Introduction
Tongue cancer is a form of oral cancer. The majority
of cases of tongue cancer are squamous cell carcinomas which
originate from the surface layer of the tongue. At present, tongue
squamous cell carcinoma is the most common form of oral cancer and
is associated with early lymph node metastasis and poor survival.
Early tongue cancer is often treatable with surgery or radiation
therapy, depending upon the location and size. Therefore, a growing
emphasis has recently been placed on inhibiting the invasion and
metastasis of tongue cancer. However, the effect of inhibition
remains unclear.
Focal adhesion kinase (FAK) (1) functions as a
phosphorylation-regulated signaling scaffold and is important for
adhesion turnover, Rho-family GTPase activation, cell migration and
cross-talk between growth-factor signalling and integrins (Mitra
et al., 2005) (2). This
ubiquitously expressed, essential protein contains an N-terminal
FERM domain, a central kinase domain, proline-rich regions and a
C-terminal focal-adhesion-targeting domain that interacts with
paxillin (3). p130Cas-Crk
signaling is regulated by interacting proteins. Cells utilize
FAK-Src activity to recruit a p130Cas-Crk complex that promotes Rac
GTPase activity, leading to lamellipodia formation in migrating
cells (4–6). Rates of focal adhesion
formation/turnover are affected by FAK-Src complex phosphorylation
of paxillin, paxillin kinase linker and p130Cas. Elevated
extracellular signal regulated kinase 2 and c-Jun N-terminal kinase
(JNK) promote increased gene transcription of target proteins
leading to enhanced secretion of matrix metalloproteinase (MMP)-2
and -9. Protease localization and activation at the leading edge of
migrating tumor cells is regulated by integrins and facilitates
matrix degradation. However, the mechanism of FAK in invasion and
metastasis of tongue cancer remains unclear.
In the present study, Tca-8113 cells were selected
to analyze the effect of FAK knockdown on tongue cancer cells,
demonstrating that FAK promotes Tca-8113 invasion and metastasis by
JNK signaling.
Materials and methods
Cell culture
Human tongue cancer TCA-8113 cells were purchased
from the Cell Bank of Type Culture Collection of the Chinese
Academy of Sciences (Shanghai, China). The cells were cultured in
complete DMEM supplemented with 10% fetal bovine serum, 2 mM
glutamine, 100 U/ml penicillin and 100 μg/ml streptomycin at 37°C,
in a 5% CO2-95% O2 atmosphere and passaged
every 3–5 days. The study was approved by the ethics committee of
Liaoning medical college, Jinzhou city, China.
Knockdown of FAK
shRNAs against FAK were designed and synthesized by
Shanghai Genechem Co., Ltd. (Shanghai, China). The sequence of the
sense strand was as follows: 5′-GCTAGTGACGTATGGATGT-3′. TCA-8113
cells in exponential growth phase were plated in six-well plates
and allowed to adhere for 24 h prior to transfection. Transfection
was performed using Lipofectamine 2000 according to the
manufacturer’s instructions (Invitrogen Life Technologies,
Carlsbad, CA, USA). Briefly, cells were cultured to 80% confluence
in a six-well culture plate and transfected at a 1:4 ratio (plasmid
DNA:transfection reagent = 5 μg:20 μl) (7). Control cells were transfected with
control RNA under the same conditions. Transfection efficiency was
analyzed by western blot analysis at 72 h.
Transwell assay
The transwell assay was performed using 24-well
transwells (Costar, Cambridge, MA, USA). Following 48 h
transfection, cells were seeded into fibronectin gel pre-coated,
porous upper chamber inserts (1×104 cells/well) and
allowed to invade for 24 h. Following 24 h, inserts were inverted
and stained with Hoechst 33258. The number of invaded cells were
observed and quantified using a Zeiss Axio Scope A1 fluorescent
microscope (Carl Zeiss AG, Oberkochen, Germany; magnification,
×100). Five fields were randomly selected and the number of
penetrated cells was quantified.
Wound healing assay
Following 48 h transfection, cells were harvested
and seeded in six-well plates (1×105 cells/well) and
cultured until cells reached. The cells were scratched using a
yellow pipette tip, washed with PBS three times and cultured in
serum-free medium for 24 h. The status of cell migration was
represented by the rate of wound closure.
