Introduction
Mesenchymal stem cells (MSCs) are multipotent cells
that may differentiate into a variety of cell lineages, including
osteocytes, adipocytes, chondrocytes, endothelial cells,
cardiomyocytes and neurons, when exposed to appropriate conditions
(1,2). Bone marrow-derived MSCs (bmMSCs) are
a commonly used source of stem cells. To date, bmMSCs have been
widely applied in tissue engineering. The migration capability of
bmMSCs is an important determinant of the efficiency of bmMSC-based
transplant therapy. A previous study showed that ~1.5% of injected
stem cells reached the injured tissue following intracoronary
injection for 2 h (3). However,
the low homing rate of bmMSCs severely limits their clinical
uses.
MicroRNAs (miRs) are endogenous, small, noncoding
RNAs in eukaryotic cells (4). miRs
are post-transcriptional regulators that negatively regulate gene
expression by binding to the target mRNA for degradation and
translational repression (4). At
present, >1000 miRs have been identified in the human and mouse
genomes, a number of which have been found to be involved in cell
migration (4,5). It has been reported that miRs,
including miR-let-7a, -16, -30a, -34a, -107, -125b, -200c, -203,
-218, -424 and -488, inhibit the migration of specific tumor cells
and other normal cell lineages (5–14).
However, other miRs, including miR-10b, -20, -21 and -144, have
been reported to promote cell migration (15–18).
To date, the effects of miR-10b on the migration and
invasion of tumor cells have been well studied (15,19,20).
However, little is known about the function of miR-10b in the
migration of bmMSCs. In the present study, the role of miR-10b in
bmMSC migration and E-cadherin expression was investigated.
Materials and methods
Isolation and culture of bmMSCs
bmMSCs were isolated and cultured as previously
described (21). In brief, bmMSCs
were isolated from bone marrow, which was harvested from mouse
tibia and femur, plated into 100-mm Petri dishes and cultured in
DMEM (Invitrogen Life Technologies, Carlsbad, CA, USA) supplemented
with 15% fetal bovine serum (Thermo Scientific HyClone, West Palm
Beach, FL, USA), 2 mM L-glutamine (Sigma-Aldrich, St. Louis, MO,
USA), 100 U/ml penicillin (Sigma-Aldrich) and 100 g/ml streptomycin
(Sigma-Aldrich) for 3 h. The non-adherent cells were removed and
the medium was replaced with fresh medium. A purified population of
bmMSCs was obtained following 3 weeks of culture. The study was
approved by the Ethics Committee of Xinxiang Medical University
(Xinxiang, China)
Flow cytometry assay
bmMSCs were harvested, washed with
phosphate-buffered saline (PBS) and incubated with CD45-FITC and
CD90-FITC antibodies (Abcam, Cambridge, MA, USA) at 4°C for 1 h.
Cells were washed and resuspended in 400 ml PBS and analyzed by
flow cytometry (Becton-Dickinson, Franklin Lakes, NJ, USA). The
flow cytometer collected ~10,000 cells.
Transfection of miR-10 mimic
bmMSCs were plated into 6-well plates. When cells
reached 60–70% confluency, they were transfected with miR-10b mimic
and negative control precursor miRNA using Lipofectamine™ 2000 in
Opti-MEM medium according to the instructions of the Lipofectamine
LTX kit (Invitrogen Life Technologies, Carlsbad, CA, USA). The
medium was replaced following 4 h of transfection. After 24 h of
transfection, the cells were used in subsequent experiments.
Quantitative polymerase chain reaction
(qPCR) for miR-10b
bmMSCs transfected with miR-10b mimic or negative
control miRNA were washed with PBS and total RNA was extracted
using TRIzol reagent (Promega Corporation, Madison, WI, USA).
miRNAs were purified using an miRNAeasy kit (Applied Biosystems,
Carlsbad, CA, USA) and cDNA was synthesized using a microRNA
reverse transcription kit (Applied Biosystems) according to the
manufacturer’s instructions. qPCR was performed using an Applied
Biosystems 7500 Real-Time PCR System (Applied Biosystems). The
miR-10b primers and U6 housekeeping primer were obtained from
Abcam.
Transwell migration assay
The migration of bmMSCs was measured using Corning
Costar transwell plates (Corning Inc., Corning, NY, USA) with 8-μm
pore filters, as previously described by Kim et al(22). In brief, bmMSCs (1×105)
were plated in the upper inserts of the transwell chamber.
