Introduction
Silicosis is a significant global health issue,
caused by long-term inhalation of high levels of dust containing
excessive free silica during the production process. Pathological
characteristics include silicotic nodule formation and pulmonary
interstitial fibrosis (1). In
addition, the incidence and prevalence of silicosis is markedly
increasing and effective therapies are not currently available.
Stem cell therapy represents a novel treatment modality associated
with increasing therapeutic potential. Pluripotent adult stem cells
located in the bone marrow are divided into two main distinct
populations, hematopoietic and bone marrow-derived mesenchymal stem
cells (BMSCs) (2). Over the last
decade, BMSCs have been exploited as therapeutic vectors or tools
for the treatment of a wide variety of diseases. Previous studies
on cell transplantation demonstrated that the administration of
BMSCs in animal models of injury exhibits protective effects
following acute spinal cord (3),
traumatic brain (4) or liver
(5) injuries. In addition, a
number of studies have revealed that exogenous delivery of BMSCs
protects against a variety of pulmonary diseases, including acute
lung injury (6,7) and chronic lung disease (8). In addition, BMSC treatment has been
identified to promote the repair of tissue structure and function
following bleomycin (BLM)- (9),
monocrotaline- (10) or
lipopolysaccharide-induced (6)
lung injury. Donor-derived BMSCs may target lung injuries, migrate
to areas of damage and then differentiate into lung-specific cells
(11), including type I and type
II alveolar epithelial cells, endothelial cells, fibroblasts and
bronchial epithelial cells, as shown in the rat model of
bleomycin-induced pulmonary fibrosis (9), demonstrating the plasticity of BMSCs
in this setting. These observations strengthen the hypothesis that
BMSC therapy may be suitable for the treatment of pulmonary
fibrosis.
At present, the majority of studies focus on the
protective mechanisms of BMSCs that have been systemically
transplanted. The protective potential of BMSCs in tissue injury
has been reported to be associated with the differentiation
potential of engrafted BMSCs into specific-cell phenotypes
(9), an increase in circulating
levels of G-CSF and GM-CSF (11)
and BMSC-driven immunomodulation caused by a shift in the Th1/Th2
balance (12). However, a newly
emerging concept in tissue repair is associated with the hypothesis
that BMSC efficacy is attributed, in part, to paracrine mechanisms
(7,13,14).
Specifically, multiple cytokines, including vascular endothelial
growth factor, stromal cell derived factor-1, insulin growth
factor-1 and basic fibroblast growth factor, are released by BMSCs
into conditioned medium in a paracrine fashion. These cytokines are
associated with an anti-apoptotic effect of BMSCs in the ischemic
myocardium (15). In addition, a
large variety of factors have been reported to be secreted from
BMSCs via a paracrine pathway, to induce growth and differentiation
and prevent injured cells from apoptotic death (16). These mediators released from BMSCs
exhibit numerous effects and exert protection on injured tissues.
Collectively, these observations indicate that the efficacy of
BMSCs may be explained by paracrine mechanisms. Notably, the
current review highlights a considerable shift from BMSCs to
BMSC-conditioned media (BMSC-CM) as an effective regenerative
therapy. In addition, a growing number of studies are reporting
that the delivery of concentrated BMSC-CM provides a significantly
superior protection against diseases compared with the effects of
BMSC administration (17,18). Mechanisms of the effects of BMSC-CM
also attribute to the production of a series of cytokines and
chemokines, released from BMSCs via the paracrine pathway (19), demonstrating that BMSCs participate
in injury repair via the secretion of specific soluble factors.
These observations indicate that the therapeutic response of BMSCs
is mediated by paracrine mechanisms. In addition, the efficacy of
BMSC-CM contributes to an improved understanding of paracrine
mechanisms.
Based on these observations, we hypothesize that
exogenous BMSC administration protects against silicosis by two
mechanisms. Firstly, by proliferation, homing and differentiation
into specific lung cell types; and secondly, by paracrine
mechanisms, involving the secretion of a wide range of soluble
factors to improve pathological injury. Therefore, the main purpose
of the present study was to evaluate the potential effects of BMSC
engraftment on silica-induced pulmonary inflammation and fibrosis,
and to determine whether BMSCs release IL1-RA into areas of lung
injury via paracrine pathways to alleviate the early inflammatory
response and the extent of fibrosis. In summary, results of this
study are likely to lay the foundations for gene and stem cell
therapy in silicosis in the future.