Western blot analysis
Following 48 h siRNA transfection, cells were
harvested, washed twice with ice-cold PBS, then lysed in RIPA
buffer [150 mM NaCl, 1% NP-40, 1% SDS, 1 mM PMSF, 10 μg/ml
leupeptin, 1 mM aprotinin and 50 mM Tris-Cl (pH 7.4)]. The cell
lysate was cleared by centrifugation at 12,000 × g for 15 min. Cell
lysate containing 50 μg protein was separated by 10% SDS-PAGE and
the protein was transferred onto polyvinylidene fluoride membranes.
Following blocking with 5% non-fat dry milk in Tris-buffered
saline, the membrane was incubated overnight with primary
antibodies against FAK (FAK sampler kit, 9330; Cell Signaling,
Danvers, MA, USA)., MMP-2 (4022; Cell Signaling), MMP-9 (3852; Cell
Signaling), MMP-14 (Ab51074; Abcam, Cambridge, MA, USA), N-cadherin
(sc-59987; Santa Cruz Biotechnology, Santa Cruz, CA, USA),
E-cadherin (sc-52328; Santa Cruz), Paxillin (2542; Cell Signaling),
p-Tyr118 (2541; Cell Signaling) and p-S178 (ab51617; Abcam).
Following this, the membrane was incubated with appropriate
secondary antibodies for 1 h and stained with ECL. Protein
expression levels of the targeted proteins were analyzed by UVP gel
analysis system and images of the membranes were captured (7).
Immunofluorescence
Following 48 h siRNA transfection, cells were
harvested and replated on fibronectin pre-coated coverslips in
6-well plates. After 24 h, cells were fixed in 3.7% formaldehyde in
PBS, permeabilized with 0.1% Triton X-100 in PBS, blocked with PBS
containing 1% BSA and incubated with specific primary antibodies
for 60 min. Next, the slides were mounted by 95% glycerol and
observed using the Zeiss A-1 fluorescent microscope (Carl Zeiss
AG).
Cytoskeleton staining
Cells were harvested and replated on
fibronectin-coated coverslips (10 μg/ml). Following 24 h, cells
were fixed in 3.7% formaldehyde in PBS, permeabilized with 0.1%
Triton X-100 in PBS, blocked with PBS containing 1% BSA and
incubated with TRITC-conjugated phalloidin (Sigma-Aldrich, St.
Louis, MO, USA) for 30 min. The slides were mounted by 95% glycerol
and observed using the Zeiss Axio Scope A1 fluorescent
microscope.
Gelatin zymography
Conditioned medium was collected and concentrated by
2-fold using a centrifugal concentrator. Equal amounts of protein
were loaded and separated by 10% polyacrylamide gel containing 1
g/l gelatin. The gels were re-natured in 2.5% Triton-X-100 with
gentle agitation for 30 min at room temperature. The gel was
pretreated with developing buffer [5 mM CaCl2, 50 mM
Tris, 0.2 mM NaCl and 0.02% Brij-35 (pH 7.5)] for 30 min at room
temperature, then developed in developing buffer overnight at 37°C.
Following this, the gel was stained with Coomassie Brilliant Blue
R-250 for 30 min and destained with destaining solution. Protease
activity was analyzed using a gel imaging and analysis system
(9,10).
Statistical analysis
SPSS 13.0 statistical software was used for analysis
of the data (SPSS, Inc., Chicago, IL, USA). Data are expressed as
the mean ± SD. P<0.05 was considered to indicate a statistically
significant difference.
