Introduction
Autism is a common neurological disorder that occurs
during childhood and is characterized by impairments in social
interaction and communication, as well as restricted and repetitive
behaviors (1,2). In 2008, the Centers for Disease
Control and Prevention (CDC; Atlanta, GA, USA) estimated that the
incidence of autism spectrum disorders was 11.4 cases per 1,000
children (8 years of age) compared with 6.4 cases per 1,000
children in 2002 (3). Since autism
was first recognized as a disorder in 1943, studies investigating
its etiology initially focused on the biological causes of the
disorder and gradually shifted to the psychological causes.
Recently, the focus of studies has shifted back to the biological
etiologies, as data in support of genetic causes have been
reported; in particular, a strong correlation between autism and
various markers on chromosome 7 (4,5).
However, the causes of 80–90% of cases of autism are unknown.
Previous studies have reported neurohistological and
anatomical alterations in autistic brains (6–8).
Biochemical studies have also reported alterations in the levels of
Bcl-2, p53, reelin, brain-derived neurotrophic factor (BDNF) and
acetylcholine receptors in the cerebellar and parietal areas of
autistic brains (9,10). In the brain of autistic
individuals, pre- and postnatal developmental abnormalities involve
multiple regions of the brain, including the cerebral cortex,
cortical white matter, amygdala, brainstem and cerebellum (8). The cerebellum is considered to be the
region of the brain that is vulnerable to autism, as well as an
important therapeutic target to improve an autistic-like state.
Abnormalities of the cerebellum in autism, Purkinje cell loss in
the cerebellar vermis and cerebellar hyperplasia and hypoplasia are
strongly associated with the impairment of gait, motor preparation,
grip, manual dexterity, ball skills, locomotion and balance
(6,7).
Apoptosis is a form of cell death that constitutes
part of a common mechanism in cell replacement, tissue remodeling
and the removal of damaged cells (11). Apoptosis consists of two main
pathways, the extrinsic and intrinsic pathway. A class of cysteine
proteases, including caspase-3, caspase-8 and caspase-9, is
commonly involved in these pathways (12). As well as the caspases, the Bcl-2
family of proteins are also important in the regulation of
apoptosis. Bcl-2 proteins are classified into anti-apoptotic
proteins, which include Bcl-2 and Bcl-2 XL, and pro-apoptotic
proteins, including Bax and Bid (13). Excessive neuronal apoptosis
contributes to the dysfunction of the central nervous system
(14,15).
Reelin is an extracellular glycoprotein that is
essential for neuronal migration and brain development (9,16).
Reelin glycoprotein is a secretory serine protease that performs
dual roles in the mammalian brain. In the embryo, it guides neurons
and radial glial cells to their correct positions in the developing
brain. In the adult brain, reelin is involved in a signaling
pathway that regulates neurotransmission, memory formation and
synaptic plasticity (16). The
functions of reelin are mediated through the receptors
apolipoprotein E receptor 2 (ApoER2) and very-low-density
lipoprotein receptor (VLDLR), which trigger a complex signaling
cascade (17,18). Fatemi et al(9) observed deficits in reelin mRNA and
protein levels in the brains of patients with schizophrenia, major
depression and autism, and suggested that the dysregulation of
reelin may be responsible for a number of the structural and
behavioral abnormalities observed in the brains of patients with
autism (9). Activation of the
reelin signaling pathway may inhibit excitotoxic neurotransmission
and Tau phosphorylation, and may activate neurogenesis. This may
lead to diminished brain injury and an increased rate of brain
injury repair (19).
Glutamate decarboxylase p67 (GAD67), a downstream
molecule of reelin, is an important enzyme that regulates GABAergic
function in the central nervous system. The predominant finding in
the postmortem brain of subjects diagnosed with schizophrenia,
autism and bipolar illness was a decrease in GAD67 mRNA levels,
which affected multiple brain regions (20). Cyclin D1 is a protein encoded by
the Bcl-1 gene and is important for regulating the cell cycle. The
expression levels of cyclin D1 and reelin are correlated with the
morphological development of the cerebellum (21).
Exercise enhances learning ability and memory
functions, provides protection from neurodegeneration, delays
age-related cognitive decline and alleviates the symptoms of
developmental and neuropsychiatric disorders (22–25).
Several studies have suggested that exercise may be a critical
mediator in treating neuropsychiatric disorders, including autism
(24,26–28).
In the present study, we evaluated the effect of
treadmill exercise on motor coordination and balance in correlation
with reelin expression and the rate of apoptosis in the cerebellum
using valproic acid-induced autistic rat pups. Motor coordination
and balance were determined using the rotarod test and the vertical
pole test. Immunofluorescence, immunohistochemistry and western
blot examinations were also conducted.
Materials and methods
Animals and the induction of autism
Male Sprague-Dawley rat pups (weight, 25±5 g; age, 2
weeks old) were used in this study and all experimental procedures
were performed in accordance with the animal care guidelines of the
National Institutes of Health (NIH) and the Korean Academy of
Medical Sciences (Seoul, Korea). The animals were housed under
controlled temperature (23±2°C) and lighting (08:00 to 20:00 h)
conditions, with food and water available ad libitum. The
animals were randomly divided into four groups (n=10 in each
group); the control group, the control and treadmill exercise
group, the valproic acid-treated group and the valproic
acid-treated and treadmill exercise group.
For the induction of the autism-like animal models,
400 mg/kg valproic acid (Sigma-Aldrich, St. Louis, MO, USA) was
dissolved in saline at a concentration of 0.1 ml/kg and was
subcutaneously injected into the rat pups on postnatal day 14,
according to a previously described method (29). The day of birth was recorded as day
0 and all pups were labeled for individual identification. Rat pups
in the control groups received subcutaneous injection of saline at
the same volume and on the same schedule as the treadmill exercise
groups.
Treadmill exercise
Rat pups in the treadmill exercise groups were
forced to run on a treadmill for 30 min, once a day, five times a
week for 4 weeks, starting at postnatal day 28. Exercise load for
the exercise groups consisted of running at a speed of 2 m/min for
the first 5 min, 5 m/min for the next 5 min and then at 8 m/min for
the last 20 min, with a 0° inclination.
Rotarod test
We performed the rotarod test (Biological Research
Apparatus, Ugo Basile, Varese, Italy) to measure motor coordination
and balance, according to a previously described method (30). Each rat pup was placed in a
separate compartment on a rotating 7-cm diameter rod. The velocity
of the rod was set to a constant 15 rpm. The time of latency until
fall-off was automatically recorded by magnetic trip plates. To
eliminate stress and fatigue, the rat pups were given a maximum
cut-off latency of 180 sec.
