Introduction
Stroke is the third leading cause of death and the
leading cause of long-term disability in the US (1). Approximately 4 million Americans live
with the negative consequences of stroke (2,3). In
addition, the lives of caregivers including spouses, children and
friends are personally affected because of this significant
disease. Current investigations have focused on stroke
rehabilitation and brain plasticity as a mechanism in recovery
(4).
Therefore, plasticity following stroke remains a
crucial issue for stroke survivors and there is invariably some
degree of functional recovery (5).
In other words, when neurons are damaged by stroke, other neurons
take over for them. This adaptive behavior assists in the
reorganization of the brain and recovery of lost skills. Brain
plasticity is therefore the reason intensive therapy is such a
critical component of stroke rehabilitation (6–8).
Plasticity after stroke has traditionally been
studied by observing changes only in the spatial distribution and
laterality of focal brain activation during affected limb movement
(9). However, neural
reorganization is multifaceted and our understanding may be
enhanced by examining dynamics of activity within large-scale
networks involved in the sensorimotor control of the limbs. In
stroke rehabilitation, functional imaging studies of the motor
system have described task-related brain activation in recovered
patients over and above control subjects in contralesional
sensorimotor and premotor cortex, ipsilesional cerebellum,
bilateral supplementary motor area (SMA) and parietal cortex
(10–14).
Functional magnetic resonance imaging (fMRI) as a
molecular magnetic resonance imaging procedure is used in various
studies for stroke plasticity. This technique is based on the fact
that the magnetic properties of deoxygenated and oxygenated
hemoglobin in the blood are different and produce different signals
(contrast) when imaged with T2* sensitive MRI sequences (15,16).
Therefore, the mapping of the brain’s networks often begins by
identifying a set of links, and then attempts to estimate the set
of connections between these nodes, based on an analysis of the
fMRI time series associated with these nodes. In most cases, the
directionality of these links exhibits ‘connectivity’ by
demonstrating how information flows through the network (17). Functional connectivity is a
promising means of assessing the consequences of a stroke lesion as
well as studying plasticity in neural networks.
A large number of neurological impairments that
involve muscle weakness, loss of range of motion, and impaired
force generation create deficits in motor control that affect the
stroke survivor’s capacity for independent living and economic
self-sufficiency (18). Tactile
sensibility of the hand is essential for identifying objects and
for motor performance. This performance is largely affected by
stroke as well as sensory perception, which is difficult to
recover. Many traditional therapeutic interventions have been used
in rehabilitation to promote functional recovery, with outcome
studies yielding inconsistent results (19). Recent evidence has demonstrated
that intensive massed and repeated practice may be necessary to
modify neural organization and effect recovery of functional motor
skills (9).
A recent preliminary study on 4 individuals
post-stroke showed that all 4 individuals improved in sensory tasks
and motor performance, effects that remained 4 weeks post-treatment
(20). In terms of traditional
therapy, which is provided in a rehabilitation center or hospital,
the patient is usually seen for half-hour sessions, once or twice a
day. This visitation is decreased to once or twice a week in
outpatient therapy. It is evident that in this service-delivery
model, it is difficult to provide the amount or intensity of
practice needed to effect neural and functional changes. Therefore,
further intervention is required, including exercise tasks
throughout the therapy. More recently, clinical studies using
robot-assisted therapy have been shown to benefit patients during
neurological recovery (21–32).
The incremental improvements in clinical scales following intensive
robotic therapy, although small, are statistically significant and
certainly meaningful to patients.
In a previous study (8), we demonstrated decreased intrinsic
neural coupling between M1 and cerebellum (Ce), which was
consistent with a dysfunctional M1 to Ce connection in stroke
patients compared to controls. Stroke patients also showed
increased SMA to M1 and SMA to cerebellum coupling, suggesting that
changes in SMA and Ce connectivity may occur to compensate for a
dysfunctional M1. In this study, we present additional findings
exploring whether training effective connectivity strengths altered
after training relative to baseline and promote functional recovery
in chronic stroke patients and healthy controls.
