
miR-1 mimic (50 nM) using the X-tremeGENE HP DNA transfection
reagent. The control group did not require transfection. Following
culture for 24 and 48 h, cell growth was measured using the MTT
method. Spent cell culture medium was replaced with 0.1 ml of fresh
medium containing 0.5 mg/ml MTT. Cells were incubated at 37°C for 4
h and the blue-violet crystal precipitate was resolved with 0.1 ml
DMSO (Sigma-Aldrich). Absorbance was measured at 570 nm.
Detection of cell cycle
At 24 and 48 h post-transfection, the cartilage
cells were harvested and resuspended in PBS, fixed with 75%
ice-cold ethanol at 4°C overnight and stained using a 10% RNase A
and 4% propidium iodide (PI) kit (Beijing Dingguo Biotechnology
Co., Ltd., Beijing, China) for 1 h at 37°C. Stained cells were
detected using flow cytometry (FCM) and the data were analyzed
using Cell-Quest software (Becton Dickinson, San Jose, CA,
USA).
IGF-1 3′UTR luciferase reporter
assay
The acquired 3′UTR sequence of IGF-1 was cloned into
the pmiR-RB-Report™ vector, downstream of the cytomegalovirus
promoter-driven firefly luciferase cassette, in order to construct
luciferase reporters with the 3′UTR of IGF-1. Cells were
transiently transfected with 50 ng of luciferase reporter plasmid
and 5 pmol of miR-1 mimic using X-tremeGENE HP DNA transfection
reagent. Following culture for 24 and 48 h, the cell proteins were
extracted. Luciferase activity was measured using the
Dual-Luciferase Reporter Assay system (Promega, Madison, WI, USA),
according to the manufacturer’s instructions. Data were normalized
by Photinus luciferase activity.
Western blot analysis
Total protein was extracted from cells using RIPA
lysis buffer with 1 mM phenylmethyl sulfonylfluoride at 0°C.
Following mixing with a loading buffer, the protein was separated
by 12% SDS-PAGE and transferred to polyvinylidene fluoride
membranes. Non-specific binding was blocked with 5% dried skim milk
in a Tris-buffered saline and Tween-20 (TBST) mixture for 2 h.
Subsequently, the membranes were incubated with primary antibodies
against IGF-1 at a 1:500 dilution and GAPDH at a 1:1,000 dilution
at 4°C overnight. Primary antibody was rabbit polyclonal anti-IGF1R
antibody or rabbit polyclonal anti-GAPDH antibody. Following
washing with TBST three times, the membranes were incubated with
the secondary antibody goat anti-rabbit IgG-HRP conjugated at a
1:2,000 dilution at 25°C for 2 h. Following washing with TBST a
further three times, immunoreactive bands were visualized using
enhanced chemiluminescence (ECL) reagents (Beijing Kang for Century
Biotechnology Co. Ltd., Beijing, China).
Statistical analysis
The data are presented as the mean ± SD. SPSS
Statistics 12.0 software was used for all statistical analyses.
P<0.05 was considered to indicate a statistically significant
difference.
Results
miRNA screening targeting the IGF-1
3′UTR
According to the analysis of >5,000 miRNAs by the
Affymetrix miRNA GeneChip technology, we identified 210 miRNAs that
exhibited a marked differential expression in the mesenchyme and
cartilage cells. Compared with the mesenchyme cells, 126 miRNAs
were upregulated in the cartilage cells and 84 were downregulated.
Furthermore, miRNA binding site analysis of the 3′UTR of IGF-1 by
TargetScan Human 5.1 software (http://www.targetscan.org) revealed that 25 miRNAs may
target the 3′UTR of IGF-1 and inhibit its expression. In order to
further investigate the mechanisms responsible for the promotion of
antler regeneration by miR-1, we sought to identify the molecular
targets of miR-1, since miRNAs function through the
post-transcriptional inhibition of their target mRNAs by binding to
the 3′UTR. Among the numerous predicted target genes in TargetScan,
the TargetScan prediction indicated that the deer IGF-1 3′UTR
contained a conserved putative miR-1 target site. The
characteristics of miR-1 conformed to the results of gene chip
screening and software analysis for the deer IGF-1 (Fig. 1). miR-1 was used in subsequent
experiments.