Vertical pole test
For the measurement of motor coordination and
balance, the vertical pole test was conducted as previously
described (31). Each rat pup was
placed face-up on a cloth tape-covered pole (3 cm diameter, 150 cm
long). The pole was held in a horizontal position and then
gradually lifted to a vertical position. The degree of angle until
fall off was recorded.
Tissue preparation
The experimental animals were fully anesthetized
using Zoletil 50® (10 mg/kg, i.p.; Vibac Laboratories,
Carros, France), transcardially perfused with 50 mM
phosphate-buffered saline (PBS) and fixed with a freshly prepared
solution consisting of 4% paraformaldehyde in 100 mM phosphate
buffer (PB; pH 7.4). The brains were dissected, post-fixed in the
same fixative overnight and transferred to 30% sucrose for
cryoprotection. Sagittal sections (thickness, 40 μm) in each
section of the cerebellum were cut with a freezing microtome
(Lieca, Nussloch, Germany).
Western blotting for reelin, GAD67,
cyclin D1, Bax and Bcl-2 expression levels
Western blotting was performed according to a
previously described method (23,32).
The cerebellar tissues were collected and immediately frozen at
−70°C. When used, the tissues were homogenized on ice and lysed in
a lysis buffer containing 50 mM HEPES (pH 7.5), 150 mM NaCl, 10%
glycerol, 1% Triton X-100, 1 mM phenylmethylsulfonylfluoride, 1 mM
EGTA, 1.5 mM MgCl2·6H2O, 1 mM sodium
orthovanadate and 100 mM sodium fluoride. The protein content was
measured using a colorimetric protein assay kit (Bio-Rad, Hercules,
CA, USA). Protein samples (30 μg) were separated on sodium dodecyl
sulfate-polyacrylamide gels and transferred onto a nitrocellulose
membrane.
Mouse β-actin antibody (1:3,000; Santa Cruz
Biotechnology, Inc., Santa Cruz, CA, USA), mouse reelin antibody
(1:1,000; Chemicon International, Temecula, CA, USA), rabbit GAD67
antibody (1:1,000; Santa Cruz Biotechnology, Inc.), mouse cyclin D1
antibody (1:1,000; Santa Cruz Biotechnology, Inc.), mouse Bcl-2
antibody (1:1,000; Santa Cruz Biotechnology, Inc.), mouse Bax
antibody (1:1,000; Santa Cruz Biotechnology, Inc.) and rabbit
cleaved caspase-3 antibody (1:1,000; Cell Signaling Technology,
Inc., Beverly, MA, USA) were used as the primary antibodies.
Horseradish peroxidase-conjugated anti-rabbit antibody for GAD67
and cleaved caspase-3 (1:4,000; Vector Laboratories, Burlingame,
CA, USA) and horseradish peroxidase-conjugated anti-mouse antibody
for actin, cyclin D1, Bcl-2 and Bax (each 1:3,000; Amersham
Pharmacia Biotechnology GmbH, Freiburg, Germany) were used as the
secondary antibodies.
The experiments were performed in normal laboratory
conditions and at room temperature, except for the membrane
transfer. The membranes were transferred at 4°C using a cold pack
and pre-chilled buffer. Band detection was performed using the
enhanced chemiluminescence (ECL) detection kit (Santa Cruz
Biotechnology, Inc.). In order to compare the relative expression
levels, the detected bands were calculated densitometrically using
Molecular Analyst™, version 1.4.1 (Bio-Rad).
Immunofluorescence for reelin, calbindin
D-28k and caspase-3 expression
Cerebellum samples were embedded and frozen at
−20°C, and mounted on positively charged slides. For the
visualization of reelin, calbindin D-28k and cleaved caspase-3
expression, immunofluorescence was performed according to a
previously described method (33).
For double immunofluorescence staining, the sections (40 μm) were
fixed with 4% paraformaldehyde and 4% sucrose in PBS at room
temperature for 40 min, permeabilized with 0.5% Nonidet P-40 in PBS
and blocked with 2.5% horse serum and 2.5% bovine serum albumin for
4 h at room temperature. The sections were incubated with
anti-calbindin D-28k rabbit polyclonal antibody (1:800; Santa Cruz
Biotechnology, Inc.), anti-reelin mouse monoclonal antibody (1:500;
Millipore Corporation, Billerica, MA, USA), anti-cleaved caspase-3
rabbit polyclonal antibody (1:400; Cell Signaling Technology, Inc.)
and then incubated with fluorescein-goat anti-mouse (1:600;
Molecular Probes, Eugene, OR, USA) or rhodamine-goat anti-rabbit
secondary antibodies (1:800; Molecular Probes) in 3% bovine serum
albumin for 1 h at room temperature, and coverslipped with gelatin
mount medium. The sections were observed using a fluorescence
microscope and images were captured using an attached camera.
Immunohistochemistry for caspase-3
expression
Caspase-3 immunohistochemistry was performed
according to a previously described method (23). In brief, the sections were
incubated overnight with mouse anti-cleaved caspase-3 antibody
(1:500; Santa Cruz Biotechnology, Inc.) and incubated for 1 h with
biotinylated mouse secondary antibody. The bound secondary antibody
was then amplified using the Vector Elite ABC kit®
(1:100; Vector Laboratories). The antibody-biotin-avidin-peroxidase
complex was visualized using 0.03% 3,3′-diaminobenzidine. The
sections were finally mounted onto gelatin-coated slides. The
slides were air-dried overnight at room temperature and the
coverslips were mounted using Permount® (Thermo Fisher
Scientific Inc., Waltham, MA, USA).
Statistical analysis
The data are expressed as the mean ± standard error
of the mean (SEM). All data were analyzed using SPSS statistical
software version 12.0 (SPSS, Inc., Chicago, IL, USA). For
comparisons among the groups, one-way ANOVA and the Duncan’s
post-hoc test were performed. P<0.05 was considered to indicate
a statistically significant result.