Results
Knockdown of FAK inhibits the invasion
and metastasis of TCA-8113
To determine whether FAK knockdown affects the
invasion and metastasis of TCA-8113, FAK knockdown was performed by
transfection of FAK-specific shRNA and the effect of RNA
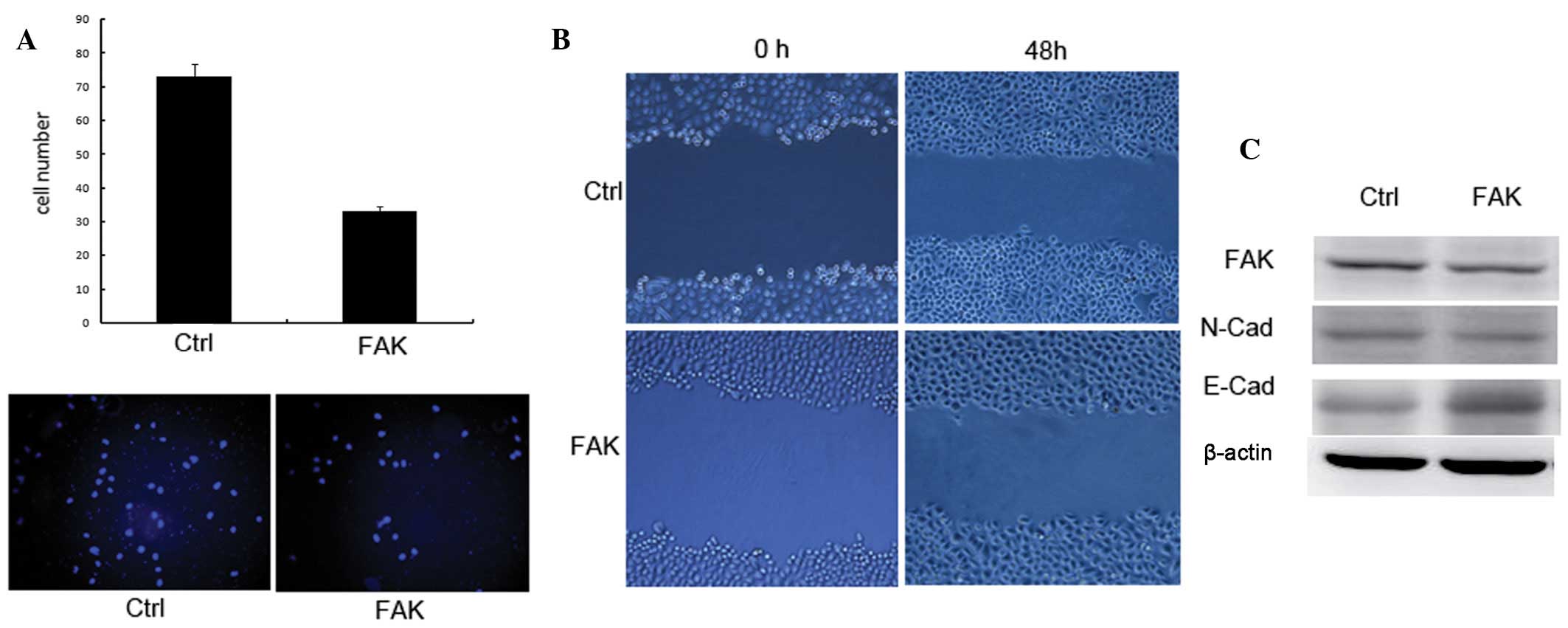
interference was determined using western blot analysis (Fig. 1C). The invasion and metastasis
potential of FAK knockdown cells was analyzed further by wound
healing and transwell assays (11). Wound healing assay revealed that
the metastasis of FAK knockdown cells was significantly decreased
(~50%) compared with the control cells (Fig. 1B). Transwell assay demonstrated
that knockdown of FAK in TCA-8113 cells reduced the invasion
potential to 46% compared with control cells (Fig. 1A).
Downregulation of FAK in TCA-8113
inhibits epithelial-mesenchymal transition (EMT)
EMT is essential for the invasion and metastasis of
TCA-8113 (12). To elucidate the
underlying mechanisms, the effects of FAK knockdown on EMT were
examined by analyzing the expression of E-cadherin and N-cadherin
in FAK knockdown cells. Knockdown of FAK increased the levels of
E-cadherin, whereas the expression of N-cadherin was reduced,
indicating that FAK knockdown inhibited EMT.
Knockdown of FAK leads to cytoskeletal
rearrangement and inhibition of cell adhesion and spreading
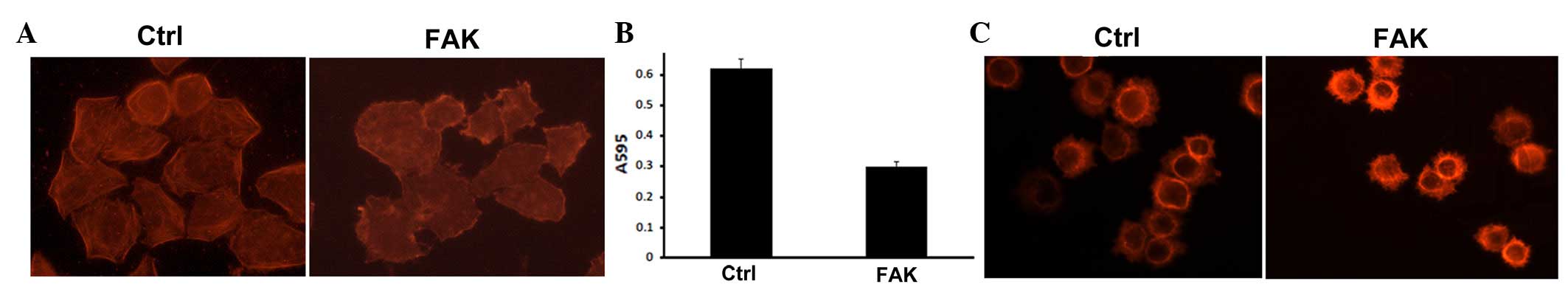
To determine the role of FAK in the regulation of
cytoskeletal dynamics, cytoskeletal dynamics (13) were analyzed by TRITC-conjugated
phalloidin staining (Fig. 2A).