Materials and methods
Cell culture
BMSCs were generated from male Sprague-Dawley (SD)
rats. Fresh bone marrow cells were collected by flushing the
medullary cavity of rat femurs with Dulbecco’s Modified Eagle’s
Medium (DMEM; Gibco-BRL, Carlsbad, CA, USA). After filtering, cells
were centrifuged at 1,000 × g for 5 min. Purified cells were
dispersed in cell culture flasks (Corning Life Sciences, Tewksbury,
MA, USA), grown in DMEM supplemented with 10% fetal calf serum
(Gibco-BRL), 100 U/ml penicillin and 100 μg/ml streptomycin
(Sigma-Aldrich, St. Louis, MO, USA) and then cultured at 37°C with
5% CO2. Following 48 h, non-adherent cells were removed
and fresh media was added. Media was replaced every 3 days. Adhered
cells were allowed to grow to ~90% confluency and then trypsinized
and reseeded. Passage 3 (P3) BMSCs were used for this experiment.
The study was approved by the Ethics committee of The School of
Basic Medical Sciences, Hebei United University, Tangshan,
China.
Flow cytometry
P3 BMSCs were harvested by trypsinization
(Gibco-BRL) and cells were fixed in neutralized 2% paraformaldehyde
solution for 30 min. Fixed cells were washed three times with PBS
and incubated with antibodies against the following antigens: CD19,
CD34, CD44 and CD90 (all from Santa Cruz Biotechnology, Santa Cruz,
CA, USA; 1:200) for 30 min. Primary antibodies were directly
conjugated with FITC. Flow cytometry was performed with a FACScan
flow cytometer (BD Biosciences, Franklin Lakes, NJ, USA).
Animals and silicosis model
BMSC donor animals were five male adult SD rats,
aged 3–5 weeks and weighing 100–120 g. BMSC recipient animals were
30 female adult SD rats, aged 6–8 weeks and weighing 200–220 g. All
animals were provided by Vital River Laboratory Animal Technology
Co., Ltd. (Beijing, China). The rat model of silicosis was induced
by 1 ml One-time infusing silica (Sigma-Aldrich) suspension (5 g/l)
using the non-exposed tracheal intubation. All rats were maintained
in a room with a reversed 12-h light-dark cycle at constant
temperature (21°C) and humidity (55%).
Groups and BMSC administration
BMSC recipient rats were randomly divided into three
groups (n=10/group); the control, model and BMSC-treated groups.
Rats in the control group were intratracheally injected with
sterile saline. Following intratracheal administration of silica
suspension, 1 ml BMSC suspension (3×106 cells/ml) was
injected through a tail vein puncture. All rats in each group were
sacrificed 14 days following surgery.
Histological analysis
Lung tissues were fixed in 4% paraformaldehyde
solution for 24 h, washed with running water for 2 h, then
dehydrated with gradient alcohol and embedded in paraffin following
the standard histology procedure. Tissues were serially sectioned
at a thickness of 5 μm. All sections were mounted on glass slides
and then stained with hematoxylin and eosin (H&E). Sections
were observed and analyzed using an optical microscope.
Immunohistochemical analysis
Surgery was performed according to the
manufacturer’s instructions obtained from the SABC
immunohistochemistry kit (Wuhan Boster Biological Technology, Ltd.,
Wuhan, China). Paraffin embedded lung tissue sections (5 μm) on
poly-1-lysine-coated slides were heated for 30 min at 60°C, dewaxed
and rehydrated, followed by microwave antigen retrieval. Endogenous
peroxidase was inactivated with 3% H2O2 for
10 min at room temperature. Sections were incubated in 5% BSA
solution for 20 min to block nonspecific binding. Next, sections
were incubated overnight at 4°C with rabbit anti-interleukin-1
receptor antagonist (IL-1RA), -interleukin-1 (IL-1) or -tumor
necrosis factor-α (TNF-α) polyclonal antibodies (Santa Cruz
Biotechnology; 1:100), and then with horseradish
peroxidase-conjugated anti-rabbit IgG antibodies for 30 min. DAB
was used to reveal the immunohistochemical reaction. PBS was
substituted for the primary antibody as the negative control.