Materials and methods
Participants
Twelve healthy volunteers and 5 chronic stroke
patients provided written informed consent to participate in this
cross-sectional study. All experiments were approved by the
Institutional Review Board at Massachusetts General Hospital and
performed at the Athinoula A. Martinos Center for Biomedical
Imaging. All participants used an MR-compatible hand induced
robotic device (MR_CHIROD) during fMRI at 45% of their maximum
strength. The brain maps and connectivity strengths of stroke
patients and healthy controls were compared prior and subsequent to
the training.
MR_CHIROD hand device
The design and testing of the hand device have been
previously described (4,8,33).
The hand device consists of three main subsystems: i) an
electrorheological fluid (ERF) resistive element; ii) handles and
iii) two sensors, an optical encoder to measure patient-induced
mobility and a second encoder functioning as a force sensor. Unlike
previously described devices (34,35),
MR_CHIROD is the first ERF-based device that has been demonstrated
to function in conjunction with fMRI for brain mapping in chronic
stroke patients (33,36). Of note, MR_CHIROD is capable of
limiting and controlling a number of factors that affect its
function, rendering it particularly useful for home-based training
given the low level of expert clinical support in the home
environment that can be accompanied by low extrinsic motivation.
MR_CHIROD can be re-engineered to improve the cost-to-benefit ratio
and therapy effectiveness by providing autonomous and recordable
training programs with extrinsic motivation through virtual reality
technology.
Process, training and MRI protocol
As described in a previous study (8), all studies were performed on a
state-of-the-art 3-T MR system in order to obtain a high
signal-to-noise ratio (SNR). We used a systematic approach to
optimize the protocol with respect to SNR by varying the number of
echoes, the echo time, the repetition time, the Generalized
Autocalibrating Partially Parallel Acquisitions (GRAPPA)
acceleration factor, the field of view (FOV), and the number of
excitations (NEX). These factors were set in such a manner that the
protocol could be completed in 45 min and a 12-channel Siemens Tim
coil was used. The functional MRI protocol was as follows:
T1-weighted MR images (a high-resolution three-dimensional
T1-weighted, MP-RAGE image was obtained for anatomical reference
and optimal gray-white matter contrast); fluid attenuation
inversion recovery (FLAIR); providing anatomical localization of
hyperintense regions and MR images of stroke lesions and Multilevel
fMRI (high-resolution GRAPPA EPI sequence for whole-brain BOLD fMRI
at optimal spatial resolution for BOLD detection).
Patients completed a single training at home and
underwent serial MR evaluation at baseline and after 8 weeks of
training. Training at home consisted of squeezing a gel exercise
ball with the paretic hand at ~75% of maximum strength for 1 h/day,
3 days/week. For each patient, reference (100%) was own maximum
force, defined as the force at which subjects were able to
completely squeeze the MR_CHIROD [group max force: 128±13 N (n=5,
male)]. All the studies were performed on a Siemens Tim Trio (3T)
and BOLD fMRI was performed using GRAPPA gradient-echo EPI (TR/TE =
3,000/30 msec, 1.56×1.56×3 mm). A block design paradigm was used
for fMRI. During the action period, subjects squeezed the MR_CHIROD
and released continuously. A fixation cross was projected during
rest. Each volunteer performed the paradigm at 45, 60, and 75% of
their maximum grip strength and fully squeezed the device at all
levels. The percentage levels compensate for performance
confounds.