Actin filaments were distributed mainly on the periphery and no
marked stress fiber formation was observed in the cell cortex in
FAK knockdown cells, indicating that knockdown of FAK inhibits
stress fiber formation. The status of cell adhesion and spreading
was analyzed further in FAK knockdown cells using cell adhesion and
spreading assays (Fig. 2B). The
cell adhesion assay revealed that knockdown of FAK inhibited cell
adhesion to fibronectin-coated substrate. In addition, the cell
spreading assay demonstrated that knockdown of FAK reduced the
spreading of tumor cells.
Knockdown of FAK induces paxillin
redistribution and inhibits the activity of JNK
To identify whether FAK knockdown affects
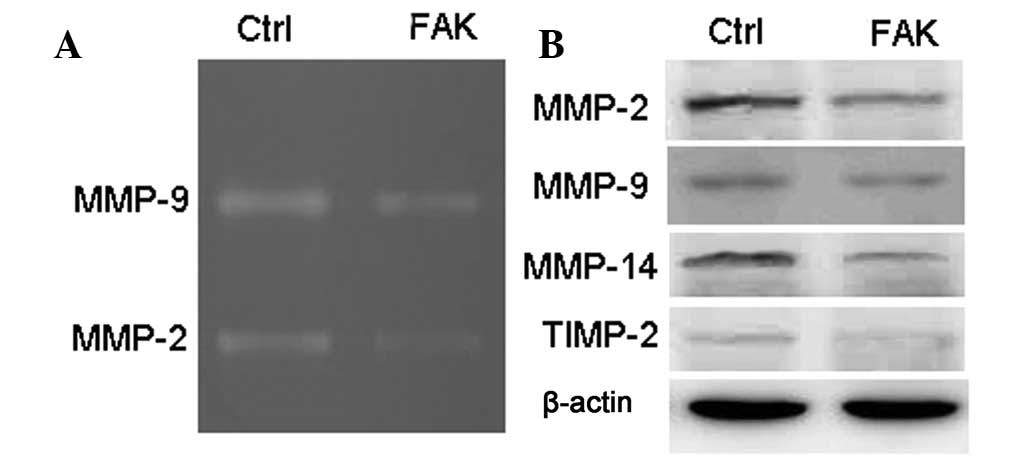
extracellular matrix degradation (14), the activity of MMP-2 and -9 was
determined by gelatin zymography in FAK knockdown cells (Fig. 3A). Knockdown of FAK decreased the
activity of MMP-2 and -9. In addition, the expression of several
MMP family members, including MMP-2, -9 and -14, and TIMP-2, was
determined in FAK knockdown cells. FAK knockdown decreased the
levels of MMP-2, -9 and -14, and TIMP-2 (Fig. 3B).