Following 6 h of transmigration, the migrated bmMSCs on the lower
side of the filter were fixed with 4% paraformaldehyde and stained
with crystal violet and viewed by an inverted microscope (Olympus,
Tokyo, Japan).
Immunofluorescence assay
Immunofluorescence staining was performed using
rabbit anti-mouse E-cadherin antibody (Santa Cruz Biotechnology,
Inc., Santa Cruz, CA, USA), as previously described (23). In brief, bmMSCs were cultured on
10-mm round coverslips and stained using standard methods. Cells
were mounted on slides using ProlongH Gold antifade reagent (Life
Technologies Corporation, Carlsbad, CA, USA) and imaged by
fluorescence microscopy (Olympus).
RT-PCR
Total RNA was isolated from bmMSCs using RNeasy mini
kits (Invitrogen Life Technologies) according to the manufacturer’s
instructions. RNA (1 μg) was applied to synthesize cDNA using the
SuperScript II First Strand DNA Synthesis kit (Invitrogen Life
Technologies). RT-PCR was performed using a 20-μl reaction volume
containing 100 ng cDNA, 10 μl 2X PCR mixture and 0.3 μM primers.
The products were separated by 1.5% agarose gel electrophoresis and
visualized by ethidium bromide on a UV transilluminator (Bio-Rad,
Hercules, CA, USA). The primers used were as follows: E-cadherin
forward, 5′-CCTGTCAACCCAAGCAC-3′ and reverse,
5′-ATTTCCTGACCCACACCAAA-3′; and β-actin forward,
5′-TTCTTTGCAGCTCCTTCGTTGCCG-3′ and reverse,