Western blot analysis
RIPA lysis buffer (1 ml) was added to 100 mg lung
tissue and the mixture was homogenated at a low temperature (4°C).
Lysates from tissue samples were cleared by centrifugation at
10,000 × g for 10 min at 4°C. The protein concentration of samples
was determined using BCA reagent (Beijing Solarbio Science &
Technology Co., Ltd., Beijing, China). Samples were subjected to
sodium dodecyl sulfate polyacrylamide gel electrophoresis. Proteins
were transferred onto PVDF membranes (Roche Diagnostics GmbH,
Mannheim, Germany). Blots were blocked with 5% fat-free dry milk
for 1 h at room temperature. Next, blots were incubated with the
following primary antibodies overnight at 4°C: rabbit polyclonal
anti-IL1-RA, -IL-1 and -TNF-α (1:400), mouse monoclonal
anti-β-actin (Santa Cruz Biotechnology; 1:500). The blots were then
incubated with alkaline phosphatase conjugated anti-rabbit IgG and
anti-mouse IgG (Cell Signaling Technology, Inc., Danvers, MA, USA;
1:3,000) for 2 h at room temperature. Blots were developed with
alkaline phosphatase color development kit (Beijing Solarbio
Science & Technology Co., Ltd., Beijing, China).
Immunofluorescence analyses
Lung tissues were fixed in 4% paraformaldehyde for
24 h, submerged in 30% sucrose solution (0.1 M PBS, pH 7.4) until
sinking to the bottom and then embedded in optimum cutting
temperature compound (OCT). Frozen sections (10 μm) were sliced
with a microtome, treated with 0.4% Triton X-100 for 10 min and
blocked in normal donkey serum for 1 h. For double labeling, frozen
sections were incubated with a mixture of goat anti-sex determining
region Y (SRY; Santa Cruz Biotechnology; 1:200) and rabbit
anti-IL-1RA (1:200) polyclonal antibodies overnight at 4°C. On the
following day, sections were incubated with a mixture of
fluorescein-conjugated anti-rabbit IgG and anti-goat IgG (Santa
Cruz Biotechnology; 1:1,000 ) for 2 h at 37°C in the dark. Images
were captured using a laser scanning confocal microscope (Olympus
FV1000; Olympus Inc., Center Valley, PA, USA). Primary antibodies
were replaced with PBS in the negative control group. Additional
immunofluorescence staining was performed in accordance with the
described experimental procedure, to detect expression of SRY in
the heart, liver, spleen, kidney and lung tissues of rats in the
BMSC-treated group.
Statistical analysis
All experiments were repeated three times and
similar results were obtained. Statistical analysis was performed
using the SPSS 16.0 statistics software (SPSS, Inc., Chicago, IL,
USA). Data are expressed as the mean ± SE and the significance of
the experimental results was determined using one-way ANOVA.
P<0.05 was considered to indicate a statistically significant
difference.
Results
BMSC growth state and surface
markers
Primary cells adhered within 48h and proliferated in
clone mode. Cell morphological characteristics included circles and
polygons, nuclear-centered and occasional polygonal cells. BMSCs
were purified by changing the medium several times and during this
process, hematopoietic stem cells and other non-adherent growth
cells were removed. Following ~2 weeks, primary cells reaching 90%
confluence were passaged. Passaged cells overcame the growth
inhibition period and underwent accelerated growth. Following
passaging, cell morphology was more uniform and consistent, forming
mainly fibroblast-like flattened cells. The identity of the
undifferentiated BMSCs was confirmed by detecting specific cell
surface markers. Flow cytometry analysis of P3 BMSCs indicated that
BMSCs were CD44- and CD90-positive and CD19- and CD34-negative.
Confirmation of construction of the
silicosis model
Successful construction of the rat model of
silicosis was confirmed by H&E staining. As demonstrated in
Fig. 1A, alveolitis change,
silicosis nodule formation and collagen deposition were observed in
the model group, confirming successful construction of the model.
By contrast, clear alveolar structures and walls were present, and
inflammatory cell infiltration was not observed in the control
group. However, following transplantation of the BMSCs,
pathological changes associated with silicosis were significantly
reduced. These results indicate that the silicosis model was
successfully constructed and delivery of BMSCs exerted a
significant protective effect. In addition, the majority of IL-1RA
released by transplanted BMSCs appeared to be mediated in a
paracrine manner.
