Introduction
Non-melanoma skin cancer (NMSC), comprising
predominantly of basal cell carcinoma and squamous cell carcinoma
(also termed epidermoid carcinoma), is one of the most common
malignancies in the United States, with more than two million novel
cases annually (1). Although skin
cancer may often be treated with surgery, chemotherapy or radiation
therapy, the risk of recurrence and metastasis remain a
concern.
Improvements in skin cancer treatment are likely to
derive from novel agents targeting the molecular pathways that
promote tumor cell growth and survival. The epidermal growth factor
receptor (EGFR) is an 170-kDa glycoprotein consisting of an
extracellular ligand-binding domain, a transmembrane domain
containing a single hydrophobic anchor sequence and an
intracellular domain with tyrosine kinase activity (2). Following ligand binding,
tyrosine-phosphorylated EGFR initiates the activation of downstream
pathways, including Janus kinase (JAK)/signal transducer and
activator of transcription (STAT), phosphatidylinositol 3-kinase
(PI3K)/AKT and mitogen-activated protein kinase (MAPK) cascades
(3). Activation of the EGFR
downstream pathways results in cell proliferation, migration,
adhesion, anti-apoptosis, angiogenesis and metastasis (4). Previously, novel therapeutic
approaches targeting the EGFR superfamily and their downstream
pathways were generated (4).
Herba Epimedii has been used traditionally as a
medicinal herb in East Asia, including in Korea, China and Japan,
to treat conditions such as hypertension, coronary heart disease,
osteoporosis, menopausal syndrome, rheumatism, neurasthenia,
bronchitis and hypogonadism. Icariin is the active ingredient of
Herba Epimedii (5) and icariside
II (IS) is a metabolite of icariin (6). A previous study demonstrated that IS
was a novel anticancer agent that induced apoptosis in certain
tumor cell lines, including PC-3 prostate cancer cells (7), U266 multiple myeloma cells (8), U937 acute myeloid leukemia cells
(8,9) and A549 lung cancer cells (10)in vitro. IS has been
demonstrated to inhibit the activation of the JAK-STAT3 signaling
pathway in U266 and U937 cells (9). As EGFR is one of the upstream
modulators of JAK-STAT, it was hypothesized that IS inhibits the
activation of the EGFR signaling pathways. A431 cells are an
established epidermoid carcinoma cell line that overexpresses EGFR.