Detection of miR-1 expression levels by
qPCR
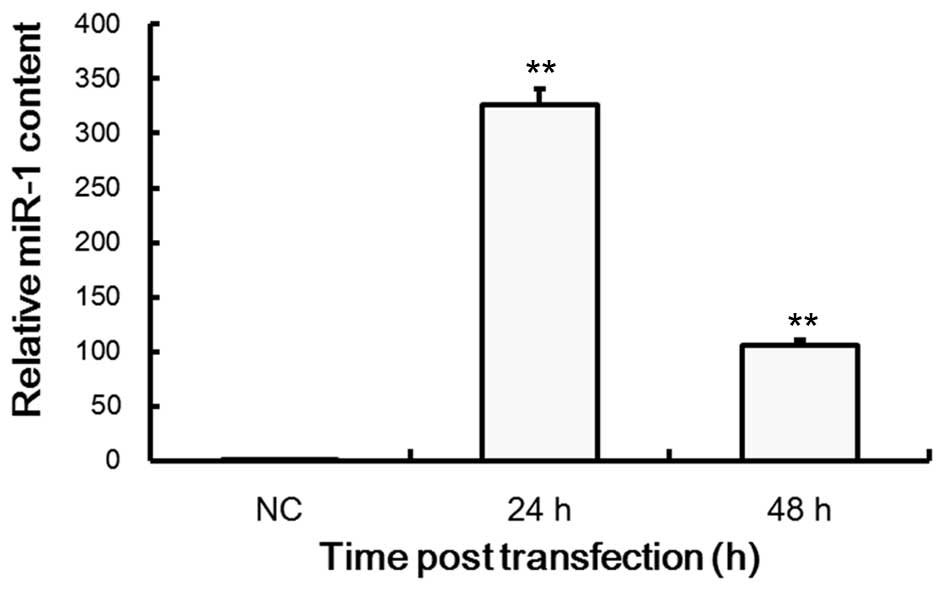
Subsequently, we further determined the effects of
miR-1 on cartilage cells. qPCR analysis revealed that the
expression levels of miR-1 in transfected cells were significantly
increased compared with the untransfected normal cells, where
β-actin was used as an internal control. As shown in Fig. 2, miR-1 expression levels decreased
after 48 h, and levels in transfected cells decreased after 24 h.
This indicated that miR-1 had been successfully transfected into
the cartilage cells and subsequent tests were performed.
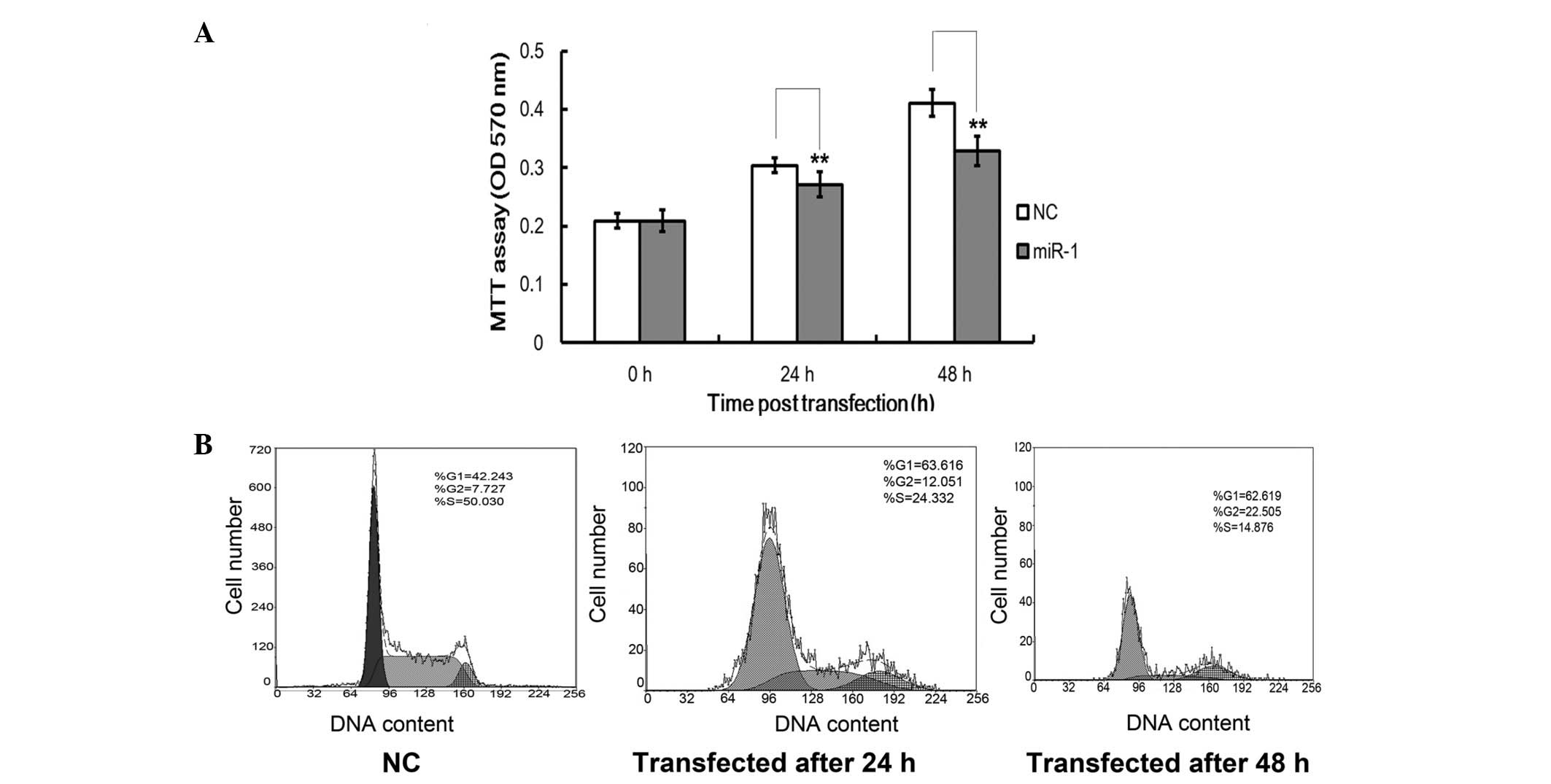
Restoration of miR-1 inhibits cartilage
cell growth
During the investigation of the molecular mechanisms
for miR-1-induced IGF-1 inhibition, the expression of miR-1 was
found to be inversely correlated with cellular IGF-1 levels. IGF-1
has an important role in antler cell growth. We sought to determine
whether miR-1 affects the proliferation rate of cartilage cells
using an MTT assay and cell cycle analysis. Cell growth curves
demonstrated that the overexpression of miR-1 significantly
suppressed cartilage cell proliferation in a time-dependent manner
when compared with the NCs, and this suppression occurred within 48
h in the cartilage cells (P<0.05; Fig. 3A). To further determine the effect
of miR-1 on cartilage cells, cell cycle analysis was performed. As