Results
Effect of treadmill exercise on motor
coordination and balance in the rotarod test and vertical pole
test
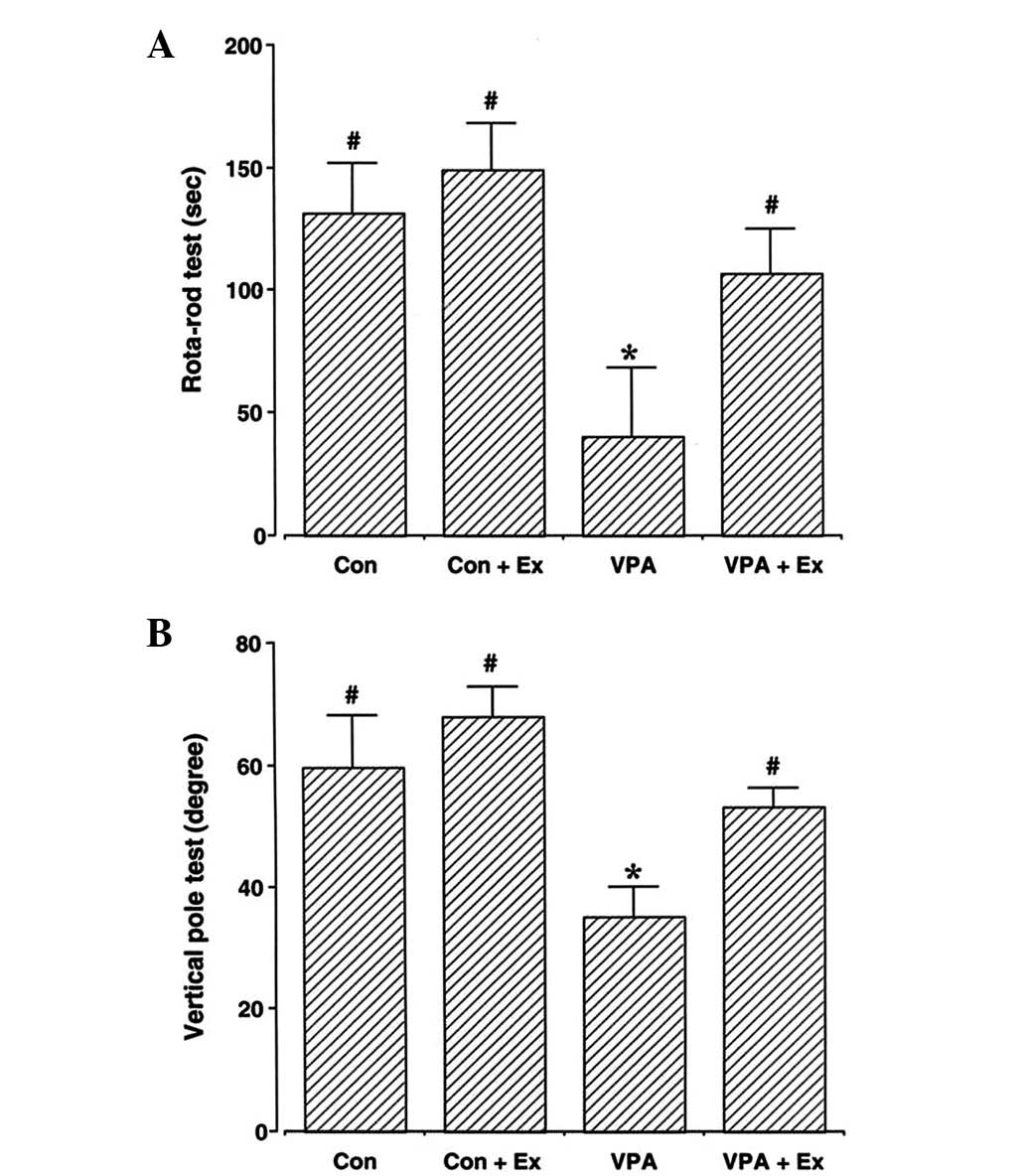
Motor coordination and balance were determined using
the rotarod test and vertical pole test (Fig. 1). The results indicated that motor
coordination and balance were affected by the induction of autism
(P<0.05). By contrast, treadmill exercise ameliorated the
disturbance of motor coordination and balance in the autistic rat
pups (P<0.05). Under normal conditions, treadmill exercise
exerted no significant effect on motor coordination and
balance.
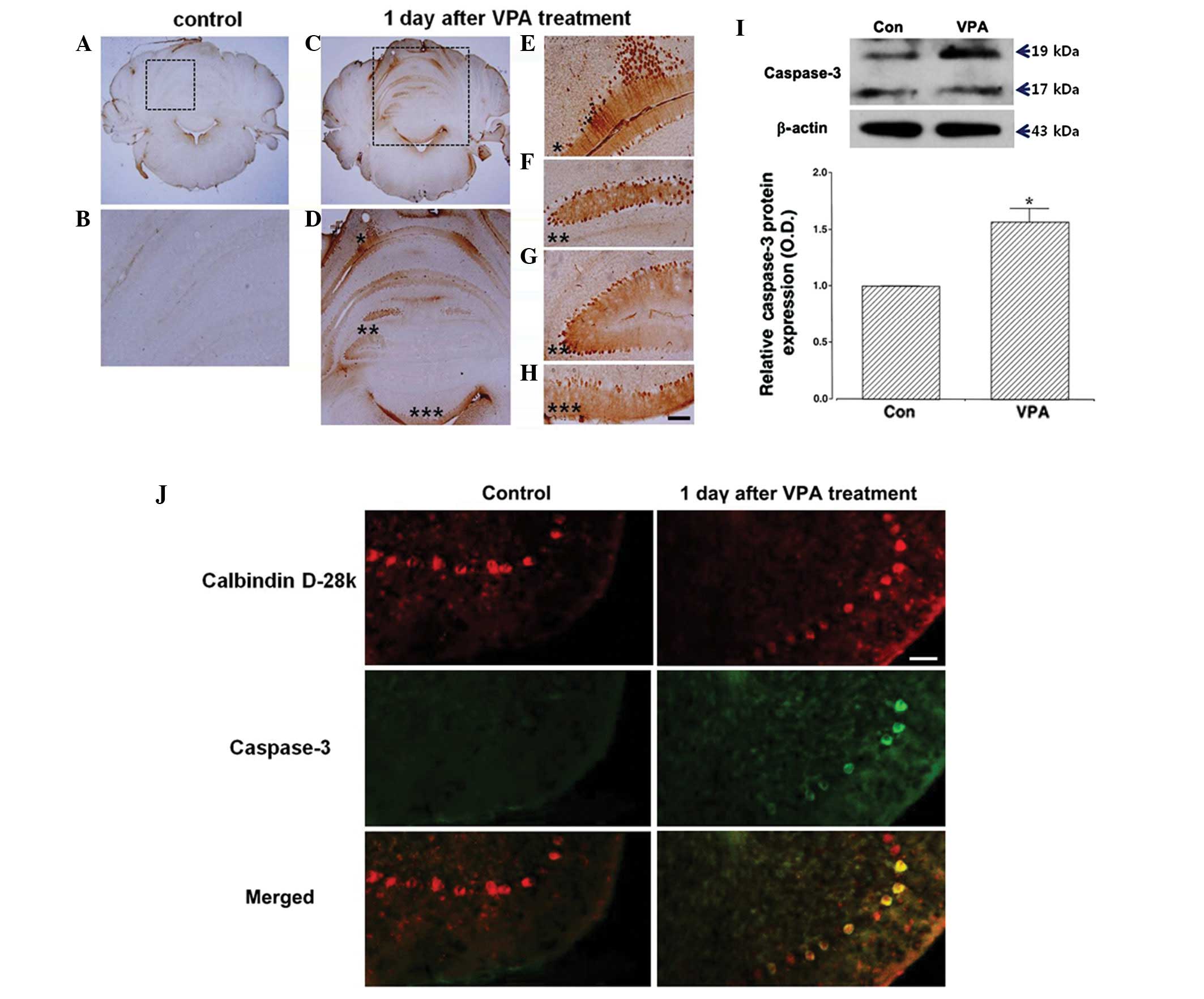
Caspase-3 expression in the cerebellum 1
day following valproic acid treatment
Representative photomicrographs of cleaved
caspase-3-positive cells in the cerebellar vermis are presented in
Fig. 2A–H. Numerous
caspase-3-labeled cells were observed in the cerebellar Purkinje
cell layer of the valproic acid-treated group, while
caspase-3-positive cells were not detected in the control group. We
quantitatively assessed caspase-3 expression in the cerebellum of
the valproic acid-treated group (Fig.