In the present study, we evaluated the antitumor effects of IS on
A431 cells in vitro and demonstrated the possible mechanism
involved in IS-induced apoptosis.
Materials and methods
Reagents and cell culture
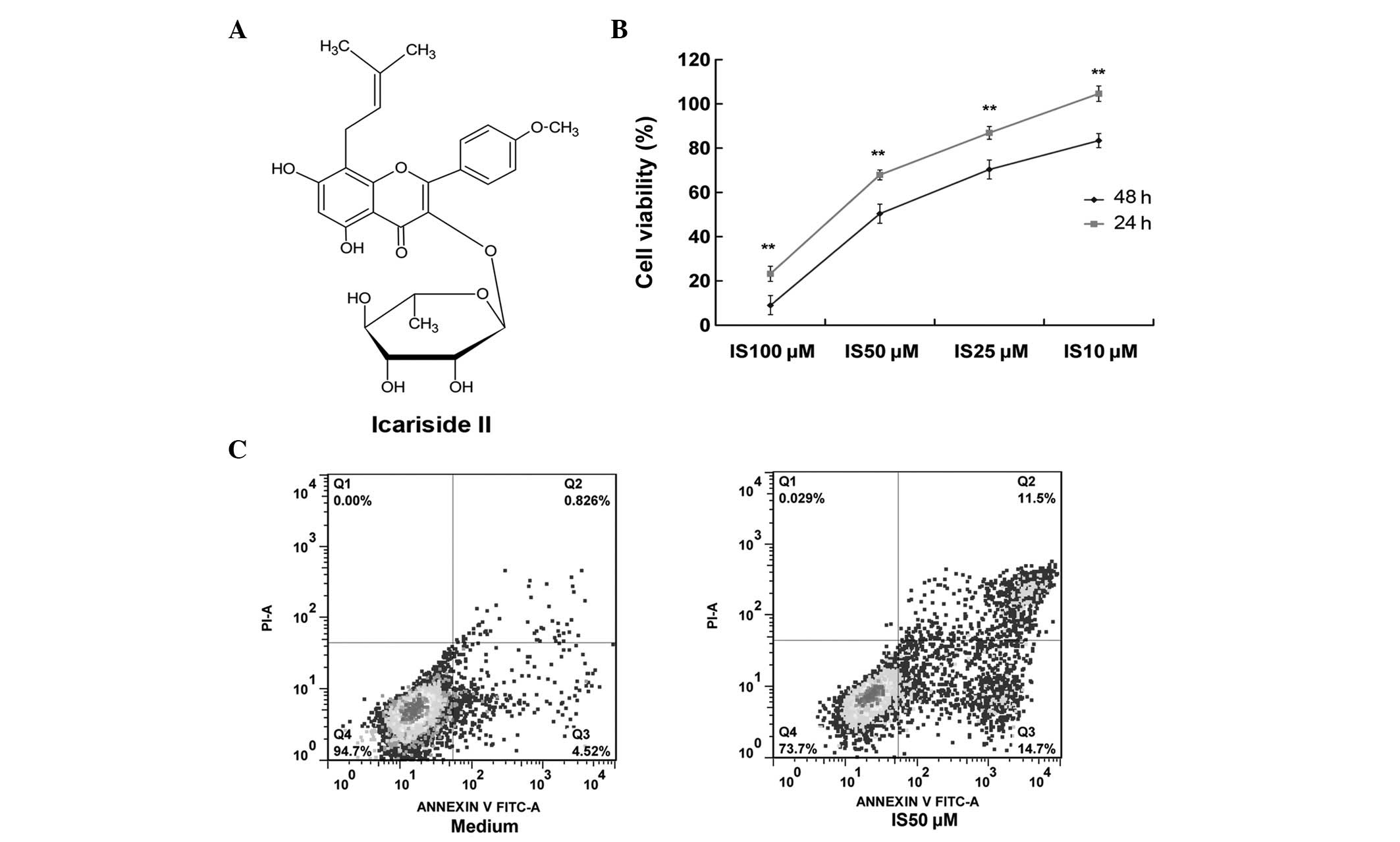
IS (purity, >98%; Fig. 1A) was isolated from the enzymatic
hydrolysis of icariin, as previously described (6). The A431 human epidermoid carcinoma
cell line was purchased from the American Type Culture Collection
(Manassas, VA, USA) and maintained in Dulbecco’s modified Eagle’s
medium (DMEM; Invitrogen, Carlsbad, CA, USA) containing 4 mM
L-glutamine, 3.7 g/l sodium bicarbonate, 4.5 g/l glucose and 10%
fetal bovine serum. Cells were maintained in a 5% CO2
humidified incubator at 37°C. WST-8 was obtained from Dojindo
Laboratories (Kumamoto, Japan); fluorescein isothiocyanate
(FITC)-conjugated Annexin V was supplied by R&D Systems
(Minneapolis, MN, USA); and antibodies against caspase-9, cleaved
caspase-9, cleaved poly ADP ribose polymerase (PARP), cleaved PARP,
EGFR, phosphorylated (P)-EGFR (Tyr1068), STAT3, P-STAT3,
extracellular signal-related kinases (ERK), P-ERK, AKT, P-AKT and
β-actin were obtained from Cell Signaling Technology, Inc.,
(Beverly, MA, USA). Human EGF was also obtained from Cell Signaling
Technology, Inc. The EGFR inhibitor (AGF1478) and the PI3K
inhibitor (LY294002) were supplied by Sigma-Aldrich (St. Louis, MO,
USA).
Cell viability assays
IS [dissolved in dimethyl sulfoxide (DMSO)] was used
for the treatment of cells. The final concentration of DMSO was
<0.1% (v/v). Cell viability was measured by the WST-8 assay
(Dojindo Laboratories) according to optimized manufacturer’s
instructions. Briefly, A431 cells were seeded at a density of 4,000
cells/100 μl/well in 96-well culture plates in DMEM, then incubated
in a humidified incubator at 37°C overnight prior to treatment with
different concentrations of IS (0, 10, 25, 50 and 100 μM).
Following 24 or 48 h of incubation post-treatment, 10 μl WST-8 was
added to each well for 1 h. Subsequently, the optical density (OD)
was measured at 450 nm. The percentage of viable cells was
determined using the formula: Ratio (%) = [OD (IS) − OD (blank)/OD
(control) − OD (blank)] × 100. The cell viability data were the
averages of three independent experiments, each containing three
replicates.