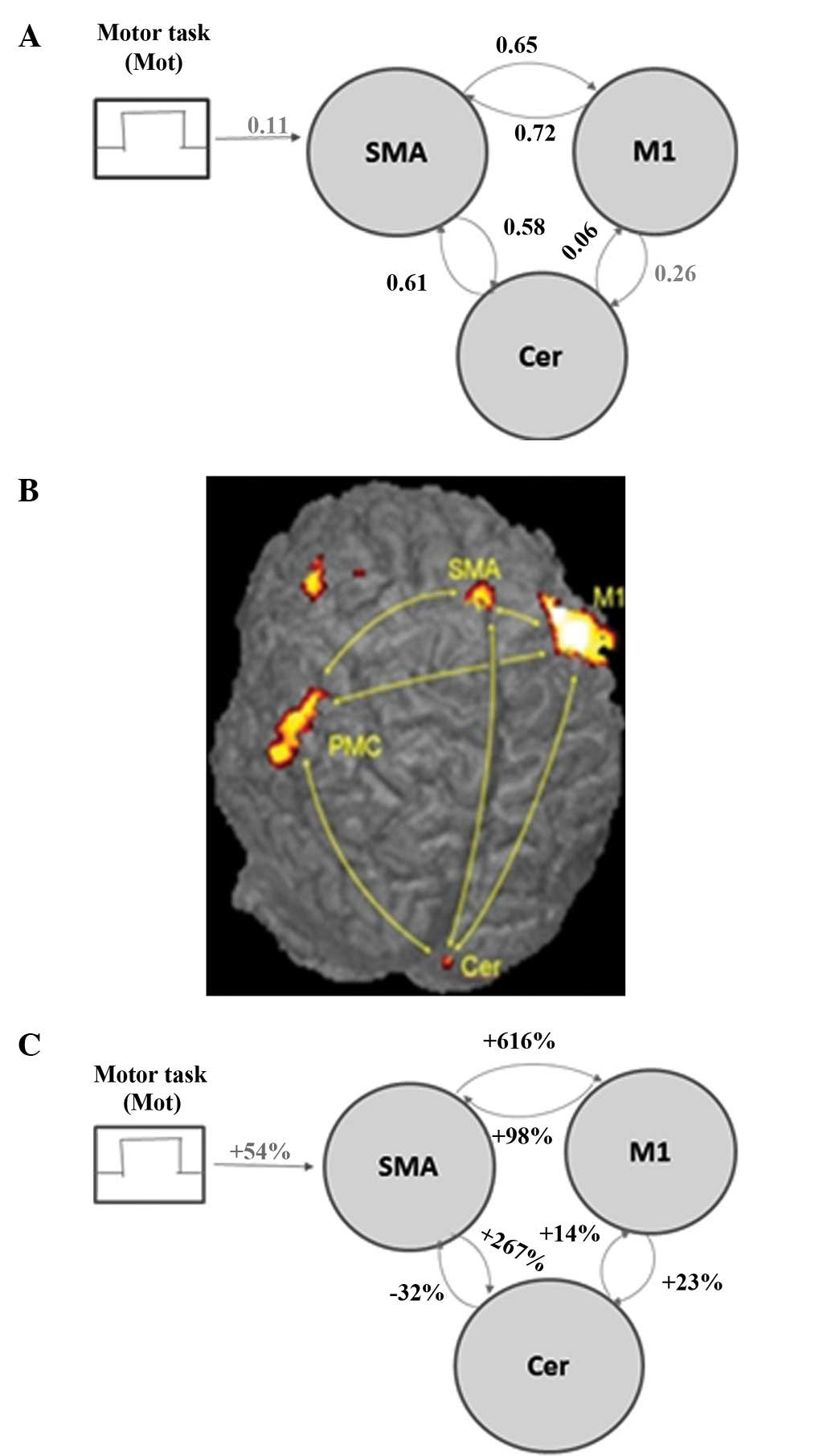
The DCM model was constructed for the connectivity
analysis using brain regions that were activated in all subjects
(Fig. 1A) and comprised three
regions: M1, SMA, and Ce. Volumes of interest were defined in these
regions using a sphere centered at the maximum activation from the
second-level analysis and with a radius of 2 voxels. Possible
connections between the brain areas were permitted to account for
plasticity changes in the stroke patients. Mot connected to the
SMA, which is the only region in the model responsible for motor
planning. Connectivity strengths and posterior probabilities were
calculated using the DCM utility in SPM5.
Statistical analysis
Statistical analysis was performed with ANOVA with
Least Significance Difference adjustment for post-hoc comparisons,
Mixed Model Procedure with Restricted Maximum Likelihood
estimation, SPSS version 12.
Results
The results suggest that a dysfunctional
connectivity between SMA and Ce and/or M1 underlies hand motor
disability after stroke. We suggest that assessing effective
connectivity by means of fMRI and dynamic causal modeling might be
used for the evaluation of training-promoting recovery of function
and neuroplasticity after stroke.