2I). When the band intensity of caspase-3 in the control group
was set at 1.00, the level of caspase-3 was 1.57±0.11 in the
valproic acid-treated group. To examine the rate of Purkinje cell
death by valproic acid treatment, prepared cerebellar tissues were
used for double immunostaining with anti-calbindin D-28k and
anti-cleaved caspase-3 antibodies. As shown in Fig. 2J, numerous cleaved
caspase-3-positive cells in the valproic acid-treated group
overlapped with Purkinje neurons; however, no overlapping was
observed in the control group. The results demonstrated the
presence of apoptotic cell death in cerebellar Purkinje cells 1 day
following valproic acid treatment.
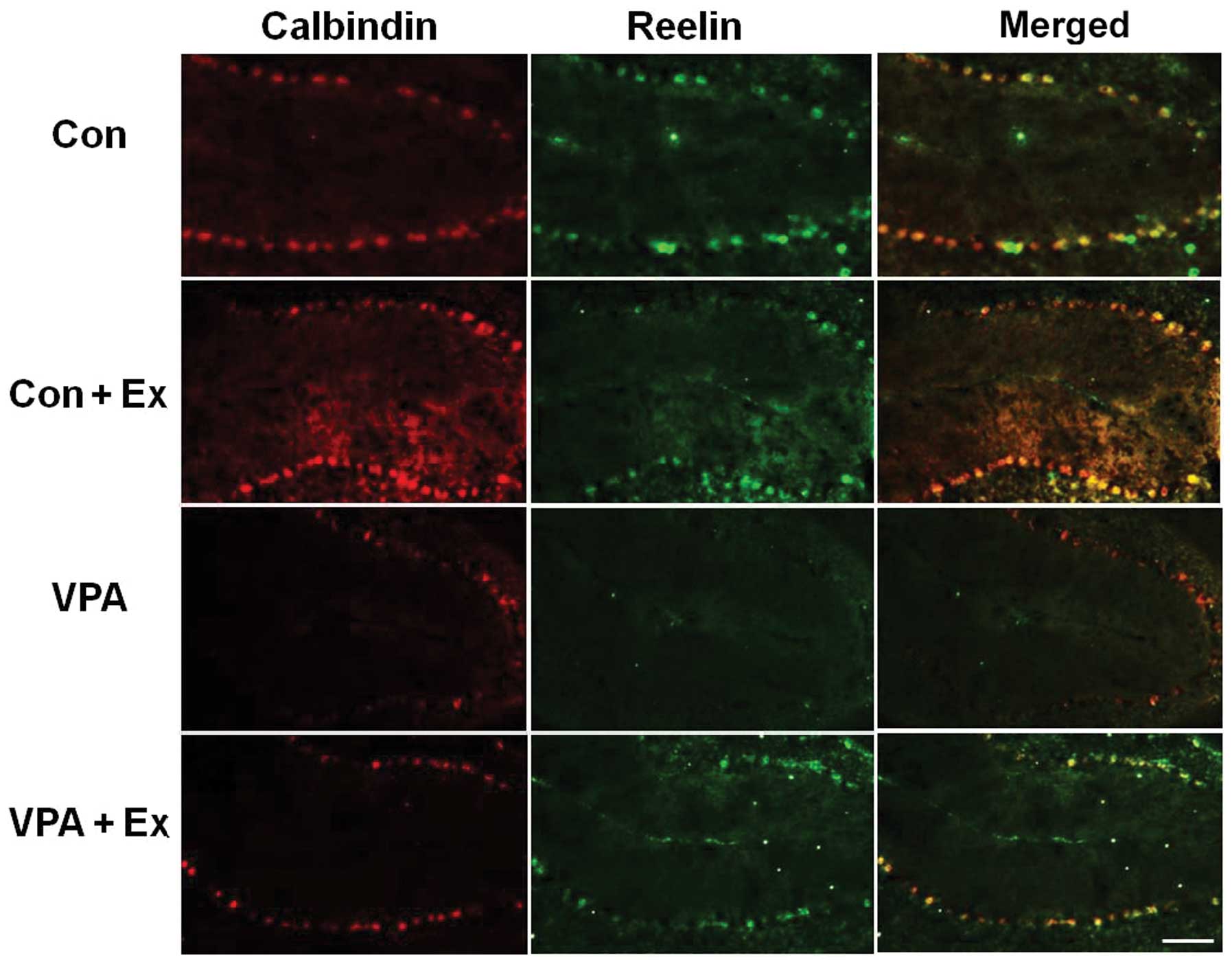
Effect of treadmill exercise on reelin
expression in the cerebellum
To examine the effect of treadmill exercise on
reelin expression, we performed double immunostaining using
anti-calbindin D-28k and anti-reelin antibodies (Fig. 3). Reelin proteins were merged with
calbindin-positive neurons in all groups. Valproic acid
downregulated the number of Purkinje neurons that overlapped with
reelin, while treadmill exercise increased the number of Purkinje
neurons, as well as the expression of reelin in the cerebellum of
the autistic rat pups. The present results indicated that reelin
expression in the cerebellum was decreased by the induction of
autism. By contrast, treadmill exercise upregulated reelin
expression in the autistic rat pups.