BMSCs upregulate IL-1RA to suppress IL-1
and TNF-α expression
Immunohistochemical analysis was used to examine the
localization of IL-1RA (Fig. 1B),
IL-1 (Fig. 1C) and TNF-α (Fig. 1D) protein expression. Brown
particles observed in cells were considered as positive results.
Brown particles appeared in macrophages, alveolar epithelial cells
and inflammatory cells. Western blot (Fig. 2) and immunohistochemical analysis
revealed trends in IL-1RA, IL-1 and TNF-α expression. IL-1RA, IL-1
and TNF-α proteins were expressed at low levels in the control
group. Their expression was increased in the model group compared
with the control group. Following treatment with BMSCs, IL-1RA
protein expression was markedly upregulated compared with the model
group. Furthermore, IL-1 and TNF-α protein expression levels were
significantly decreased compared with the model group; however,
these remained higher than levels in the control group. Overall,
these findings indicate that the release of IL-1RA blocks the
production and/or activity of IL-1 and TNF-α proteins.
BMSCs release IL-1RA into injured
lungs
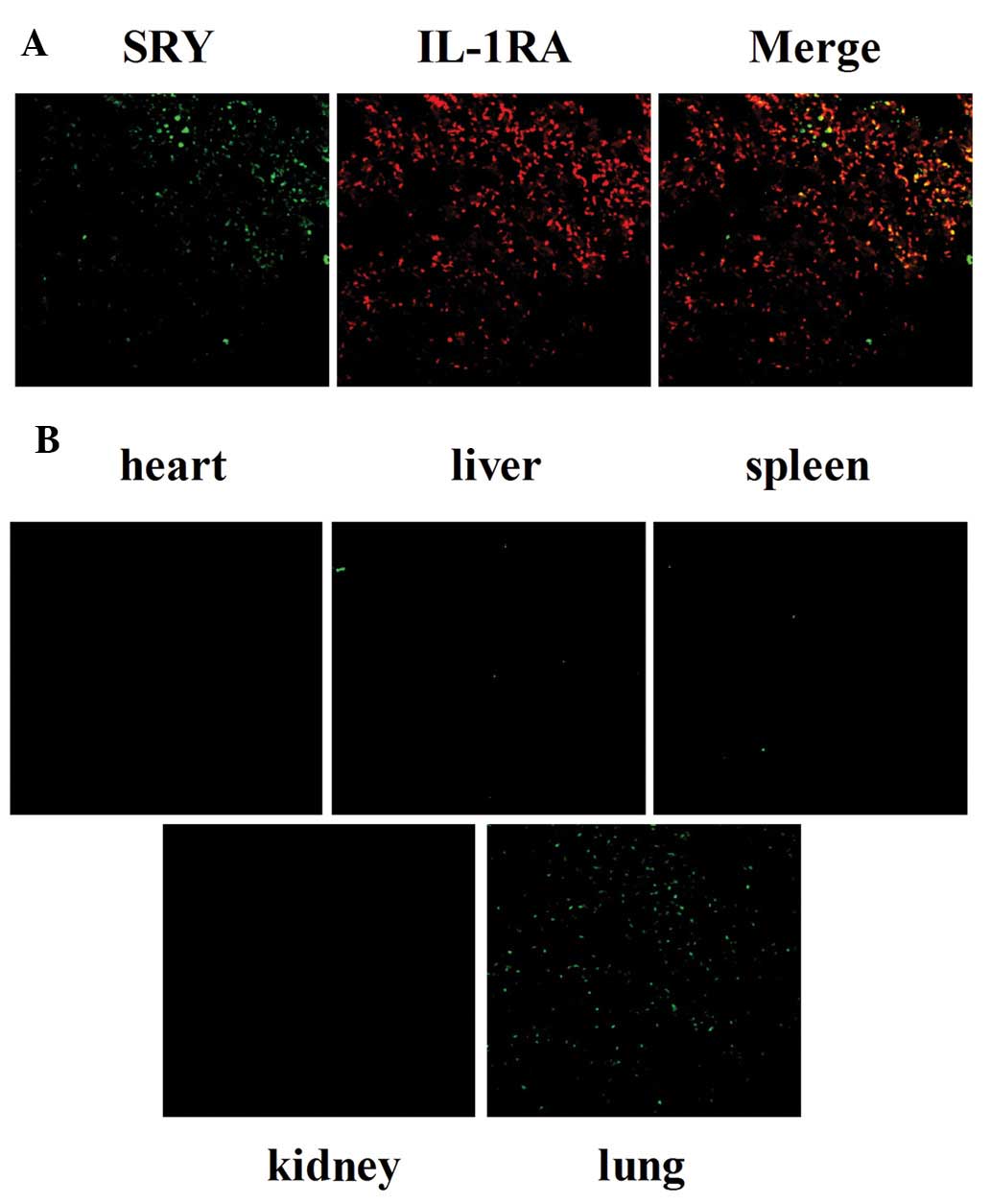
Double immunofluorescence staining was performed to
investigate the co-localization of SRY and IL-1RA expression. As
shown in Fig. 3A, SRY was stained
with goat anti-SRY and fluorescently labeled (green) secondary
antibodies. IL-1RA was stained with rabbit anti-IL1RA and
fluorescently labeled (red) secondary antibodies. Fluorescence was
observed under a laser scanning confocal microscope; yellow
fluorescence indicated co-localized expression in merged images.