Table I summarizes
the findings of the study. More specifically, the fMRI analysis
revealed activations in M1, SMA, premotor cortex and Ce in both
stroke patients and controls (Fig.
1B). Greater connection strength translated into a greater
absolute value of the parameter shown, and thus a more prominent
effect of one area on another (Table
I). Connectivity strengths of healthy subjects are shown in
Fig. 1A and percentage changes in
connectivity strengths after training relative to the baseline are
shown in Fig. 1C. The DCM analysis
produced the following three noteworthy results: i) in healthy
subjects performing a simple motor task, there was minimum
effective connectivity from Ce to M1 (Fig. 1A); ii) training significantly
increased coupling between M1 and SMA, suggesting an induction of
SMA recruitment (Fig. 1C). This
possibility has been suggested by earlier fMRI studies in healthy
subjects (37). iii) SMA-Ce
coupling and Ce-M1 coupling were induced by training (Fig. 1C).
 | Table IConnectivity strengths in chronic
stroke patients for the selected intrinsic model. |
Table I
Connectivity strengths in chronic
stroke patients for the selected intrinsic model.
| Pathway | Baselinea | After
traininga | % Difference from
baselineb | P-value |
|---|
| M1→SMA | 0.50±0.05 | 49.49±0.07 | +98c | <0.001 |
| SMA→M1 | 0.37±0.07 | 2.65±0.05 | +616c | <0.001 |
| SMA→Ce | 0.32±0.06 | 0.17±0.08 | +267c | <0.05 |
| Ce→SMA | 0.41±0.04 | 0.29±0.06 | −32 | <0.05 |
| Ce→M1 | 0.35±0.03 | 0.40±0.05 | 14 | NS |
| M1→Ce | 0.39±0.06 | 0.48±0.07 | 23 | NS |
Discussion
Results of this study indicate that fMRI is a
promising molecular imaging procedure and show connectivity
alterations in motor-related areas suggesting functional
reorganization of motor systems in stroke (8). Of note, enhancement of SMA activity
through training has been suggested as a potential means for
ameliorating M1 dysfunction after stroke. These results emphasize
the importance of the role, of SMA not only for the preparation and
execution of intended movements, but also for suppressing movements
that are represented in the motor system but are not to be
performed. These results also demonstrate that connectivity
alterations between motor areas may help balance a functionally
abnormal M1 in chronic stroke patients. In a previous study, Ce
hyperactivity was also documented in Parkinson’s disease patients,
where it was suggested to represent a compensatory mechanism for
defective basal ganglia (38). In
the present study, Ce hyperactivity reflects efforts by stroke
patients to improve motor balance and function. Our results confirm
data of a previous study (6).
Moreover, in this study, data suggest that a dysfunction between
ipsilesional and contralesional M1, and between ipsilesional SMA
and contralesional M1 underlies hand motor disability following
stroke. Assessing effective connectivity by means of fMRI and
dynamic causal modeling might be used in the future for the
evaluation of interventions promoting recovery of function.
The present results confirm and extend previous
findings in stroke rehabilitation and plasticity. A meta-analysis
focusing on 10 studies of robot-assisted therapy on motor and
functional recovery in 218 stroke patients showed a significant
effect on motor recovery in the upper paretic limb but no
significant effect on functionality (39). In another similar report, the
authors found no significant improvement in daily activities,
although motor function and arm motor strength improved (40). A more recent meta-analysis of 11
eligible studies that included 328 patients showed significant
improvements in motor function and strength of the paretic arm with
electromechanical and robot-assisted arm training, but there were
no improvements in activities of daily living. Robot-assisted
therapy has been shown to benefit patients during neurological
recovery (21,25,29,32).
Specifically, individuals who received robotic therapy exhibited
improved gain-in-motor coordination and muscle strength of the
exercised shoulder and elbow relative to control subjects (32). Furthermore, Volpe et
al(25) reported that these
improvements were sustained over a 3-year period following
inpatient discharge from the hospital.