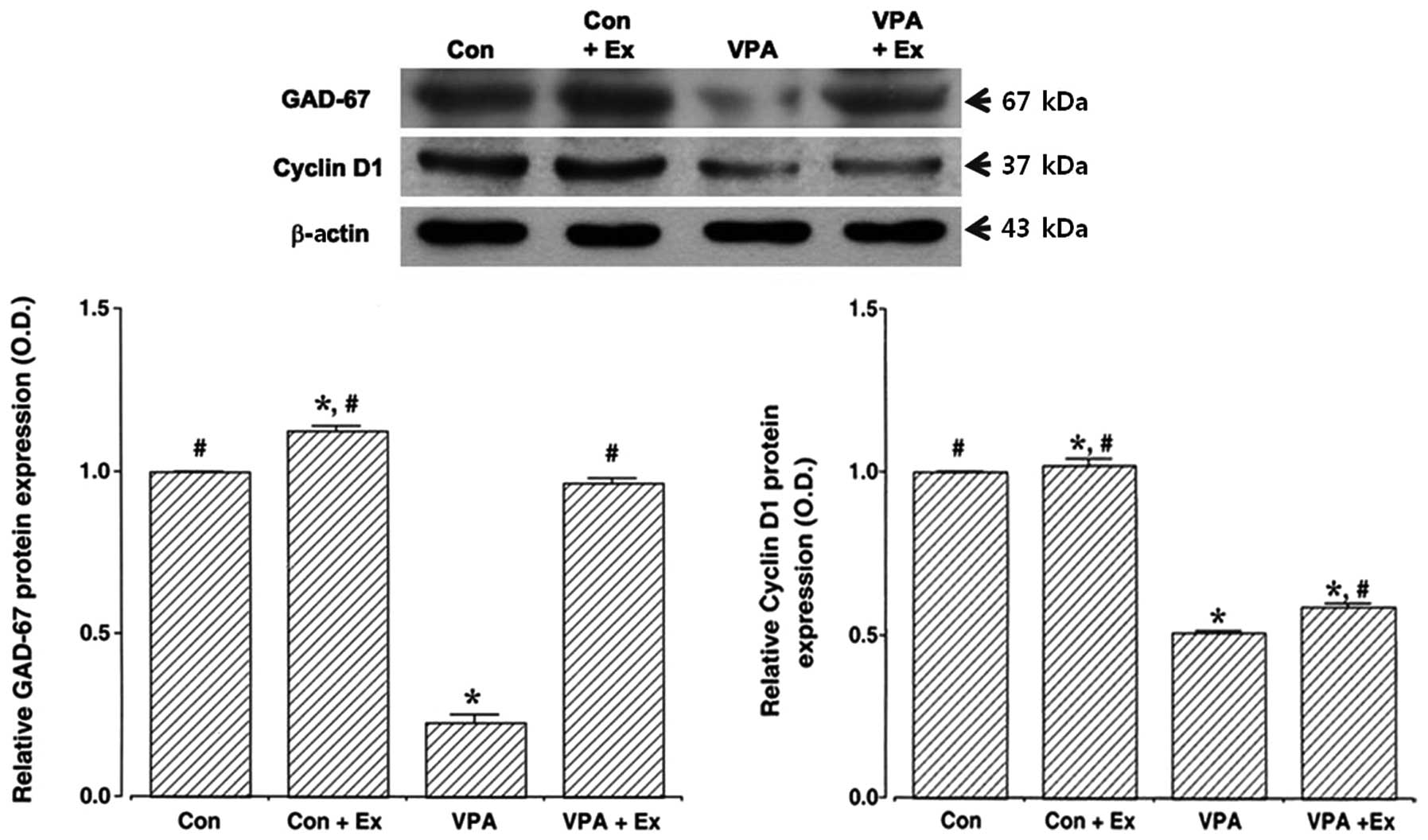
Effect of treadmill exercise on GAD67 and
cyclin D1 expression in the cerebellum
To examine the effect of treadmill exercise on the
expression of GAD67 and cyclin D1, both of which are downstream
molecules of reelin, we analyzed the relative expression of GAD67
and cyclin D1 proteins by western blotting (Fig. 4). The results indicated that GAD67
and cyclin D1 expression levels in the cerebellum were decreased
following the induction of autism (P<0.05). By contrast,
treadmill exercise upregulated GAD67 and cyclin D1 expression
levels in the autistic rat pups (P<0.05). Under normal
conditions, treadmill exercise increased the expression levels of
GAD67 and cyclin D1 (P<0.05).