shown in Fig. 3B and C, compared
with the NCs, miR-1 induced a significant decrease in the cell
cycle process in the cartilage cells. Compared with the control
group, the number of cells at the G1 and G2
phases were markedly increased. Furthermore, the number of cells at
the S-phase decreased (P<0.05, Fig.
3B). Taken together, these results demonstrated that miR-1
acted as an inhibitor and was capable of markedly reducing the
proliferation of antler cells.
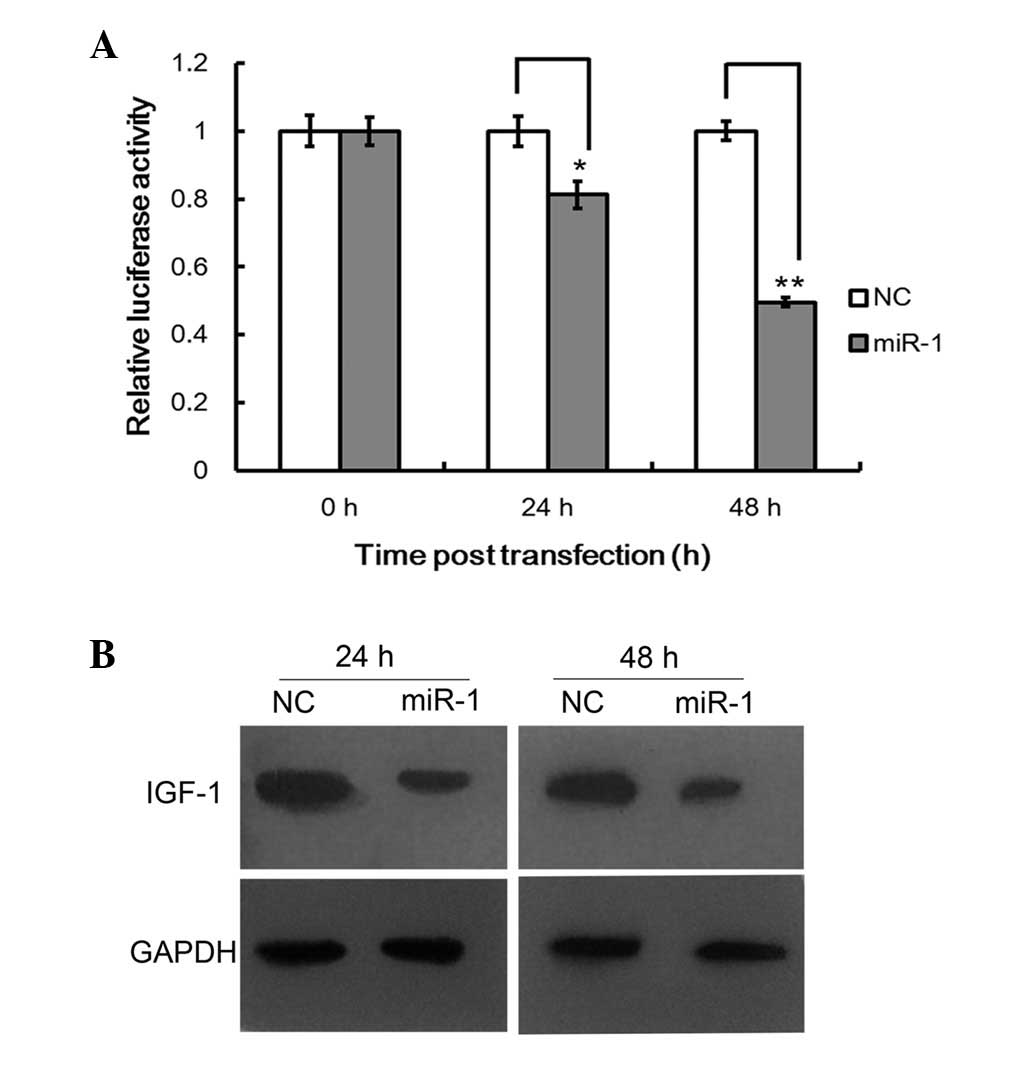
miR-1 regulates IGF-1 by binding to its
3′UTR
miRNAs regulate the expression of target genes by
binding to the 3′UTR of specific mRNAs, triggering mRNA degradation
or translational repression (14).
To confirm whether IGF-1 is a direct target of miR-1 in cartilage
cells, a 510 bp fragment of the 3′UTR of IGF-1 containing the
target sequence was cloned into a luciferase reporter vector, and
the effect of miR-1 on the luciferase activity of each construct in
the cartilage cells was tested. As shown in Fig. 4A, miR-1 suppressed the luciferase
activity of pmiR-IGF-1 by ~50% compared with the NCs (P<0.05).
Western blot analysis demonstrated that the IGF-1 expression level
decreased markedly compared with the NCs (Fig. 4B). Taken together, our results
suggested that the 3′UTR of IGF-1 contains the miR-1 binding site
and miR-1 is capable of inhibiting the expression of proteins with
similar binding sites.
Discussion
miRNAs that are important in the regulation of gene
expression have been discovered in various organisms. The main
effect of miRNA is to silence the expression of target genes by
complementary association with the 3′UTR on the mRNA of the target
gene. A number of scholars hypothesize that there are thousands of
miRNAs, and that these may control ~30% of gene transcription in
the human genome (20).
Numerous studies have confirmed that IGF-1, an
important cell factor, rapidly improves the growth of deer antlers
(3–5). Although the open reading frame (ORF)
section of IGF-1 mRNA is widely conserved in all species, the 3′UTR
section is not, such that the homology of Homo sapiens and
the Chinese sika deer is <80%. Furthermore, the most effective
part of the miRNA is known as the ‘seed’. miRNA recognizes target
mRNAs with seed-complementary sequences to direct
post-transcriptional repression. Many downregulated targets against
prediction algorithms have been developed based on this
complementarity. This suggests that the miRNA seed sequence, which
is the main regulating site, is necessary for regulating the
expression of the target gene. The results also indicate that the
3′UTR of target gene, which is a complementary sequence of the
miRNA seed region, may be evolutionarily well adapted various
species.