Flow cytometric analysis
Following the treatment of A431 cells with IS (0 and
50 μM) for 24 h, 1×106 cells were harvested and washed
once with binding buffer [Hepes buffer: 10 mM HEPES/NaOH (pH 7.4),
150 mM NaCl, 5 mM KCl, 1 mM MgCl2 and 1.8 mM
CaCl2]. Following aspiration of the supernatant, the
cells were resuspended in 100 μl binding buffer containing 1 μl
FITC-conjugated Annexin V antibody and 5 μl PI for exactly 5 min in
the dark at room temperature. The cells were then analyzed on a
FACSCalibur cytometer (BD Biosciences, San José, CA, USA). The data
were analyzed using FlowJo software v6.0 (Tree Star, Inc., Ashland,
OR, USA).
Western blot analysis
A431 cells were treated with different
concentrations of IS (0, 10, 25 and 50 μM) for 24 h, or pretreated
with IS at various concentrations (0, 25, 50 and 100 μM) for 2 h
prior to the treatment with EGF (100 ng/ml) for 10 min. The cells
were then resuspended in lysis buffer [150 mmol/l NaCl, 1% NP-40,
0.5% sodium deoxycholate, 0.1% sodium dodecyl sulfate (SDS), 50
mmol/l Tris-Cl (pH 8.0), 2 μg/ml aprotinin, 2 μg/ml leupeptin, 40
mg/ml phenylmethylsulfonyl fluoride and 2 mmol/l dithiothreitol]
and centrifuged at 12,000 × g for 15 min to remove the nuclei and
cell debris. The supernatants were immediately frozen at −80°C
until use. The protein concentrations were determined by the
Bradford assay (Bio-Rad, Hercules, CA, USA) and 30 μg cellular
proteins were electroblotted onto a polyvinylidene difluoride
(PVDF) membrane following separation by 10% SDS-polyacrylamide gel
electrophoresis. The immunoblot was incubated for 1 h with 5% milk
at room temperature, followed by overnight incubation at 4°C at a
1:1,000 dilution of primary antibody against caspase-9, cleaved
caspase-9, PARP, cleaved PARP, EGFR, P-EGFR (Tyr1068), STAT3,
P-STAT3, ERK, P-ERK, AKT, P-AKT or β-actin. Blots were washed twice
with Tris-buffered saline and Tween-20 (TBST) prior to the addition
of a 1:1000 dilution of horseradish peroxidase-conjugated secondary
antibody for 1 h at room temperature. Blots were washed again with
TBST prior to development by enhanced chemiluminescence using
Supersignal West Femto chemiluminescent substrate (Pierce,
Rockford, IL, USA). Band intensities were quantified using
UN-SCAN-IT gel analysis software (version 6; Silk Scientific, Orem,
Utah, USA). The optical density for target protein is shown as a
proportion of the β-actin optical density. The western blot
analysis was repeated three times.
Statistical analysis
Data are presented as the mean ± standard deviation.
Data analysis was performed by one-way analysis of variance. For
the comparison of two groups, a Student’s t-test was used.
P<0.05 was considered to indicate a statistically significant
difference.
Results
IS is cytotoxic to A431 cells in
vitro
The viability of A431 cells following treatment with
increasing concentrations of IS (0, 10, 25, 50 and 100 μM) for 24
or 48 h was investigated. As demonstrated by the WST-8 assay,
treatment with IS resulted in significantly decreased cell
viability in a dose-dependent manner (Fig. 1B). For example, treatment with 10
μM IS for 24 h did not decrease the cell viability. However,
treatment with 10 μM IS for 48 h significantly decreased the cell
viability compared with that at 24 h (83.3%). In addition,
treatment with 100μM IS for 24 h markedly decreased the cell
viability (to 23.9%), while treatment with 100 μM IS for 48 h
resulted in a further decrease (to 8.9%).
IS increases the apoptosis of A431
cells
To determine whether the cytotoxicity of IS occurred
by apoptosis, the percentage of Annexin V-positive and PI-negative
A431 cells was measured following treatment with increasing
concentrations of IS (0–50 μM, Fig.
1C). Treatment with 50 μM IS for 24 h resulted in an increased
number of apoptotic cells (26.2%) compared with that of the medium
control group (5.3%).