Our results show the importance of exercise and
training after stroke, potentially crucial for a rapid recovery.
Recent studies have shown that individuals with stroke, given the
opportunity to exercise after stroke, maintain their functional
status after the initial rehabilitation and improve function
(41,42). In this study, training suggests
reorganization in M1, SMA, premotor cortex and Ce, which has been
documented in other studies. Reorganization of brain networks has
already been explored in humans (43), non-human primates (44) and rats (45). Of note, despite the disrupted motor
patterns following stroke, motor system reorganization has been
demonstrated in stroke patients (46,47),
confirming results of the present study. Our results suggest that
patients with minor corticospinal system damage show plasticity in
the process of recovery. In a previous study, non-invasive
transcranial magnetic stimulation have been used successfully for
the activation of SMA, resulting in M1 improvement (48). Since stroke recovery may vary among
different cultures, future investigations are required on specific
training approaches that should be matched to the individual case
characteristics.
In recent years, there has been an explosive
research trend to rehabilitative evidence, which formulated a large
platform of new technologies (e.g., robotics) and systems for
stroke recovery. Virtual reality can engage patients, increase
their attention during the task, and improve motivation, thus
increasing the effectiveness of rehabilitation. However, more
investigation is required for the development of a united code
applicable to all settings that potentially lead to the best
possible outcomes for stroke survivors.
In conclusion, we suggest that assessing changes in
connectivity by means of fMRI and MR_CHIROD might be used in the
future to demonstrate the neural network plasticity that underlies
functional recovery in chronic stroke patients. Our findings
suggest that rehabilitative exercise training might induce
functional connectivity alterations after training in both stroke
and healthy subjects. Thus, we purport that fMRI as a molecular
imaging biomarker of functional reorganization of motor systems in
stroke is a clinically relevant molecular medicine approach and it
may allow caregivers to select the most appropriate rehabilitation
approach for each patient and to fine-tune this approach based on
brain maps obtained before and after a short trial of therapy. This
is a new concept of personalized molecular medicine combining motor
fMRI with a novel MR-compatible hand-induced robotic device
training in chronic stroke that can be applied in other motor
pathologies.
Acknowledgements
This study was supported in part by a grant from the
National Institute of Biomedical Imaging and Bioengineering of the
National Institutes of Health (grant no. R21 EB004665-01A2) to Dr
A. Aria Tzika. We thank Dr Mavroidis, the principal investigator of
the subcontract to Northeastern University, for construction of the
MR_CHIROD. Thanks is extended to Ann Power Smith Ph.D. of Write
Science Right for editorial assistance.
References
|
1
|
Hall MJ, Levant S and DeFrances CJ:
Hospitalization for stroke in U.S. hospitals, 1989–2009. NCHS Data
Brief: 1–8. 2012
|
|
2
|
Evers SM, Ament AJ and Blaauw G: Economic
evaluation in stroke research: a systematic review. Stroke.
31:1046–1053. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Payne KA, Huybrechts KF, Caro JJ, Craig
Green TJ and Klittich WS: Long term cost-of-illness in stroke: an
international review. Pharmacoeconomics. 20:813–825. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Astrakas LG, Naqvi SH, Kateb B and Tzika
AA: Functional MRI using robotic MRI compatible devices for
monitoring rehabilitation from chronic stroke in the molecular
medicine era (Review). Int J Mol Med. 29:963–973. 2012.PubMed/NCBI
|
|
5
|
Twitchell TE: Variations and abnormalities
of motor development. Phys Ther. 45:424–430. 1965.PubMed/NCBI
|
|
6
|
Grefkes C, Nowak DA, Eickhoff SB, et al:
Cortical connectivity after subcortical stroke assessed with
functional magnetic resonance imaging. Ann Neurol. 63:236–246.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Wang LE, Tittgemeyer M, Imperati D, et al:
Degeneration of corpus callosum and recovery of motor function
after stroke: a multimodal magnetic resonance imaging study. Hum
Brain Mapp. 33:2941–2956. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Mintzopoulos D, Astrakas LG, Khanicheh A,
et al: Connectivity alterations assessed by combining fMRI and
MR-compatible hand robots in chronic stroke. Neuroimage. 47(Suppl
2): T90–T97. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Murphy TH and Corbett D: Plasticity during
stroke recovery: from synapse to behaviour. Nat Rev Neurosci.