However, there have been few reports with regard to
Chinese sika deer miRNAs. In the present study, sequences for miR-1
of a known species were the same as those determined by TargetScan
Human 5.1 Data Base analysis. Furthermore, the sequences of miR-1
from the total RNA of the Chinese sika deer corresponded with that
of other species according to Affymetrix microRNA GeneChip
analysis. This implies that miR-1 is widespread in different
species and its sequence is conserved during evolution. We
performed luciferase reporter screening in order to determine
whether IGF-1 is targeted by miRNAs. We selected a pool of 25
miRNAs that had the potential to target IGF-1 according to the
microRNA GeneChip, which is reported to have the best performance
(21,22). We found that miR-1 was capable of
targeting the 3′UTR of IGF-1. Therefore, we proposed that the
effect of miR-1 on IGF-1 expression results from their near-perfect
complementarity, which leads to the degradation of IGF-1 mRNA.
miR-1 expression profiles, available from the miRZ database
(23), indicate that miR-1 is
preferentially expressed in mesenchyme and cartilage cells (data
not shown), suggesting that miR-1 may be involved in antler
regeneration. This study provides initial evidence that miR-1
directly regulates the expression of IGF-1 by binding to the IGF-1
3′UTR, in order to promote antler growth and regeneration.
The results of this study showed that miR-1 affected
the proliferation of antler cartilage cells. Transfection with an
miR-1 mimic significantly inhibited the proliferation of cartilage
cells compared with the NC group. These results suggested that a
loss of miR-1 contributes to the promotion of cartilage cell
proliferation. Furthermore, we found that the cell cycle
distribution was affected by miR-1 expression in cartilage cells.
The percentage of cells in the S-phase is an important indicator
for determining the proliferation of cells. The smaller the
percentage of cells in the S-phase, the lower the cell
proliferation. FCM analysis indicated that the number of S-phase
cells markedly decreased, and miR-1 transfection interacted with
and disrupted the antler cells during mitosis. Therefore, this
study provides evidence for the first time that miR-1 inhibits
proliferation and cell cycle progression in the cartilage cells of
deer antlers.
Furthermore, western blot analysis revealed that the
expression levels of IGF-1 in cells transfected with the miR-1
mimic was lower than those in the NC group. This further
illustrated the inhibitory effect of miR-1 on the expression of
IGF-1. Sun et al(22)
reported that miR-1 was able to regulate the expression of human
IGF-1. In this study, miR-1 repressed the cellular mRNA and protein
levels of IGF-1 by directly targeting the binding site within the
3′UTR, which subsequently led to a reduction in cell viability.
These results suggested that miR-1 negatively regulates IGF-1
expression at the post-transcriptional level. The repression of the
target by miRNA depends on the degree of complementarity between
the miRNA and the target. In animals, mature miRNAs suppress gene
expression by imperfect base pairing to the 3′UTR of the target
mRNAs, leading to the repression of protein production and in
certain cases, mRNA degradation. Since the miRNA-mediated
suppression of protein production occurs through a mechanism that
operates following the initiation of protein synthesis, a reduction
in protein production is not necessarily accompanied by a change in
corresponding mRNA levels (25,26).
The underlying mechanisms that mediate miR-1
deregulation in deer antler development remain elusive. This study
suggests that miR-1 has a crucial role in antler regeneration.
Since TargetScan predicted hundreds of potential targets of miR-1,
and a single miRNA is capable of targeting multiple mRNAs in order
to regulate gene expression (27), other miR-1 targets may also
participate in antler development. Based on this theory, further
studies are required to identify the additional roles of miR-1 in
antler growth. In the future, we aim to determine whether the
deregulation of miR-1 expression in antler growth is mediated by
epigenetic alterations, including deregulated DNA methylation or
histone modification.
Acknowledgements
This study was supported by a grant from the
National Natural Science Foundation of China (no. 30972083) and was
performed by the Jilin Provincial Science Technology Development
Foundation of China (no. 20090574).
References
|
1
|
Miller SC, Bowman BM and Jee WS: Available
animal models of osteopenia - small and large. Bone. 17:S117–S123.
1995. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Kierdorf U, Li C and Price JS: Improbable
appendages: deer antler renewal as a unique case of mammalian
regeneration. Semin Cell Dev Biol. 20:535–542. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
King GL, Kahn CR, Samuels B, et al:
Synthesis and characterization of molecular hybrids of insulin and
insulin-like growth factor I. The role of the A-chain extension
peptide. J Biol Chem. 257:10869–10873. 1982.PubMed/NCBI
|
|
4
|
Panagakos FS: Insulin-like growth
factors-I and -II stimulate chemotaxis of osteoblasts isolated from
fetal rat calvaria. Biochimie. 75:991–994. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Werther GA, Russo V, Baker N and Butler G:
The role of the insulin-like growth factor system in the developing
brain. Horn Res Paed. 49:37–40. 2004.
|
|
6
|
Zheng WH, Kar S, Dore S and Quirion R:
Insulin-1ike growth factor-1 (IGF-1): a neuro protective trophic
factor acting via the Akt kinase pathway. J Neural Transm Suppl.