IS induces apoptosis by increasing the
levels of cleaved caspase-9 and cleaved PARP in the A431 cells
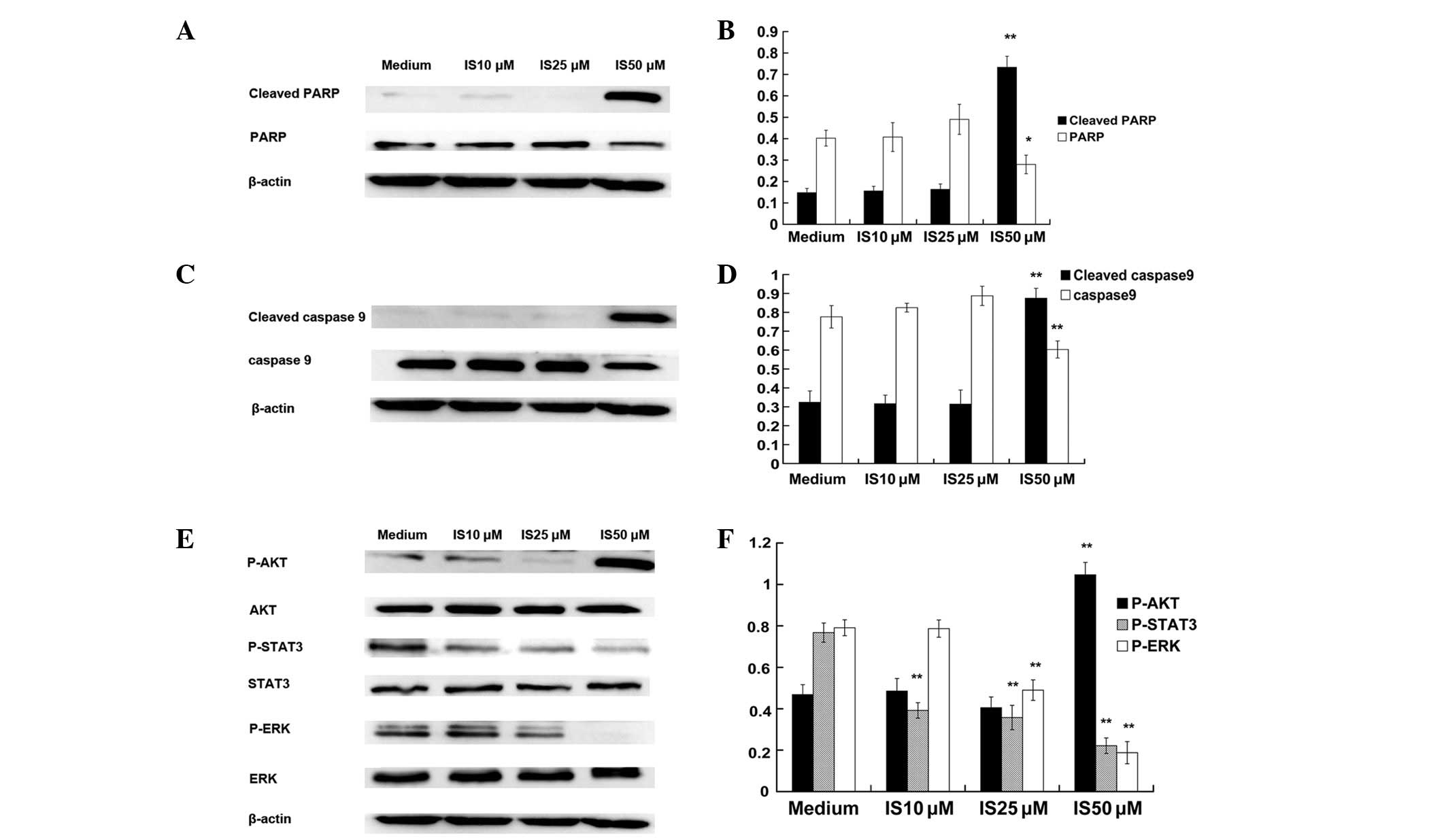
PARP and caspase-9 are terminal pro-apoptotic
proteins (11), and are cleaved to
produce the active forms. The effect of IS on the expression of
PARP (Fig. 2A and B) and caspase-9
(Fig. 2C and D) was determined.
Protein expression in A431 cells treated with various
concentrations of IS (0, 10, 25 and 50 μM) for 24 h was measured by
western blot analysis. As predicted, treatment with 50 μM IS
significantly increased the expression of cleaved caspase-9 and
cleaved PARP and significantly decreased the expression of
caspase-9 and PARP. However, treatment with 10 and 25 μM IS for 24
h did not increase the expression of cleaved caspase-9 and cleaved
PARP.