10:861–872. 2009. View
Article : Google Scholar : PubMed/NCBI
|
|
10
|
Chollet F, DiPiero V, Wise RJ, Brooks DJ,
Dolan RJ and Frackowiak RS: The functional anatomy of motor
recovery after stroke in humans: a study with positron emission
tomography. Ann Neurol. 29:63–71. 1991. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Weiller C: Imaging recovery from stroke.
Exp Brain Res. 123:13–17. 1998.(In German).
|
|
12
|
Cramer SC: Stroke recovery. Lessons from
functional MR imaging and other methods of human brain mapping.
Phys Med Rehabil Clin N Am. 10:875–886. 1999.PubMed/NCBI
|
|
13
|
Cramer SC: Brain repair after stroke. N
Engl J Med. 362:1827–1829. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Seitz RJ: Stroke recovery: the pyramid in
focus. Neurology. 74:276–277. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Ogawa S and Lee TM: Magnetic resonance
imaging of blood vessels at high fields: in vivo and in vitro
measurements and image simulation. Magn Reson Med. 16:9–18. 1990.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Amaro E Jr and Barker GJ: Study design in
fMRI: basic principles. Brain Cogn. 60:220–232. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Smith SM: The future of FMRI connectivity.
Neuroimage. 62:1257–1266. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Brodal A: Self-observations and
neuro-anatomical considerations after a stroke. Brain. 96:675–694.
1973. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Smith PS, Dinse HR, Kalisch T, Johnson M
and Walker-Batson D: Effects of repetitive electrical stimulation
to treat sensory loss in persons poststroke. Arch Phys Med Rehabil.
90:2108–2111. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Ietswaart M, Johnston M, Dijkerman HC, et
al: Mental practice with motor imagery in stroke recovery:
randomized controlled trial of efficacy. Brain. 134:1373–1386.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Aisen ML, Krebs HI, Hogan N, McDowell F
and Volpe BT: The effect of robot-assisted therapy and
rehabilitative training on motor recovery following stroke. Arch
Neurol. 54:443–446. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Volpe BT, Krebs HI, Hogan N, Edelstein OL,
Diels C and Aisen M: A novel approach to stroke rehabilitation:
robot-aided sensorimotor stimulation. Neurology. 54:1938–1944.
2000. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Volpe BT, Krebs HI, Hogan N, Edelsteinn L,
Diels CM and Aisen ML: Robot training enhanced motor outcome in
patients with stroke maintained over 3 years. Neurology.
53:1874–1876. 1999.PubMed/NCBI
|
|
24
|
Volpe BT, Krebs HI and Hogan N: Is
robot-aided sensorimotor training in stroke rehabilitation a
realistic option? Curr Opin Neurol. 14:745–752. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Volpe BT, Ferraro M, Lynch D, et al:
Robotics and other devices in the treatment of patients recovering
from stroke. Curr Neurol Neurosci Rep. 5:465–470. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Volpe BT, Ferraro M, Krebs HI and Hogan N:
Robotics in the rehabilitation treatment of patients with stroke.
Curr Atheroscler Rep. 4:270–276. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Ferraro M, Palazzolo JJ, Krol J, Krebs HI,
Hogan N and Volpe BT: Robot-aided sensorimotor arm training
improves outcome in patients with chronic stroke. Neurology.
61:1604–1607. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Fasoli SE, Krebs HI, Stein J, Frontera WR,
Hughes R and Hogan N: Robotic therapy for chronic motor impairments
after stroke: follow-up results. Arch Phys Med Rehabil.