These results suggest that the majority of IL-1RA was released by
transplanted BMSCs in a paracrine manner.
BMSC aggregation in injured lungs
SRY expression was determined by immunofluorescent
staining 14 days after administration of BMSCs. SRY protein was
stained using goat anti-SRY and secondary antibodies labeled with
green fluorescence. As revealed in Fig. 3B, BMSCs expressing SRY (green) were
identified in lung tissues, but not in tissues of the heart, liver,
spleen and kidneys in BMSC-treated rats. These observations
indicated that injured tissues were ‘sensed’ by engrafted BMSCs.
Subsequently, BMSCs homed and localized to the injured lung tissue,
exerting important biological functions in damaged areas.
Discussion
Silicosis is associated with significant levels of
morbidity and mortality. In addition, the development of fibrosis
during silicosis is associated with a complex pathogenesis
(1). Therefore, treatment
strategies require a multi-angle, multi-link and multi-targeted
approach. To date, no efficient clinical treatments are available,
and the identification of safe and effective therapeutic strategies
for this pathology is of considerable urgency. Over previous years,
we have been committed to the study of the prevention and treatment
of silicosis. Exogenous BMSC transplantation therapies may
represent a novel therapeutic strategy for lung diseases currently
lacking efficient treatments and are likely to be important in
silicosis clinical care in the future.
The primary objective of the present study was to
verify our hypothesis that the protective effects of BMSCs on
silicosis injury are mediated, in part, by the secretion of IL-1RA
in a paracrine manner. It was previously reported that IL-1RA
exhibits a wide range of biological functions, including
competitively inhibiting IL-1 activity (20) and blocking the production and/or
activity of IL-1 and TNF-α (21).
In the current study, BMSCs were selected as a novel method for
silicosis treatment due to their capacity to undergo self-renewal,
proliferation and differentiation into multiple cell lineages. More
importantly, BMSCs are associated with low immunogenicity and no
immune rejection, enabling BMSC therapies to be applied for daily
clinical applications. As a result of these advantageous
properties, BMSCs have been applied in a number of disease types in
animals and human patients. However, the mechanisms responsible for
their therapeutic effect remain unknown.
In the current study, BMSCs were cultured and
passaged using the cell adherent method (22) and P3 BMSCs were used in the
experiments. Cells were identified via flow cytometry analysis and
results confirmed that the BMSCs expressed characteristic surface
markers, CD44 and CD90, and were CD19- and CD34 negative,
indicating the multilineage potential of BMSCs. Distribution of
BMSCs in recipient rats was determined by immunofluorescence
staining. SRY (green) was only observed in the lung tissue,
indicating that BMSCs were able to ‘sense’ the environment and
respond according to the requirements of the organism for survival
(23). Following this, BMSCs
target the site of lung injury, assume specific and distinct lung
cell phenotypes and regulate inflammatory responses, to promote
structural and functional repair (9). Finally, intravenous administration of
BMSCs was revealed to exhibit a marked therapeutic potential for
silicosis in rats. These observations indicate that injured lung
tissue produces chemokines responsible for the mobilization and
homing of BMSCs towards the site of damage. In addition, the fate
of engrafted BMSCs in vivo is largely regulated by the
microenvironment.