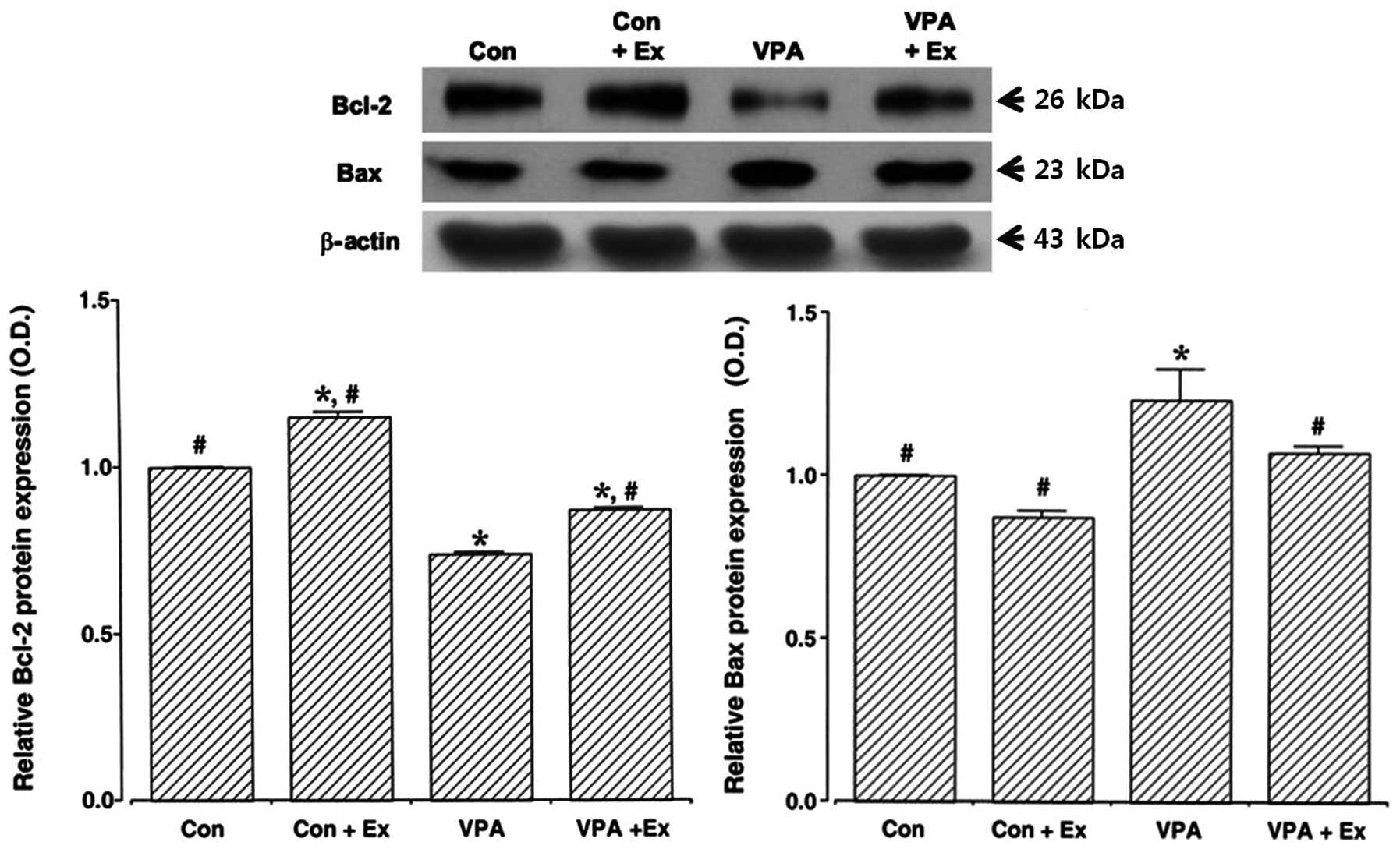
Effect of treadmill exercise on Bcl-2 and
Bax expression in the cerebellum
To examine the effect of treadmill exercise on Bcl-2
and Bax expression, we analyzed the relative expression of Bcl-2
and Bax proteins using western blotting (Fig. 5). The results indicated that Bcl-2
expression levels in the cerebellum were decreased and Bax
expression levels in the cerebellum were increased by the induction
of autism (P<0.05). By contrast, treadmill exercise enhanced
Bcl-2 expression and suppressed Bax expression in the autistic rat
pups (P<0.05). Under normal conditions, treadmill exercise
increased Bcl-2 expression; however, treadmill exercise exerted no
significant effect on Bax expression.
Discussion
Cerebellar structural abnormalities in autism
include the loss of granular and Purkinje cells (34), and atrophy of Purkinje cells
(35). In the present study, the
loss of cerebellar Purkinje cells and caspase-3 activation were
observed 1 day following 400 mg/kg valproic acid injection.
Caspase-3 is the most widely studied member of the caspase family
and is a key executor of apoptosis (36). Enhanced caspase-3 activation by
valproic acid injection may contribute to Purkinje cell loss in the
cerebellum.
Valproic acid has been used as an anticonvulsant and
mood stabilizer for seizures and epilepsy (37,38).
Exposure to valproic acid during pregnancy leads to the retardation
of embryonic motor function and cognition; in a study in which
maternal rats received 350 mg/kg of valproic acid on days 11.5 (the
day of neural tube closure), 12 or 12.5 of gestation, autism-like
brain lesions were detected in the rat pups (39). Postnatal administration of valproic
acid induced behavioral and neuroanatomical abnormalities similar
to those observed in autism (40).
Notably, Wagner et al(29)
studied the dose-dependent effect of valproic acid on the
neurobehavioral alterations representing autism using postnatal day
14 mice. This study demonstrated that mice treated with 400 mg/kg
valproic acid exhibited more severe autism-like symptoms. In the
present study, a dose of 400 mg/kg valproic acid initiated
apoptotic Purkinje cell death and this dosage was sufficient to
induce autism in the rat pups.
To examine motor abnormality and imbalance, we used
the rotarod test and vertical pole test. The latency in the rotarod
test and the degree of angle in the vertical pole test prior to
fall-off were decreased in the valproic acid-induced autistic rat
pups. By contrast, treadmill exercise increased the time of latency
in the rotarod test and the degree of angle in the vertical pole
test in the autistic rat pups. The cerebellum is important in motor
coordination and balance and has been recognized as a prominent
contributor to a wide array of cognitive and emotional functions
(2,41). Motor function disturbances,
including a disturbance of motor anticipation, postural and gait
abnormalities, various degrees of dystonia, bradykinesia and
hyperkinesia, and abnormality of muscle tone are important aspects
of the description of autism (2,42).
The results of the present study also demonstrate
that cerebellar Purkinje cell loss was accompanied with a decrease
in reelin expression in the valproic acid-induced autistic rat
pups. By contrast, treadmill exercise alleviated Purkinje cell loss
with increased reelin expression in the autistic rat pups. Death of
cerebellar Purkinje neurons in autism is strongly associated with
the reduced activation of the reelin signaling pathway in several
psychiatric disorders, including depression, schizophrenia and
autism (5,43). Reelin is a serine protease and an
important glycoprotein involved in the specific layering of the
developing brain (9,44); it is preferentially secreted by
cortical GABAergic interneurons. Disruption of the reelin signaling
pathway is strongly associated with the onset of neuropsychiatric
disorders, including autism and schizophrenia (16).
In the present study, the expression levels of GAD67
and cyclin D1 were suppressed in the cerebellum of valproic
acid-induced autistic rat pups. By contrast, treadmill exercise
increased GAD67 and cyclin D1 expression levels in the cerebellum
of the autistic rat pups. In a previous study, GAD67 mRNA levels
were reduced by 40% in the autistic group, suggesting that reduced
Purkinje cell GABA input to the cerebellar nuclei potentially
disrupts cerebellar output to higher association cortices,
affecting motor and/or cognitive function. The authors of this
study suggested that the dysregulation of reelin and GAD67
expression may be responsible for a number of the brain structural
abnormalities observed in autism (45). The dysregulation of cyclin D1
expression, resulting in neuro-cardio-facial-cutaneous syndromes,
is associated with developmental abnormalities, cognitive deficits
and autism (46).