IS inhibits the activation of STAT3 and
ERK but promotes the activation of AKT in A431 cells
STAT3, ERK and AKT have been demonstrated to be
constitutively active in numerous types of tumors and to promote
tumorigenesis by preventing apoptosis (4). Therefore, western blot analysis was
conducted to determine the expression levels of STAT3, ERK and AKT
and their activated (phosphorylated) counterparts. It was
demonstrated that IS (at 10, 25 and 50 μM) significantly reduced
the phosphorylated forms of STAT3 and that IS (at 25 and 50 μM)
significantly reduced the phosphorylated forms of ERK. However,
treatment with 50 μM IS significantly promoted the activation of
AKT (Fig. 2E and F).
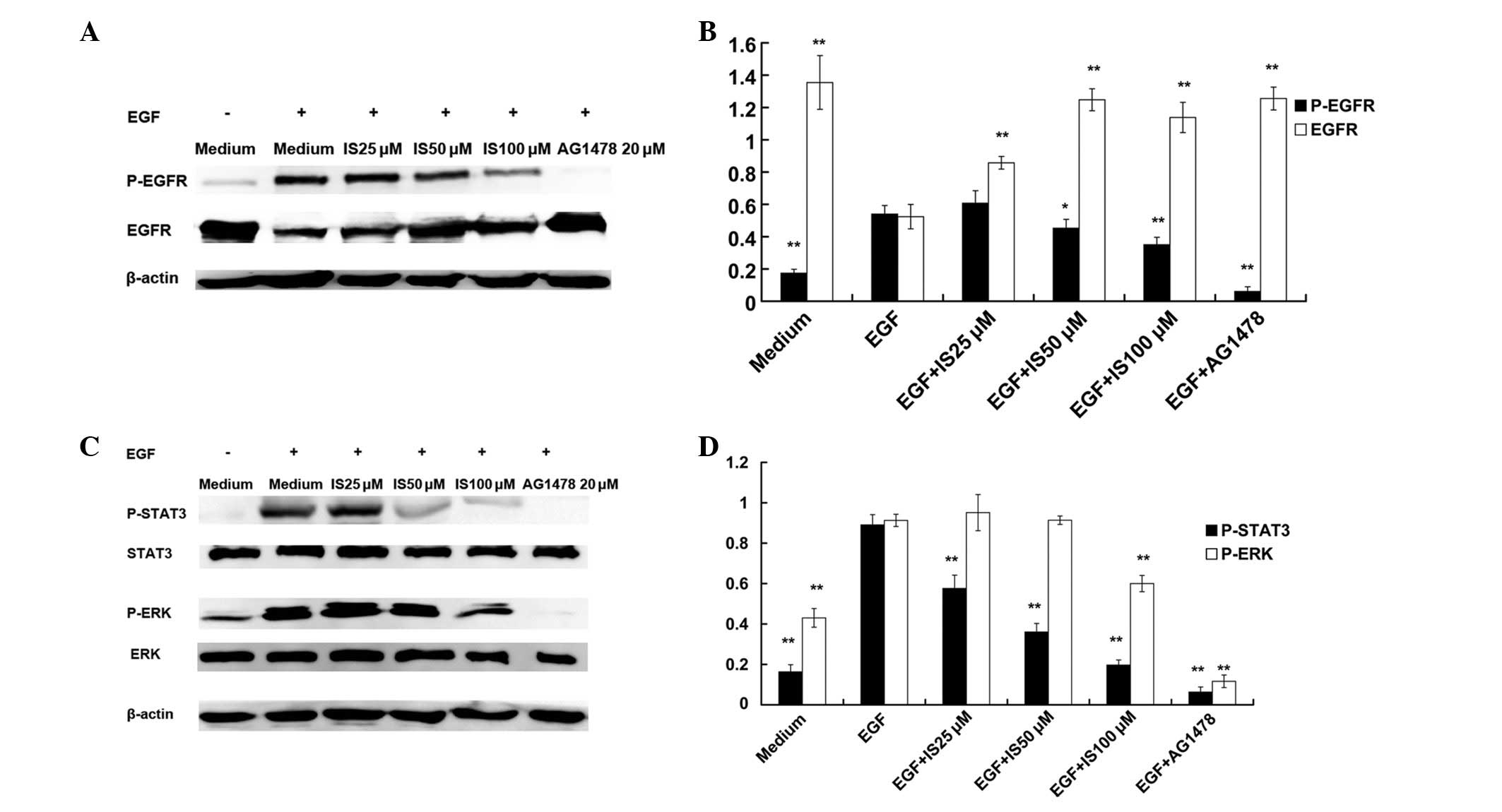
IS inhibits the EGF-induced activation of
EGFR signaling pathways in A431 cells
A431 cells are an established epidermoid carcinoma
cell line, that overexpresses EGFR. EGF activates EGFR signaling
pathways, including JAK-STAT and MAPK-ERK. Western blot analyses
demonstrated that EGF (100 ng/ml, 10 min) induced significant
increases in the expression of P-EGFR, P-STAT3 and P-ERK in A431
cells, compared with that of the medium control. IS (50 and100 μM)
pretreatment for 2 h resulted in significant decreases in the
expression of P-EGFR (Fig. 3A and
B) and P-STAT3 (Fig. 3C and
D), compared with that of the EGF alone group. Only
pretreatment with 100 μM IS inhibited the EGF-induced activation of
ERK (Fig. 3C and D). In addition,
IS (at 50 and 100 μM) pretreatment significantly inhibited the
EGF-induced decreases in EGFR.