Based on these observations, the differentiation of
BMSCs into specific cell phenotypes appears to be crucial in injury
repair. However, differentiation does not fully account for the
strong therapeutic response observed, indicating that other
specific mechanisms may be involved in the repair of lung injury by
BMSCs. Notably, an important observation of the present study was
that the efficiency of BMSCs in injury repair was more likely to be
due to paracrine effects. It was previously reported that BMSCs
secrete a wide array of growth factors, cytokines and
immunomodulatory factors (24) and
release numerous angiogenic, anti-apoptotic and mitogenic factors
(25), which may afford protection
to injured tissue. However, little is known with regard to
variations in the secretion of paracrine factors between various
subpopulations of BMSCs. Substrate-dependent paracrine signaling
has been demonstrated between subpopulations of BMSCs, which may
affect their formation or perhaps malformation (26). In addition, Bakondi et
al(18) examined typical,
CD133-derived and p75LNGFR-derived BMSCs, observing different
secretion responses in each population in terms of the levels of
secreted growth factors and cytokines when exposed to a hypoxic
environment. Consistent with these observations, in the current
study, intravenous administration of BMSCs as a novel method of
treatment for silicosis protected lungs not only through
multi-lineage differentiation mechanisms, but also through
paracrine mechanisms.
In this study, the tracking of BMSCs was essential
for evaluation of their early migration and distribution patterns
in rats. Therefore, BMSCs isolated from male rats were injected
into female rats in vivo. Next, the expression of SRY was
detected by laser scanning confocal microscopy to track BMSC
survival and further co-localization with paracrine factors. The
results of H&E staining revealed the marked infiltration of
inflammatory cells and silicotic nodule formation in the model
group, indicating that the rat model of silicosis was constructed
successfully. Following administration of BMSCs, these pathological
changes were reduced, indicating that BMSCs significantly reduced
inflammation and the extent of fibrosis. Prevention of the
inflammatory response and inhibition of collagen accumulation and
matrix metalloproteinase activation by BMSCs has also been
demonstrated in BLM-induced lung injury in mice (9). In this study, administration of rat
BMSCs 24 h following silica treatment was observed to result in a
significant reduction in IL-1 and TNF-α levels, explained, in part,
by an increase in IL-1RA expression. In addition, the fluorescent
double-labeled co-localization of SRY (green) and IL-1RA (red)
expression was observed in the BMSC-treated group. Co-localization
of SRY and IL-1RA appeared yellow following the merging of images.
Consistent with our hypothesis that BMSCs may secrete IL-1RA into
damaged lung tissue via a paracrine pathway, Ortiz et
al(21) reported that the
expression and secretion of IL-1RA is restricted to a unique
subpopulation of BMSCs. In addition, a previous study by Mei et
al(27) demonstrated that
human BMSCs possessed direct antimicrobial activity, which was
mediated, in part, by the secretion of cathelicidin hCAP-18/LL-37,
improving survival and enhancing bacterial clearance. More
specifically, results of immunohistochemistry and western blot
analysis indicated that IL-1RA released by BMSCs not only
antagonized the function of IL-1, but also blocked the release of
TNF-α from activated macrophages. IL-1 and TNF-α, two fundamental
pro-inflammatory cytokines in the lung, are vital in the pathogenic
process of pulmonary fibrosis. Inflammatory responses in silicosis
are mediated by the release of pro-inflammatory cytokines, IL-1 and
TNF-α, from activated macrophages and other leukocytes (28). IL-1 and TNF-α also directly or
indirectly stimulate proliferation of endothelial cells and
fibroblasts, extracellular matrix deposition and collagen synthesis
(29). Finally, these cytokines
promote the formation and development of pulmonary fibrosis.
However, IL-1RA, a protein present in normal conditions, is known
to reduce the degree of pulmonary fibrosis (30). Previous studies have reported that
injection of IL-1RA intraperitoneally by implantation of a
permeability micropump device revealed a marked protective
potential from BLM- or silicon dioxide-induced pulmonary fibrosis
(31), indicating that IL-1RA may
block lung inflammation and fibrosis induced by exposure to silica.
These studies provided a realistic and viable basis for the present
study.