Paxillin is an important signal transduction adaptor
protein involved in the regulation of tumor invasion and metastasis
(15). The distribution of
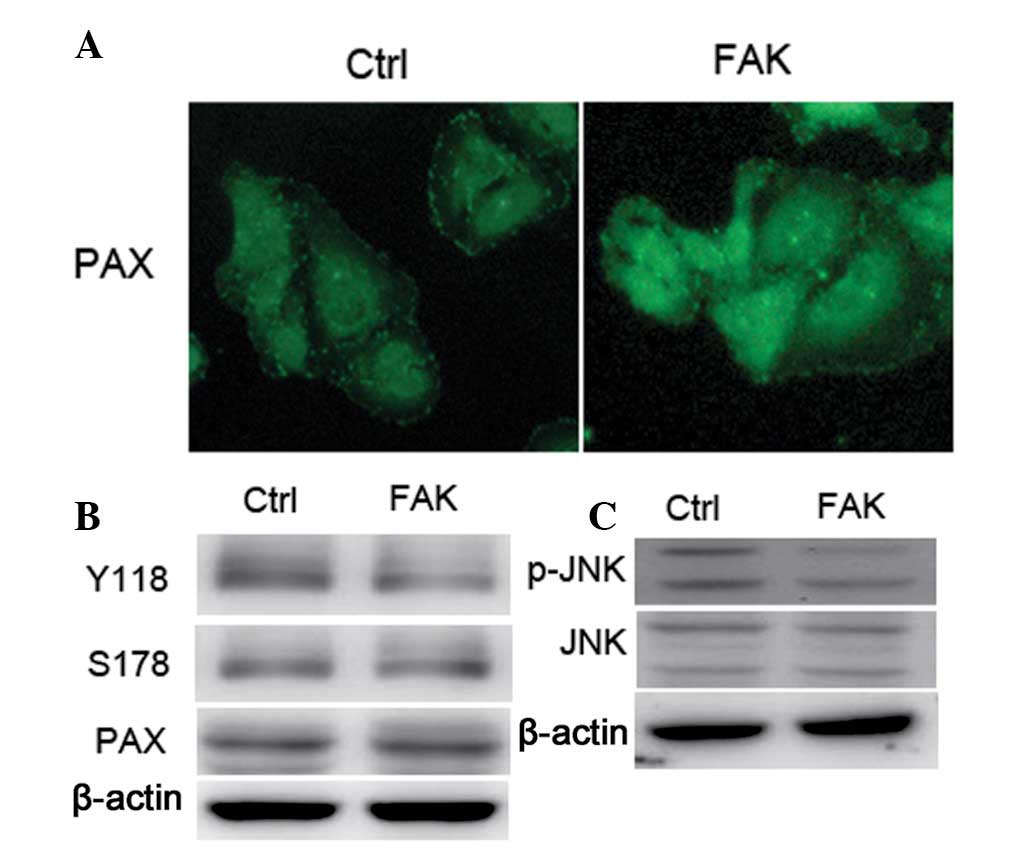
paxillin in FAK knockdown cells was analyzed and paxillin was
observed to be distributed homogeneously in the cytoplasm in FAK
knockdown cells. However, paxillin was found to accumulate at the
cell membrane in control cells. In addition, the phosphorylation
levels of paxillin at residues Y118 and S178 were analyzed by
western blot analysis (16).
Western blot analysis revealed that the phosphorylation levels of
Y118 and S178 were significantly decreased (Fig. 4).
JNK is a well-known Ser and Thr protein kinase.
Therefore, JNK activity was analyzed in FAK knockdown cells by
western blot analysis. The results revealed that the
phosphorylation level of JNK was significantly decreased in FAK
knockdown cells as compared with control cells, indicating that FAK
knockdown inhibited the activity of JNK. However, the expression of
JNK was comparable in FAK knockdown and control cells.
Discussion
Due to its characteristic feature of early lymph
node metastasis, tongue cancer is difficult to treat. Of note,
surgical resection has been found to correlate with tissue and
organ dysfunction leading to a poorer quality of life for patients
(17,18). Therefore, the identification of
novel therapeutic target molecules to inhibit invasion and
metastasis in tongue cancer is vital. At present, FAK is known to
be overexpressed in various forms of cancer (19,20).
A number of studies have demonstrated that FAK is useful as a
molecular marker of nodal metastasis in cancer cells (21–23).
In the present study, shFAK was used to treat Tca-8113 cells to
determine the mechanisms associated with invasion and metastasis of
tongue cancer.
Firstly, western blot analysis was used to analyze
the knockdown efficiency of shFAK in Tca-8113. Next, the invasive
abilities of shFAK-treated and control Tca-8113 cells was
determined by wound healing assay. shFAK was found to inhibit the
invasion of Tca-8113 cells. To further validate this result, the
activity of a number of MMPs was analyzed by western blot analysis.
MMPs are important for tumor invasion and metastasis, and among
numerous MMPs, MMP-2 and -9 are well characterized in invasion and
metastasis (24). Western blot
analysis revealed that shFAK decreases the expression of MMP-2, -9
and -14 in cells. In addition, knockdown of FAK was found to
decrease the expression of TIMP-2.