We additionally demonstrated that Bcl-2 expression
was decreased and Bax expression was increased in the cerebellum of
valproic acid-induced autistic rat pups. By contrast, treadmill
exercise increased Bcl-2 expression and decreased Bax expression in
the cerebellum of the autistic rat pups. In previous comparative
studies of autistic and normal control cerebellar cortices, the
expression levels of reelin and Bcl-2 in the autistic cerebellum
were reduced compared with the controls (9), and it was suggested that the
dysregulation of reelin and Bcl-2 may be responsible for a number
of brain structural and behavioral abnormalities observed in
autism.
The anti-apoptotic and neuron maturation effects of
exercise on various neuropsychiatric diseases are well documented
(14,24,32,33,47).
Celiberti et al(26)
reported that regular exercise suppressed autism-like symptoms in a
5-year-old male with autism. Following bouts of physical activity,
children with autism experienced a decrease in the levels of
negative behaviors, including stereotypy and an incremental change
in positive behaviors, including time on task (27,48).
Petrus et al(28) suggested
that exercise provides a reduction of stereotypic behaviors in
children with autism spectrum disorder. Children with autism are
more physically inactive compared with their non-autistic peers
(49).
In conclusion, we demonstrated that treadmill
exercise is capable of ameliorating motor dysfunction in autistic
rat pups. The therapeutic effect of treadmill exercise on motor
deficits may be due to the reelin-mediated anti-apoptotic effect of
treadmill exercise on cerebellar Purkinje neurons. These results
support the theory that exercise may provide a potential
therapeutic strategy for the alleviation of motor symptoms in
autistic patients.
Acknowledgements
This study was supported by the National Research
Foundation of Korea grant funded by the Korean Government
(NRF-2010-0022895).
References
|
1
|
Hardan AY, Kilpatrick M, Keshavan MS and
Minshew NJ: Motor performance and anatomic magnetic resonance
imaging (MRI) of the basal ganglia in autism. J Child Neurol.
18:317–324. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Vernazza-Martin S, Martin N, Vernazza A,
Lepellec-Muller A, Rufo M, Massion J and Assaiante C: Goal directed
locomotion and balance control in autistic children. J Autism Dev
Disord. 35:91–102. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Kuehn BM: Data on autism prevalence,
trajectories illuminate socioeconomic disparities. JAMA.
307:2137–2138. 2012.PubMed/NCBI
|
|
4
|
Badner JA and Gershon ES: Regional
meta-analysis of published data supports linkage of autism with
markers on chromosome 7. Mol Psychiatry. 7:56–66. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Fatemi SH: The role of Reelin in pathology
of autism. Mol Psychiatry. 7:919–920. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Allen G and Courchesne E: Differential
effects of developmental cerebellar abnormality on cognitive and
motor functions in the cerebellum: an fMRI study of autism. Am J
Psychiatry. 160:262–273. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Gowen E and Miall RC: Behavioural aspects
of cerebellar function in adults with Asperger syndrome.
Cerebellum. 4:279–289. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Schmitz C and Rezaie P: The neuropathology
of autism: where do we stand? Neuropathol Appl Neurobiol. 34:4–11.
2008.PubMed/NCBI
|
|
9
|
Fatemi SH, Stary JM, Halt AR and Realmuto
GR: Dysregulation of Reelin and Bcl-2 proteins in autistic
cerebellum. J Autism Dev Disord. 31:529–535. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Perry EK, Lee ML, Martin-Ruiz CM, Court
JA, Volsen SG, Merrit J, Folly E, Iversen PE, Bauman ML, Perry RH
and Wenk GL: Cholinergic activity in autism: abnormalities in the
cerebral cortex and basal forebrain. Am J Psychiatry.
158:1058–1066. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Thompson CB: Apoptosis in the pathogenesis
and treatment of disease. Science. 267:1456–1462. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Nuñez G, Benedict MA, Hu Y and Inohara N:
Caspases: the proteases of the apoptotic pathway. Oncogene.
17:3237–3245. 1998.
|
|
13
|
Kuwana T and Newmeyer DD: Bcl-2-family
proteins and the role of mitochondria in apoptosis. Curr Opin Cell
Biol. 15:691–699. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Baek SS, Jun TW, Kim KJ, Shin MS, Kang SY
and Kim CJ: Effects of postnatal treadmill exercise on apoptotic
neuronal cell death and cell proliferation of maternal-separated
rat pups. Brain Dev. 34:45–56. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Kuhn HG, Biebl M, Wilhelm D, Li M,
Friedlander RM and Winkler J: Increased generation of granule cells
in adult Bcl-2-overexpressing mice: a role for cell death during
continued hippocampal neurogenesis. Eur J Neurosci. 22:1907–1915.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Fatemi SH: Reelin glycoprotein: structure,
biology and roles in health and disease. Mol Psychiatry.
10:251–257. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Beffert U, Morfini G, Bock HH, Reyna H,
Brady ST and Herz J: Reelin-mediated signaling locally regulates
protein kinase B/Akt and glycogen synthase kinase 3beta. J Biol
Chem. 277:49958–49964. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Beffert U, Weeber EJ, Durudas A, Qiu S,
Masiulis I, Sweatt JD, Li WP, Adelmann G, Frotscher M, Hammer RE
and Herz J: Modulation of synaptic plasticity and memory by Reelin
involves differential splicing of the lipoprotein receptor Apoer2.
Neuron. 47:567–579. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Delahaye NF, Coltel N, Puthier D, Barbier
M, Benech P, Joly F, Iraqi FA, Grau GE, Nguyen C and Rihet P: Gene
expression analysis reveals early changes in several molecular
pathways in cerebral malaria-susceptible mice versus cerebral
malaria-resistant mice. BMC Genomics. 8:4522007. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Akbarian S and Huang HS: Molecular and
cellular mechanisms of altered GAD1/GAD67 expression in
schizophrenia and related disorders. Brain Res Rev. 52:293–304.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Sergaki MC, Guillemot F and Matsas R:
Impaired cerebellar development and deficits in motor coordination
in mice lacking the neuronal protein BM88/Cend1. Mol Cell Neurosci.
44:15–29. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Cotman CW, Berchtold NC and Christie LA:
Exercise builds brain health: key roles of growth factor cascades
and inflammation. Trends Neurosci. 30:464–472. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Kim SE, Ko IG, Kim BK, Shin MS, Cho S, Kim
CJ, Kim SH, Baek SS, Lee EK and Jee YS: Treadmill exercise prevents
aging-induced failure of memory through an increase in neurogenesis
and suppression of apoptosis in rat hippocampus. Exp Gerontol.