5′-TGGATGGCTACGTACATGGCTGGG-3′.
Western blotting
Proteins were extracted from bmMSCs and separated by
12% SDS-PAGE. Following electrophoresis, proteins were transferred
to PVDF membranes. The membranes were blocked with 5% non-fat milk
in TBS-T and incubated with rabbit anti-mouse E-cadherin antibody
at 4°C overnight. Blots were incubated with HRP-conjugated duck
anti-rabbit secondary antibody (Santa Cruz Biotechnology, Inc.) for
1 h at room temperature. The immunoreactive bands were visualized
by enhanced chemiluminescence.
Statistical analysis
Statistical analysis was performed with SPSS 11.5
software (SPSS Inc., Chicago, IL, USA). Data are presented as the
mean ± SD. Univariate comparison of means was evaluated using the
Student’s t-test. P<0.05 was considered to indicate a
statistically significant difference.
Results
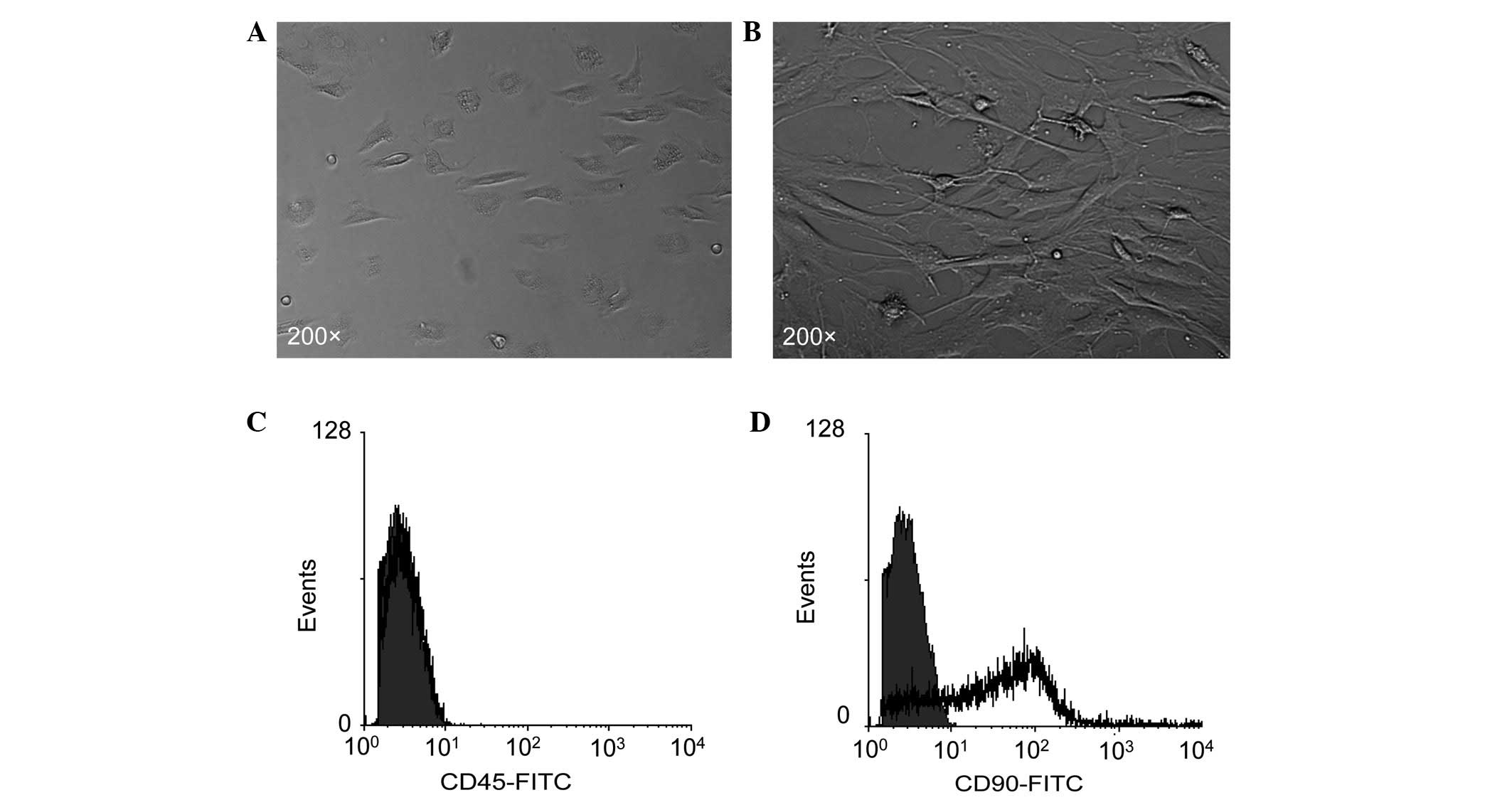
Morphology of bmMSCs and expression of
MSC markers
Consistent with previous studies (21), primary bmMSCs showed a
spindle-shaped or triangular morphology (Fig. 1A) and the passaged (the third
passage) bmMSCs exhibited a typical fibroblast-like morphology
(Fig. 1B). Flow cytometry revealed
that bmMSCs negatively expressed the leukocyte antigen molecule
CD45 (Fig. 1C), but positively
expressed the MSC marker molecule CD90 (Fig. 1D).
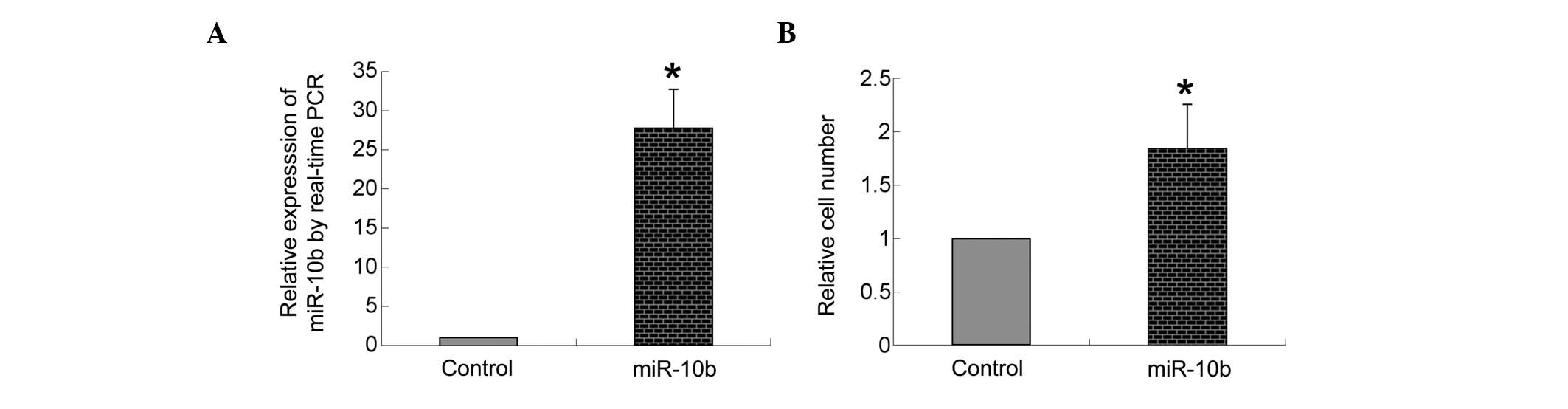
miR-10b expression following
transfection
As shown in Fig.