To elucidate the effect of shFAK on the cytoskeleton
(25,26), shFAK-treated TCA-8113 cells were
compared with control cells. The results indicate that shFAK
inhibits stress fiber formation and results in rearrangement of the
cytoskeleton. In addition, shFAK was found to initiate paxillin
redistribution and promote changes in paxillin expression from the
cell membrane to the cytoplasm. These observations indicate that
paxillin functions as an anchor for the FAK-Src complex and
activates downstream signaling (27–29).
Downregulated FAK promotes paxillin redistribution. Western blot
analysis also revealed that inhibition of FAK downregulates
paxillin expression and phosphorylation.
To elucidate the mechanism by which shFAK inhibits
the invasion and metastasis of tongue cancer cells, the expression
of JNK was analyzed as JNK is known to regulate MMP-2 and -9. shFAK
was found to inhibit the activity of JNK. As JNK cannot activate
c-jun, which is downstream of JNK, the expression of MMP-2/9 and
MMP-14 was downregulated.
In summary, the results of the current study
indicate that the downregulation of FAK inhibits Tca-8113 invasion
and metastasis by JNK and c-Jun signaling. Elevated JNK promotes
increased gene transcription of target proteins leading to enhanced
secretion of MMP-2, -9 and -14 (30). Following this, the leading edge of
migrating tumor cells is activated, cytoskeletal rearrangement
occurs and invadopodia formation is enhanced. The inhibition of FAK
leads to the reduction of JNK phosphorylation, inhibiting c-jun
activity, which is an important TF in the nucleus. The reduction of
c-jun inhibits MMP-2 activity, as well as decreasing cell
invasion
As shFAK inhibits the invasion and metastasis of
tongue cancer cells, the molecular pathway may provide a potential
therapeutic target for tongue tumor.
References
|
1
|
Schaller MD, Borgan CA, Cobb BS, et al:
Focal adhesion kinase and associated proteins. Curr Opin Cell Biol.
6:705–710. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Mitra SK, Hanson DA and Schlaepfer DD:
Focal adhesion kinase: in command and control of cell motility. Nat
Rev Mol Cell Biol. 6:56–68. 2005. View
Article : Google Scholar : PubMed/NCBI
|
|
3
|
Jelena P and Derrick B: Paxillin
phosphorylation and complexing with Erk and FAK are regulated by
PLD activity in MDA-MB-231 cells. Cell Signal. 24:1531–1540. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Abdelkader H, Maya B, Monique D, et al:
Regulation of focal adhesion dynamics and disassembly by
phosphorylation of FAK at tyrosine 397. J Cell Sci. 118:4415–4425.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Vita MG, Sheila F, Baotran TH, et al: A
small molecule focal adhesion kinase (FAK) inhibitor, targeting
Y397 site: 1-(2-hydroxyethyl)-3, 5, 7-triaza-1-azoniatricyclo
[3.3.1.1(3,7)]decane; bromide effectively inhibits FAK
autophosphorylation activity and decreases cancer cell viability,
clonogenicity and tumor growth in vivo. Carcinogenesis.
33:1004–1013. 2012.PubMed/NCBI
|
|
6
|
Tanja P, Virginia E, Kai K, et al: Sulfur
mustard induces differentiation in human primary keratinocytes:
Opposite roles of p38 and ERK1/2 MAPK. Toxicol Lett. 204:43–51.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Hochwald SN, Nyberg C, Zheng M, et al: A
novel small molecule inhibitor of FAK decreases growth of human
pancreatic cancer. Cell Cycle. 8:2435–2443. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Berger S, Dyugovskaya L, Polyakov A and
Lavie L: Short-term fibronectin treatment induces endothelial-like
and angiogenic properties in monocyte-derived immature dendritic
cells: Involvement of intracellular VEGF and MAPK regulation. Eur J
Cell Biol. 91:640–653. 2012. View Article : Google Scholar
|
|
9
|
Tanjoni I, Walsh C, Uryu S, et al:
PND-1186 FAK inhibitor selectively promotes tumor cell apoptosis in
three-dimensional environments. Cancer Biol Ther. 9:764–777. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Beierle EA, Ma X, Stewart J, et al:
Inhibition of focal adhesion kinase decreases tumor growth in human
neuroblastoma. Cell Cycle. 9:1005–1015. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Broughton G 2nd, Janis JE and Attinger CE:
The basic science of wound healing. Plast Reconstr Surg. 117(7
Suppl): 12S–34S. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Creighton CJ, Chang JC, Rosen JM, et al: A
Gene Expression Signature Associated with “K-Ras Addiction” Reveals
Regulators of EMT and Tumor Cell Survival. Cancer Cell. 15:489–500.