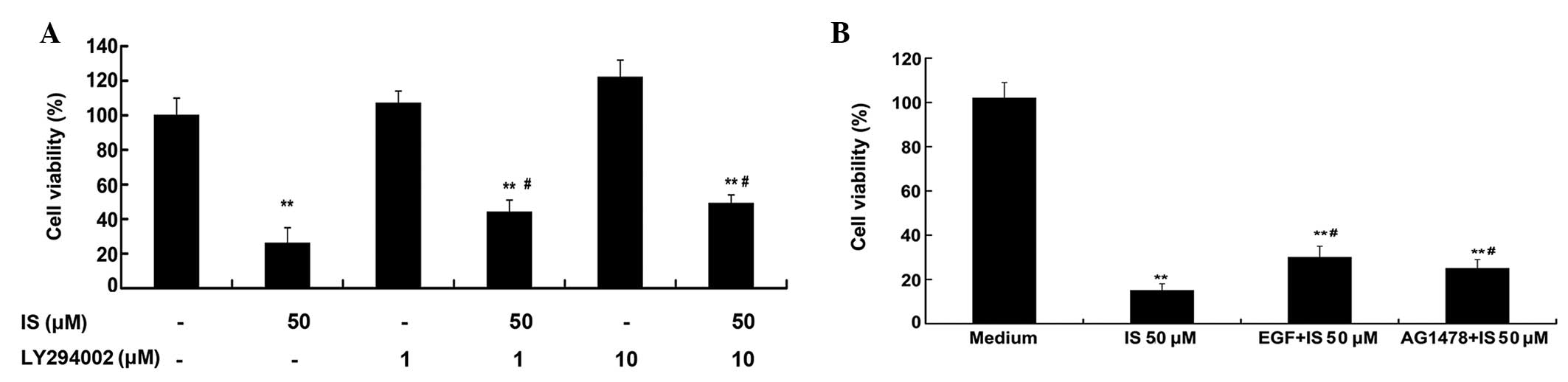
IS-induced decreases in cell viability
are partially reversed by LY294002, EGF and AG1478
As demonstrated by the WST-8 assay (Fig. 4A), treatment with 50 μM IS resulted
in a significantly decreased cell viability (P<0.01). The PI3K
inhibitor (LY294002; 1 and 10 μM) treatment alone did not decrease
the cell viability; however, pretreatment with LY294002 (1 and 10
μM) for 1 h could partially reverse the IS induced-decreases of
cell viability (P<0.05). Fig.
4B shows that treatment with 50 μM IS alone resulted in a
marked decreased cell viability (P<0.01), but EGF (20 ng/ml) and
AG1478 (1 μM) pretreatment partially reversed the IS-induced
decreases in cell viability (P<0.05).
Discussion
IS is obtained from Epimedium plants. This
flavonol glycoside has been demonstrated to have apoptotic
potential against PC-3 prostate cancer cells (7), U266 multiple myeloma cells (8), U937 acute myeloid leukemia cells
(8,9) and A549 lung cancer cells (10)in vitro. In the present study,
similar results with A431 epidermoid carcinoma cells were
demonstrated, suggesting that IS induces apoptosis in tumor cells
in general. In addition, IS-induced apoptosis via the activation of
caspase-9 and PARP in A431 cells was also observed in this
study.
Constitutive activation of several signaling
pathways, such as JAK-STAT, MAPK, PI3K-AKT and nuclear factor-κB
(NF-κB) have been determined to confer survival and proliferative
advantages to tumor cells (12,13).