45:357–365. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Kim H, Heo HI, Kim DH, Ko IG, Lee SS, Kim
SE, Kim BK, Kim TW, Ji ES, Kim JD, Shin MS, Choi YW and Kim CJ:
Treadmill exercise and methylphenidate ameliorate symptoms of
attention deficit/hyperactivity disorder through enhancing dopamine
synthesis and brain-derived neurotrophic factor expression in
spontaneous hypertensive rats. Neurosci Lett. 504:35–39. 2011.
View Article : Google Scholar
|
|
25
|
Seo JH, Kim TW, Kim CJ, Sung YH and Lee
SJ: Treadmill exercise during pregnancy ameliorates post-traumatic
stress disorder-induced anxiety-like responses in maternal rats.
Mol Med Rep. 7:389–395. 2013.PubMed/NCBI
|
|
26
|
Celiberti DA, Bobo HE, Kelly KS, Harris SL
and Handleman JS: The differential and temporal effects of
antecedent exercise on the self-stimulatory behavior of a child
with autism. Res Dev Disabil. 18:139–150. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Lang R, Koegel LK, Ashbaugh K, Regester A,
Ence W and Smith W: Physical exercise and individuals with autism
spectrum disorders: A systematic review. Res Autism Spectr Disord.
4:565–576. 2010. View Article : Google Scholar
|
|
28
|
Petrus C, Adamson SR, Block L, Einarson
SJ, Sharifnejad M and Harris SR: Effects of exercise interventions
on stereotypic behaviours in children with autism spectrum
disorder. Physiother Can. 60:134–145. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Wagner GC, Reuhl KR, Cheh M, McRae P and
Halladay AK: A new neurobehavioral model of autism in mice: pre-
and postnatal exposure to sodium valproate. J Autism Dev Disord.
36:779–793. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Uz T, Dimitrijevic N, Tueting P and Manev
H: 5-lipoxygenase (5LOX)-deficient mice express reduced
anxiety-like behavior. Restor Neurol Neurosci. 20:15–20.
2002.PubMed/NCBI
|
|
31
|
Bellum S, Thuett KA, Grajeda R and Abbott
LC: Coordination deficits induced in young adult mice treated with
methylmercury. Int J Toxicol. 26:115–121. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Sung YH, Kim SC, Hong HP, Park CY, Shin
MS, Kim CJ, Seo JH, Kim DY, Kim DJ and Cho HJ: Treadmill exercise
ameliorates dopaminergic neuronal loss through suppressing
microglial activation in Parkinson’s disease mice. Life Sci.
91:1309–1316. 2012.PubMed/NCBI
|
|
33
|
Shin MS, Kim BK, Lee SH, Kim TS, Heo YM,
Choi JH, Kim CJ and Lim BV: Treadmill exercise enhances motor
coordination and ameliorates Purkinje cell loss through inhibition
on astrocyte activation in the cerebellum of methimazole-induced
hypothyroidism rat pups. J Exerc Nutr Biochem. 16:73–84. 2012.(In
Korean).
|
|
34
|
Ritvo ER, Freeman BJ, Scheibel AB, Duong
T, Robinson H, Guthrie D and Ritvo A: Lower Purkinje cell counts in
the cerebella of four autistic subjects: initial findings of the
UCLA-NSAC Autopsy Research Report. Am J Psychiatry. 143:862–866.
1986. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Fatemi SH, Halt AR, Realmuto G, Earle J,
Kist DA, Thuras P and Merz A: Purkinje cell size is reduced in
cerebellum of patients with autism. Cell Mol Neurobiol. 22:171–175.
2002. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Cohen GM: Caspases: the executioners of
apoptosis. Biochem J. 326:1–16. 1997.
|
|
37
|
Calleja S, Salas-Puig J, Ribacoba R and
Lahoz CH: Evolution of juvenile myoclonic epilepsy treated from the
outset with sodium valproate. Seizure. 10:424–427. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Coppola G, Auricchio G, Federico R,
Carotenuto M and Pascotto A: Lamotrigine versus valproic acid as
first-line monotherapy in newly diagnosed typical absence seizures:
an open-label, randomized, parallel-group study. Epilepsia.
45:1049–1053. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Rodier PM, Ingram JL, Tisdale B, Nelson S
and Romano J: Embryological origin for autism: developmental
anomalies of the cranial nerve motor nuclei. J Comp Neurol.
370:247–261. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Ciesielski KT and Knight JE: Cerebellar
abnormality in autism: a nonspecific effect of early brain damage?
Acta Neurobiol Exp (Wars). 54:151–154. 1994.PubMed/NCBI
|
|
41
|
Tiemeier H, Lenroot RK, Greenstein DK,
Tran L, Pierson R and Giedd JN: Cerebellum development during
childhood and adolescence: a longitudinal morphometric MRI study.
Neuroimage. 49:63–70. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Kohen-Raz R, Volkmar FR and Cohen DJ:
Postural control in children with autism. J Autism Dev Disord.
22:419–432. 1992. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Maloku E, Covelo IR, Hanbauer I, Guidotti
A, Kadriu B, Hu Q, Davis JM and Costa E: Lower number of cerebellar
Purkinje neurons in psychosis is associated with reduced reelin
expression. Proc Natl Acad Sci USA. 107:4407–4411. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Quattrocchi CC, Wannenes F, Persico AM,
Ciafré SA, D’Arcangelo G, Farace MG and Keller F: Reelin is a
serine protease of the extracellular matrix. J Biol Chem.
277:303–309. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Yip J, Soghomonian JJ and Blatt GJ:
Decreased GAD67 mRNA levels in cerebellar Purkinje cells in autism:
pathophysiological implications. Acta Neuropathol. 113:559–568.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Pucilowska J, Puzerey PA, Karlo JC, Galán
RF and Landreth GE: Disrupted ERK signaling during cortical
development leads to abnormal progenitor proliferation, neuronal
and network excitability and behavior, modeling human
neuro-cardio-facial-cutaneous and related syndromes. J Neurosci.
32:8663–8677. 2012. View Article : Google Scholar
|
|
47
|
Kim SE, Ko IG, Park CY, Shin MS, Kim CJ
and Jee YS: Treadmill and wheel exercise alleviate
lipopolysaccharide-induced short-term memory impairment by
enhancing neuronal maturation in rats. Mol Med Rep. 7:31–36.
2013.
|
|
48
|
Levinson LJ and Reid G: The effects of
exercise intensity on the stereotypic behaviors of individuals with
autism. Adapt Phys Activ Q. 10:255–268. 1993.
|
|
49
|
Macdonald M, Esposito P and Ulrich D: The
physical activity patterns of children with autism. BMC Res Notes.
4:4222011. View Article : Google Scholar : PubMed/NCBI
|