In conclusion, results of the current study
demonstrate that BMSCs have a comprehensive biological effect on
silicosis. Secretion of IL1-RN by BMSCs in a paracrine manner
protected rats from silica-induced lung injury by blocking the
production and/or activity of IL-1 and TNF-α. This may perform an
anti-inflammatory role in early stages of the disease and an
antifibrotic role at later stages in silicosis rats. In short,
BMSCs exhibit a potential beneficial effect on silicosis through
paracrine mechanisms. This study may provide valuable insight into
the prevention and treatment of silicosis and present novel
prospects for cell therapy.
Acknowledgements
The present study was supported by grants from the
Science and Technology Support Key Funding Project of Hebei
Province (no. 09276191D), the State Production Safety Supervision
Administration Science and Technology Development Funding Project
(no. 06-549) and the Hebei Province Science and Technology Support
for Major Projects (no. 13277709D).
Abbreviations:
|
BMSCs
|
bone marrow-derived mesenchymal stem
cells
|
|
IL-1
|
interleukin-1
|
|
IL-1RA
|
interleukin-1 receptor antagonist
|
|
TNF-α
|
tumor necrosis factor α
|
|
SRY
|
sex determining region Y
|
References
|
1
|
Rimal B, Greenberg AK and Rom WN: Basic
pathogenetic mechanisms in silicosis: current understanding. Curr
Opin Pulm Med. 11:169–173. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Herzog EL, Chai L and Krause DS:
Plasticity of marrow-derived stem cells. Blood. 102:3483–3493.
2003. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Nishida H, Nakayama M, Tanaka H, et al:
Safety of autologous bone marrow stromal cell transplantation in
dogs with acute spinal cord injury. Vet Surg. 41:437–442. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Osanai T, Kuroda S, Sugiyama T, et al:
Therapeutic effects of intra-arterial delivery of bone marrow
stromal cells in traumatic brain injury of rats - in vivo cell
tracking study by near-infrared fluorescence imaging. Neurosurgery.
70:435–444. 2012. View Article : Google Scholar
|
|
5
|
Zhao L, Feng Z, Hu B, Chi X and Jiao S: Ex
vivo-expanded bone marrow mesenchymal stem cells facilitate
recovery from chemically induced acute liver damage.
Hepatogastroenterology. 59:2389–2394. 2012.PubMed/NCBI
|
|
6
|
Islam MN, Das SR, Emin MT, et al:
Mitochondrial transfer from bone-marrow-derived stromal cells to
pulmonary alveoli protects against acute lung injury. Nat Med.
18:759–765. 2012. View
Article : Google Scholar : PubMed/NCBI
|
|
7
|
Luan Y, Zhang X, Kong F, Cheng GH, Qi TG
and Zhang ZH: Mesenchymal stem cell prevention of vascular
remodeling in high flow-induced pulmonary hypertension through a
paracrine mechanism. Int Immunopharmacol. 14:432–437. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Aslam M, Baveja R, Liang OD, et al: Bone
marrow stromal cells attenuate lung injury in a murine model of
neonatal chronic lung disease. Am J Respir Crit Care Med.
180:1122–1130. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Ortiz LA, Gambelli F, McBride C, et al:
Mesenchymal stem cell engraftment in lung is enhanced in response
to bleomycin exposure and ameliorates its fibrotic effects. Proc
Natl Acad Sci USA. 100:8407–8411. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Luan Y, Zhang ZH, Wei DE, et al:
Implantation of mesenchymal stem cells improves right ventricular
impairments caused by experimental pulmonary hypertension. Am J Med
Sci. 343:402–406. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Rojas M, Xu J, Woods CR, et al: Bone
marrow-derived mesenchymal stem cells in repair of the injured
lung. Am J Respir Cell Mol Biol. 33:145–152. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Nemeth K, Keane-Myers A, Brown JM, et al:
Bone marrow stromal cells use TGF-beta to suppress allergic
responses in a mouse model of ragweed-induced asthma. Proc Natl
Acad Sci USA. 107:5652–5657. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Wang PP, Xie DY, Liang XJ, et al: HGF and
direct mesenchymal stem cells contact synergize to inhibit hepatic
stellate cells activation through TLR4/NF-kB pathway. PLoS One.
7:e434082012. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Jin P, Zhang X, Wu Y, et al:
Streptozotocin-induced diabetic rat-derived bone marrow mesenchymal
stem cells have impaired abilities in proliferation, paracrine,
antiapoptosis, and myogenic differentiation. Transplant Proc.