2A, compared with the transfection of negative control
precursor miRNA, transfection of miR-10b mimic markedly increased
the expression of miR-10b in bmMSCs (P<0.05).
Overexpression of miR-10b promotes
migration of bmMSCs
A transwell system was used to measure the
transmigration ability of bmMSCs. As shown in Fig. 2B, compared with the transfection of
negative control miRNA, transfection of the miR-10b mimic
significantly increased the number of bmMSCs transmitted to the
lower side of the well filters (P<0.05).
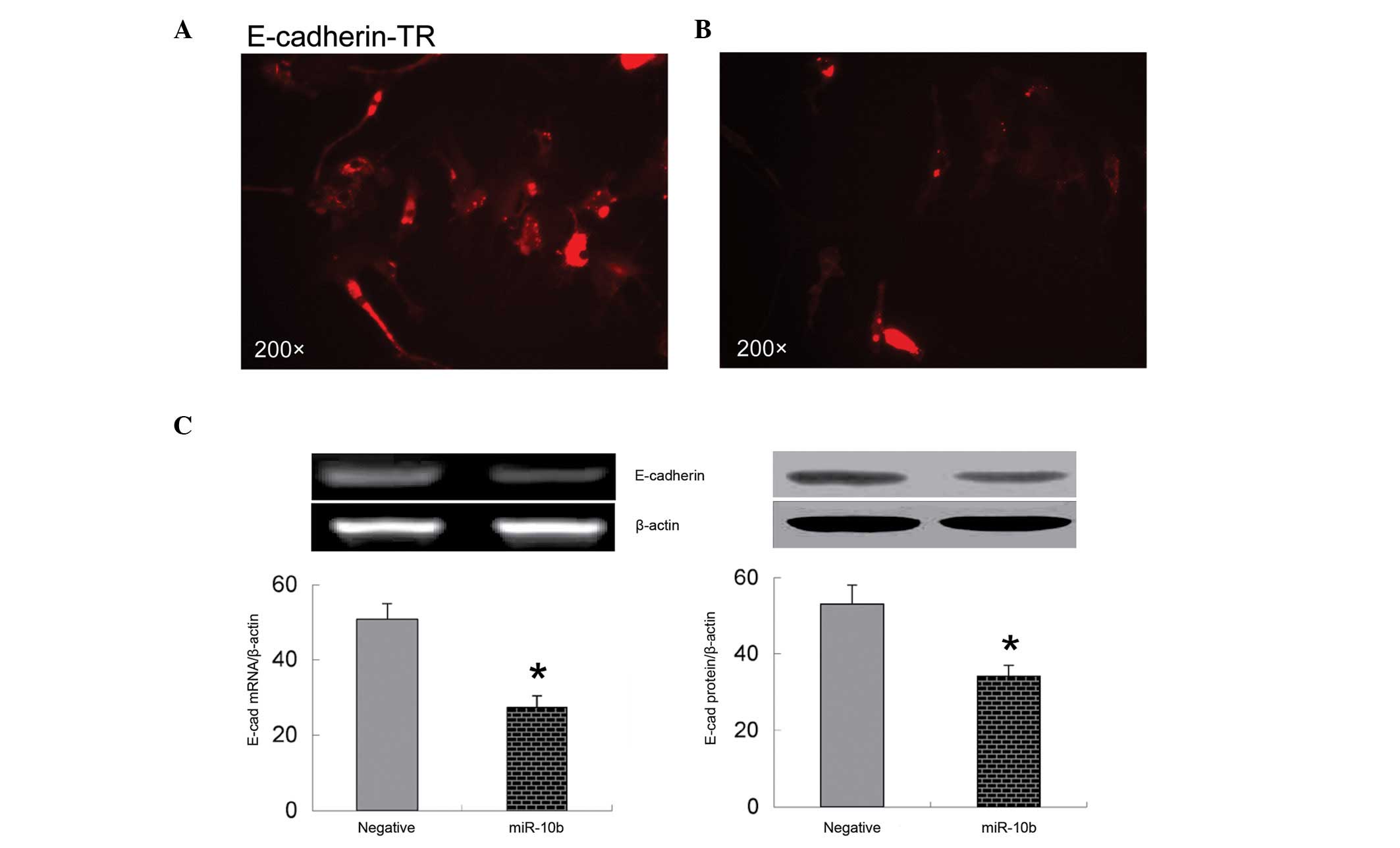
Overexpression of miR-10b decreases
expression of E-cadherin
It is known that E-cadherin is an important
regulator of cell migration (24).