85:1106–1111. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Daly JJ, Hogan N, Perepezko EM, et al:
Response to upper-limb robotics and functional neuromuscular
stimulation following stroke. J Rehabil Res Dev. 42:723–736. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Macclellan LR, Bradham DD, Whitall J, et
al: Robotic upper-limb neurorehabilitation in chronic stroke
patients. J Rehabil Res Dev. 42:717–722. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Finley MA, Fasoli SE, Dipietro L, et al:
Short-duration robotic therapy in stroke patients with severe
upper-limb motor impairment. J Rehabil Res Dev. 42:683–692. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Prange GB, Jannink MJ, Groothuis-Oudshoorn
CG, Hermens HJ and Ijzerman MJ: Systematic review of the effect of
robot-aided therapy on recovery of the hemiparetic arm after
stroke. J Rehabil Res Dev. 43:171–184. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Khanicheh A, Mintzopoulos D, Weinberg B,
Tzika AA and Mavroidis C: MR_CHIROD 2: magnetic resonance
compatible smart hand rehabilitation device for brain imaging. IEEE
Trans Neural Syst Rehabil Eng. 16:91–98. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Tsekos N, Khanicheh A, Christoforou E and
Mavroidis C: Magnetic resonance-compatible robotic and mechatronics
systems for image-guided interventions and rehabilitation: a review
study. Annu Rev Biomed Eng. 9:351–387. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Siekierka EM, Eng K, Bassetti C, et al:
New technologies and concepts for rehabilitation in the acute phase
of stroke: a collaborative matrix. Neurodegener Dis. 4:57–69. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Mintzopoulos D, Khanicheh A, Konstas A, et
al: Functional MRI of rehabilitation in chronic stroke patients
using novel MR-compatible hand robots. Open Neuroimag J. 2:94–101.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Cramer SC, Weisskoff RM, Schaechter JD, et
al: Motor cortex activation is related to force of squeezing. Hum
Brain Mapp. 16:197–205. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Yu H, Sternad D, Corcos DM and
Vaillancourt DE: Role of hyperactive cerebellum and motor cortex in
Parkinson’s disease. Neuroimage. 35:222–233. 2007.
|
|
39
|
Kwakkel G, Kollen BJ and Krebs HI: Effects
of robot-assisted therapy on upper limb recovery after stroke: a
systematic review. Neurorehabil Neural Repair. 22:111–121. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Mehrholz J, Werner C, Kugler J and Pohl M:
Electromechanical-assisted gait training with physiotherapy may
improve walking after stroke. Stroke. Apr 3–2008.(Epub ahead of
print).
|
|
41
|
Langhammer B and Stanghelle JK: Can
physiotherapy after stroke based on the Bobath concept result in
improved quality of movement compared to the motor relearning
programme. Physiotherapy research international. Physiother Res
Int. 16:69–80. 2011. View
Article : Google Scholar
|
|
42
|
Langhammer B, Stanghelle JK and Lindmark
B: An evaluation of two different exercise regimes during the first
year following stroke: a randomised controlled trial. Physiother
Theory Pract. 25:55–68. 2009.PubMed/NCBI
|
|
43
|
Xerri C, Merzenich MM, Peterson BE and
Jenkins W: Plasticity of primary somatosensory cortex paralleling
sensorimotor skill recovery from stroke in adult monkeys. J
Neurophysiol. 79:2119–2148. 1998.PubMed/NCBI
|
|
44
|
Aizawa H, Inase M, Mushiake H, Shima K and
Tanji J: Reorganization of activity in the supplementary motor area
associated with motor learning and functional recovery. Exp Brain
Res. 84:668–671. 1991. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Jones TA and Schallert T: Overgrowth and
pruning of dendrites in adult rats recovering from neocortical
damage. Brain Res. 581:156–160. 1992. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Boyd LA, Edwards JD, Siengsukon CS, Vidoni
ED, Wessel BD and Linsdell MA: Motor sequence chunking is impaired
by basal ganglia stroke. Neurobiol Learn Mem. 92:35–44. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Ward NS: Mechanisms underlying recovery of
motor function after stroke. Postgrad Med J. 81:510–514. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Hummel F and Cohen LG: Improvement of
motor function with noninvasive cortical stimulation in a patient
with chronic stroke. Neurorehabil Neural Repair. 19:14–19. 2005.
View Article : Google Scholar : PubMed/NCBI
|