42:2745–2752. 2010. View Article : Google Scholar
|
|
15
|
Xu M, Uemura R, Dai Y, Wang Y, Pasha Z and
Ashraf M: In vitro and in vivo effects of bone marrow stem cells on
cardiac structure and function. J Mol Cell Cardiol. 42:441–448.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Mezey E: The therapeutic potential of bone
marrow-derived stromal cells. J Cell Biochem. 112:2683–2687. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Reis LA, Borges FT, Simões MJ, Borges AA,
Sinigaglia-Coimbra R and Schor N: Bone marrow-derived mesenchymal
stem cells repaired but did not prevent gentamicin-induced acute
kidney injury through paracrine effects in rats. PLoS One.
7:e440922012. View Article : Google Scholar
|
|
18
|
Bakondi B, Shimada IS, Perry A, et al:
CD133 identifies a human bone marrow stem/progenitor cell
sub-population with a repertoire of secreted factors that protect
against stroke. Mol Ther. 17:1938–1947. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Burdon TJ, Paul A, Noiseux N, Prakash S
and Shum-Tim D: Bone marrow stem cell derived paracrine factors for
regenerative medicine: current perspectives and therapeutic
potential. Bone Marrow Res. 2011:Dec 6–2010.(Epub ahead of
print).
|
|
20
|
Arend WP: Interleukin 1 receptor
antagonist. A new member of the interleukin 1 family. J Clin
Invest. 88:1445–1451. 1991. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Ortiz LA, Dutreil M, Fattman C, et al:
Interleukin 1 receptor antagonist mediates the antiinflammatory and
antifibrotic effect of mesenchymal stem cells during lung injury.
Proc Natl Acad Sci USA. 104:11002–11007. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Smajilagić A, Aljičević M, Redžić A,
Filipović S and Lagumdžija A: Rat bone marrow stem cells isolation
and culture as a bone formative experimental system. Bosn J Basic
Med Sci. 13:27–30. 2013.PubMed/NCBI
|
|
23
|
Abkowitz JL, Robinson AE, Kale S, Long MW
and Chen J: Mobilization of hematopoietic stem cells during
homeostasis and after cytokine exposure. Blood. 102:1249–1253.
2003. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Kinnaird T, Stabile E, Burnett MS, et al:
Local delivery of marrow-derived stromal cells augments collateral
perfusion through paracrine mechanisms. Circulation. 109:1543–1549.
2004. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Nagaya N, Kangawa K, Itoh T, et al:
Transplantation of mesenchymal stem cells improves cardiac function
in a rat model of dilated cardiomyopathy. Circulation.
112:1128–1135. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Sharma RI and Snedeker JG: Paracrine
interactions between mesenchymal stem cells affect substrate driven
differentiation toward tendon and bone phenotypes. PLoS One.
7:e315042012. View Article : Google Scholar
|
|
27
|
Krasnodembskaya A, Song Y, Fang X, et al:
Antibacterial effect of human mesenchymal stem cells is mediated in
part from secretion of the antimicrobial peptide LL-37. Stem Cells.
28:2229–2238. 2010. View
Article : Google Scholar : PubMed/NCBI
|
|
28
|
Zhang K and Phan SH: Cytokines and
pulmonary fibrosis. Biol Signals. 5:232–239. 1996. View Article : Google Scholar
|
|
29
|
Kolb M, Margetts PJ, Anthony DC, Pitossi F
and Gauldie J: Transient expression of IL-1beta induces acute lung
injury and chronic repair leading to pulmonary fibrosis. J Clin
Invest. 107:1529–1536. 2001. View
Article : Google Scholar : PubMed/NCBI
|
|
30
|
Zhang Y, Lee TC, Guillemin B, Yu MC and
Rom WN: Enhanced IL-1 beta and tumor necrosis factor-alpha release
and messenger RNA expression in macrophages from idiopathic
pulmonary fibrosis or after asbestos exposure. J Immunol.
150:4188–4196. 1993.PubMed/NCBI
|
|
31
|
Piguet PF, Vesin C, Grau GE and Thompson
RC: Interleukin 1 receptor antagonist (IL-1ra) prevents or cures
pulmonary fibrosis elicited in mice by bleomycin or silica.
Cytokine. 5:57–61. 1993. View Article : Google Scholar : PubMed/NCBI
|