2009.
|
|
13
|
Bannasch P, Zerban H and Mayer D: The
cytoskeleton in tumor cells. Pathology. 175:196–211. 1982.
|
|
14
|
Stetler-Stevenson WG, Aznavoorian S and
Liotta LA: Tumor cell interactions with the extracellular matrix
during invasion and metastasis. Annu Rev Cell Biol. 9:541–573.
1993. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Turner CE: Paxillin and focal adhesion
signalling. Nat Cell Biol. 2:E231–E236. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Chen J and Gallo KA: MLK3 Regulates
Paxillin Phosphorylation in Chemokine-Mediated Breast Cancer Cell
Migration and Invasion to Drive Metastasis. Cancer Res.
72:4130–4140. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Ludmila TT, Plamen IP, Daniel K, et al:
Endosonographic assessment of rectal cancer after neoadjuvant
radiotherapy. Med Ultrason. 14:19–23. 2012.PubMed/NCBI
|
|
18
|
Tankova L, Stoilov G, Kovatchki D, et al:
Comparative evaluation of angiogenesis in rectal cancer using
Doppler ultrasound and immunohistochemical assessment. Compt Rend
Acad Bulg Sci. 63:163–166. 2010.
|
|
19
|
Sudakoff GS, Gasparaitis A, Michelassi F,
et al: Endorectal color Doppler imaging of primary and recurrent
rectal wall tumours: Preliminary experience. AJR Am J Roentgenol.
166:55–61. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Xiaoban X, Lijun Z, Caleb M, Reyes, et al:
APPL1 mediates adiponectin-stimulated p38 MAPK activation by
scaffolding the TAK1-MKK3-p38 MAPK pathway. Am J Physiol Endocrinol
Metab. 300:E103–110. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Ito R, Oue N, Zhu X, et al: Expression of
integrin-linked kinase is closely correlated with invasion and
metastasis of gastric carcinoma. Virchows Arch. 442:118–123.
2003.PubMed/NCBI
|
|
22
|
Owens LV, Xu L, Dent GA, Yang X, et al:
Focal adhesion kinase as a marker of invasive potential in
differentiated human thyroid cancer. Ann Surg Oncol. 3:100–105.
1996. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Miyazaki T, Kato H, Nakajima M, et al: FAK
overexpression is correlated with tumour invasiveness and lymph
node metastasis in oesophageal squamous cell carcinoma. Br J
Cancer. 89:140–145. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Masson V, de la Ballina LR, Munaut C, et
al: Contribution of host MMP-2 and MMP-9 to promote tumor
vascularization and invasion of malignant keratinocytes. FASEB J.
19:234–236. 2005.PubMed/NCBI
|
|
25
|
Zhou L, Deepa SS, Etzler JC, et al:
Adiponectin activates AMP-activated protein kinase in muscle cells
via APPL1/LKB1-dependent and phospholipase
C/Ca2_/Ca2_/calmodulin-dependent protein kinase kinase-dependent
pathways. J Biol Chem. 284:22426–22435. 2009. View Article : Google Scholar
|
|
26
|
Heneghan JP, Salem RR, Lange RC, et al:
Transrectal sonography in staging rectal carcinoma: The role of
gray-scale, color-flow and Doppler imaging analysis. AJR Am J
Roentgenol. 169:1247–1252. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Liu M, Wilk SA, Wang A, et al: Resveratrol
inhibits mTOR signaling by promoting the interaction between mTOR
and DEPTOR. J Biol Chem. 285:36387–36394. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Schaller MD: Paxillin: a focal
adhesion-associated adaptor protein. Oncogene. 20:6459–6472. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Bae GU, Lee JR, Kim BG, et al: Cdo
interacts with APPL1 and activates AKT in myoblast differentiation.
Mol Biol Cell. 21:2399–2411. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Cheung LW, Leung PC and Wong AS:
Gonadotropin-Releasing Hormone Promotes Ovarian Cancer Cell
Invasiveness through c-Jun NH2-Terminal Kinase–Mediated Activation
of Matrix Metalloproteinase (MMP)-2 and MMP-9. Cancer Res.
66:10902–10910. 2006.PubMed/NCBI
|