IS has been demonstrated to inhibit the activation of the JAK-STAT3
signaling pathway in U266 and U937 cells (9). Similar results with A431 epidermoid
carcinoma cells were observed in the present study. The MAPK family
consists of three predominant members: ERKs (ERK1 and ERK2), c-Jun
NH2-terminal kinases (JNK1, JNK2 and JNK3) and p38 MAPK (14). A previous study has demonstrated
that the apoptotic effect of IS was dependent on the activation of
JNK and p38 MAPK in A549 cells, which was almost completely
inhibited by SB203580 (an inhibitor of p38 MAPK) and SP600125 (an
inhibitor of JNK) (10). In the
present study, it was demonstrated that IS induced apoptosis via
the inactivation of MAPK-ERK. Thus, the activation of JNK and p38,
as well as the inactivation of ERK, may be required for IS to
induce apoptosis. In addition, this study demonstrated that IS
activated the PI3K-AKT signaling pathway in A431 cells.
Constitutive activation of PI3K-AKT is involved in the survival of
tumors; therefore, the upregulation of PI3K-AKT may not be
beneficial in the induction of tumor apoptosis (15). However, the present study
demonstrated that pretreatment with LY294002, a PI3K inhibitor,
partially reversed IS-induced decreases in cell viability. A
previous study indicated that the PI3K-AKT pathway negatively
regulated STAT-transcriptional activities in tumor cells,
suggesting that a mechanism for the effects of IS may be AKT
activation, which induces JAK-STAT signaling (16).
EGFR is closely associated with various tumors of
epithelial origin, including breast (17), colon (18), ovarian (19) and lung (20) cancer. Recently, novel therapeutic
approaches targeting the EGFR and its downstream pathways have been
demonstrated. In addition, certain natural products, including
rhein (21), magnolol (22), vicenin (23) and shikonin (24), have been shown to induce tumor
apoptosis via inhibition of the EGFR signaling pathways. IS is a
metabolite of icariin, which is derived from Herba Epimedii
(6), and has been demonstrated to
inhibit the activation of JAK-STAT and MAPK-ERK signaling pathways.
As EGFR is one of the upstream modulators of JAK-STAT and MAPK-ERK,
it was hypothesized that IS inhibits the activation of the EGFR
signaling pathways. The present study demonstrated that IS
inhibited the EGF-induced activation of the EGFR, JAK-STAT and
MAPK-ERK pathways in A431 cells.
It was also determined that pretreatment with EGF
(20 ng/ml) and AG1478 (1 μM) partially reversed the IS-induced
decreases in cell viability. Therefore, pretreatment with EGF may
have partially reversed the IS-induced inactivation of EGFR and its
downstream survival pathways. Alternatively, IS may have a lower
binding affinity for EGFR than that of AG1478. Thus, pretreatment
with AG1478 decreased the level of IS available to bind to
EGFR.
In conclusion, IS significantly inhibited the cell
viability of A431 cells in vitro through the regulation of
apoptosis. These effects were mediated, at least in part, by
inhibiting the activation of the EGFR signal transduction
pathways.
Acknowledgements
This study was funded by a grant from the National
Natural Science Foundation of China (grant no. 81102541).
References
|
1
|
Rogers HW, Weinstock MA, Harris AR, et al:
Incidence estimate of nonmelanoma skin cancer in the United States,
2006. Arch Dermatol. 146:283–287. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Ullrich A, Coussens L, Hayflick JS, et al:
Human epidermal growth factor receptor cDNA sequence and aberrant
expression of the amplified gene in A431 epidermoid carcinoma
cells. Nature. 309:418–425. 1984. View
Article : Google Scholar : PubMed/NCBI
|
|
3
|
Uribe P and Gonzalez S: Epidermal growth
factor receptor (EGFR) and squamous cell carcinoma of the skin:
molecular bases for EGFR-targeted therapy. Pathol Res Pract.
207:337–342. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Lurje G and Lenz HJ: EGFR signaling and
drug discovery. Oncology. 77:400–410. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Zhang DW, Cheng Y, Wang NL, et al: Effects
of total flavonoids and flavonol glycosides from Epimedium koreanum
Nakai on the proliferation and differentiation of primary
osteoblasts. Phytomedicine. 15:55–61. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Xia Q, Xu D, Huang Z, et al: Preparation
of icariside II from icariin by enzymatic hydrolysis method.