60:261–272. 2000.PubMed/NCBI
|
|
7
|
Rubin J, Ackert-Bicknell CL, Zhu L, et al:
IGF-I regulates osteoprotegerin (OPG) and receptor activator of
nuclear factor-kappaB ligand in vitro and OPG in vivo. J Clin
Endocrinol Metab. 87:4273–4279. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Suttie JM, White RG, Breier BH and
Gluckman PD: Photoperiod associated changes in insulin-like growth
factor-I in reindeer. Endocrinology. 129:679–682. 1991. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Elliott JL, Oldham JM, Ambler GR, et al:
Presence of insulin-like growth factor-I receptors and absence of
growth hormone receptors in the antler tip. Endocrinology.
130:2513–2520. 1992.PubMed/NCBI
|
|
10
|
Sadighi M, Haines SR, Skottner A, Harris
AJ and Suttie JM: Effects of insulin-like growth factor-I (IGF-I)
and IGF-II on the growth of antler cells in vitro. J Endocrinol.
143:461–469. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Li G, Gao X, Wang K, Gao Y and Zhao J:
Research on the relationship of the serum concentrations of insulin
growth factor 1 and the levels of nutrients in the sika deer and
Chinese wapiti. Spec Wild Econ Anim Plant Res. 26:1–6. 2004.
|
|
12
|
Hu W, Meng X, Tian Y and Liu N: Cloning of
a full-length cDNA encoding insulin-like growth factor I and its
expression in antler tissue. J Northeast Forestry Univ. 11:71–75.
2011.
|
|
13
|
Shabalina SA and Spiridonov NA: The
mammalian transcriptome and the function of non-coding DNA
sequences. Genome Biol. 5:1052004. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Lee RC, Feinbaum RL and Ambros V: The C.
elegans heterochronic gene lin-4 encodes small RNAs with antisense
complementarity to lin-14. Cell. 75:843–854. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Rajewsky N: MicroRNA target predictions in
animals. Nat Genet. 38:S8–S13. 2006. View
Article : Google Scholar
|
|
16
|
Zeng Y: Principles of microRNA production
and maturation. Oncogene. 25:6156–6162. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Tang F, Kaneda M, O’Carroll D, et al:
Maternal microRNAs are essential for mouse zygotic development. Gen
Dev. 21:644–648. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Stark A, Brennecke J, Bushati N, Russell
RB and Cohen SM: Animal microRNAs confer robusness to gene
expression and have a significant on 3′UTR evolution. Cell.
123:1133–1146. 2005.PubMed/NCBI
|
|
19
|
Tay Y, Zhang J, Thoinson AM, Lim B and
Rigoutsos I: MicroRNAs to Nanog, Oct4 and Sox2 coding regions
modulate embryonic stem cell differentiation. Nature.
455:1124–1128. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Tian S, Huang S, Wu S, Guo W, Li J and He
X: MicroRNA-1285 inhibits the expression of p53 by directly
targeting its 3′ untranslated region. Biochem Biophys Res Commun.
396:435–439. 2010.PubMed/NCBI
|
|
21
|
Baek D, Villén J, Shin C, Camargo FD, Gygi
SP and Bartel DP: The impact of microRNAs on protein output.
Nature. 455:64–71. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Sun Y, Ge Y, Drnevich J, Zhao Y, Band M
and Chen J: Mammalian target of rapamycin regulates miRNA-1 and
follistatin in skeletal myogenesis. J Cell Biol. 189:1157–1169.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Hausser J, Berninger P, Rodak C, Jantscher
Y, Wirth S and Zavolan M: MirZ: an integrated microRNA expression
atlas and target prediction resource. Nucleic Acids Res.
37:W266–W272. 2009.PubMed/NCBI
|
|
24
|
Yang M and Mattes J: Discovery, biology
and therapeutic potential of RNA interference, microRNA and
antagomirs. Pharmacol Ther. 117:94–104. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Nilsen TW: Mechanisms of microRNA-mediated
gene regulation in animal cells. TRENDS Genet. 23:243–249. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Selbach M, Schwanhäusser B, Thierfelder N,
Fang Z, Khanin R and Rajewsky N: Widespread changes in protein
synthesis induced by microRNAs. Nature. 455:58–63. 2008. View Article : Google Scholar : PubMed/NCBI
|