Fitoterapia. 81:437–442. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Lee KS, Lee HJ, Ahn KS, et al:
Cyclooxygenase-2/prostaglandin E2 pathway mediates icariside II
induced apoptosis in human PC-3 prostate cancer cells. Cancer Lett.
280:93–100. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Kim SH, Ahn KS, Jeong SJ, et al: Janus
activated kinase 2/signal transducer and activator of transcription
3 pathway mediates icariside II-induced apoptosis in U266 multiple
myeloma cells. Eur J Pharmacol. 654:10–16. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Kang SH, Jeong SJ, Kim SH, et al:
Icariside II induces apoptosis in U937 acute myeloid leukemia
cells: role of inactivation of STAT3-related signaling. PloS One.
7:e287062012. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Song J, Shu L, Zhang Z, et al: Reactive
oxygen species-mediated mitochondrial pathway is involved in
Baohuoside I-induced apoptosis in human non-small cell lung cancer.
Chem Biol Interact. 199:9–17. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Fan TJ, Han LH, Cong RS and Liang J:
Caspase family proteases and apoptosis. Acta Biochim Biophys Sin
(Shanghai). 37:719–727. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Ravandi F, Talpaz M and Estrov Z:
Modulation of cellular signaling pathways: prospects for targeted
therapy in hematological malignancies. Clin Cancer Res. 9:535–550.
2003.PubMed/NCBI
|
|
13
|
Chalandon Y and Schwaller J: Targeting
mutated protein tyrosine kinases and their signaling pathways in
hematologic malignancies. Haematologica. 90:949–968.
2005.PubMed/NCBI
|
|
14
|
Nickischer D, Laethem C, Trask OJ Jr, et
al: Development and implementation of three mitogen-activated
protein kinase (MAPK) signaling pathway imaging assays to provide
MAPK module selectivity profiling for kinase inhibitors: MK2-EGFP
translocation, c-Jun, and ERK activation. Methods Enzymol.
414:389–418. 2006. View Article : Google Scholar
|
|
15
|
Davies MA, Stemke-Hale K, Lin E, et al:
Integrated molecular and clinical analysis of AKT activation in
metastatic melanoma. Clin Cancer Res. 15:7538–7546. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Krasilnikov M, Ivanov VN, Dong J and Ronai
Z: ERK and PI3K negatively regulate STAT-transcriptional activities
in human melanoma cells: implications towards sensitization to
apoptosis. Oncogene. 22:4092–4101. 2003. View Article : Google Scholar
|
|
17
|
Normanno N, Campiglio M, Maiello MR, et
al: Breast cancer cells with acquired resistance to the EGFR
tyrosine kinase inhibitor gefitinib show persistent activation of
MAPK signaling. Breast Cancer Res Treat. 112:25–33. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Shimizu M, Deguchi A, Lim JT, et al:
(−)-Epigallocatechin gallate and polyphenon E inhibit growth and
activation of the epidermal growth factor receptor and human
epidermal growth factor receptor-2 signaling pathways in human
colon cancer cells. Clin Cancer Res. 11:2735–2746. 2005.
|
|
19
|
Zhang X, Ling MT, Feng H, et al: Id-I
stimulates cell proliferation through activation of EGFR in ovarian
cancer cells. Br J Cancer. 91:2042–2047. 2004.PubMed/NCBI
|
|
20
|
Gadgeel SM, Ali S, Philip PA, et al:
Response to dual blockade of epidermal growth factor receptor
(EGFR) and cycloxygenase-2 in nonsmall cell lung cancer may be
dependent on the EGFR mutational status of the tumor. Cancer.
110:2775–2784. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Lin YJ and Zhen YS: Rhein lysinate
suppresses the growth of breast cancer cells and potentiates the
inhibitory effect of Taxol in athymic mice. Anticancer Drugs.
20:65–72. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Lee DH, Szczepanski MJ and Lee YJ:
Magnolol induces apoptosis via inhibiting the EGFR/PI3K/Akt
signaling pathway in human prostate cancer cells. J Cell Biochem.
106:1113–1122. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Nagaprashantha LD, Vatsyayan R, Singhal J,
et al: Anti-cancer effects of novel flavonoid vicenin-2 as a single
agent and in synergistic combination with docetaxel in prostate
cancer. Biochem Pharmacol. 82:1100–1109. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Singh F, Gao D, Lebwohl MG and Wei H:
Shikonin modulates cell proliferation by inhibiting epidermal
growth factor receptor signaling in human epidermoid carcinoma
cells. Cancer Lett. 200:115–121. 2003. View Article : Google Scholar : PubMed/NCBI
|