Loss of cell surface E-cadherin suppresses cell adhesion and
promotes cell migration (24). The
present immunofluorescence data show that overexpression of miR-10b
significantly decreases E-cadherin expression on the surface of
bmMSCs (Fig. 3A and B). These
observations were further confirmed by RT-PCR and western blot
analysis, which indicate that mRNA and protein expression of
E-cadherin are significantly downregulated in bmMSCs transfected
with miR-10b mimic compared with those transfected with negative
control miRNA (P<0.05; Fig.
3C).
Discussion
In the present study, upregulation of miR-10b was
shown to promote the migration of bmMSCs in vitro for the
first time. In addition, overexpression of miR-10b was observed to
markedly decrease the expression of E-cadherin, a critical
regulator of cell migration. These observations indicate that
miR-10b positively regulates bmMSC migration, which may depend on
its role in regulating E-cadherin expression.
miRs are small, noncoding RNA molecules that
participate in multiple pathophysiological processes, including
cell differentiation, migration, proliferation, apoptosis and
inflammation (25). miR-10b is the
a well-studied member of the miR family in cell metastasis. It has
been shown that upregulation of miR-10b facilitates migration of
several types of tumor cell lineages (19,20).
For example, Tian et al(15) reported that overexpression of
miR-10b increases the metastases of KYSE140 cells. Guessous et
al(26) observed that miR-10b
expression is increased in human glioblastoma tissues and
glioblastoma stem cells, and inhibition of miR-10b markedly reduces
the invasion and migration of glioblastoma stem cells. In the
present study, overexpression of miR-10b was observed to
significantly increase the migration of bmMSCs in the transwell
assay.
E-cadherin is a transmembrane cell adhesion molecule
that is important for multiple physiological processes, including
cell migration, morphology and polarity (27). Downregulation of E-cadherin has
been observed to decrease cell-cell adhesion and increase cell
migration (28). In the present
study, overexpression of miR-10b was observed to significantly
decrease the expression of E-cadherin. miR-10b-mediated bmMSC
migration is hypothesized to be involved in the downregulation of
E-cadherin.
In summary, the current study supports the
hypothesis that miR-10b promotes the migration of bmMSCs in
vitro. The present observations indicate that the upregulation
of miR-10b expression may be a viable approach to increase the
migration capacity of bmMSCs in transplantation therapy and an
alternative to improve the therapeutic efficiency of
transplantation.
References
|
1
|
Tokaer-Keskin Z, Akar AR, Ayaloglu-Butun
F, et al: Timing of induction of cardiomyocyte differentiation for
in vitro cultured mesenchymal stem cells: a perspective for
emergencies. Can J Physiol Pharmacol. 87:143–150. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Oswad J, Boxberger S, Jørgensen B, et al:
Mesenchymal stem cells can be differentiated into endothelial cells
in vitro. Stem Cells. 22:377–384. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Hu X, Wei L, Taylor TM, et al: Hypoxic
preconditioning enhances bone marrow mesenchymal stem cell
migration via Kv2.1 channel and FAK activation. Am J Physiol Cell
Physiol. 301:C362–C372. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Li C, Feng Y, Coukos G and Zhang L:
Therapeutic microRNA strategies in human cancer. AAPS J.
11:747–757. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Kim SJ, Shin JY, Lee KD, et al: MicroRNA
let-7a suppresses breast cancer cell migration and invasion through
downregulation of C-C chemokine receptor type 7. Breast Cancer Res.
14:R142012. View
Article : Google Scholar : PubMed/NCBI
|
|
6
|
Cheng CW, Wang HW, Chang CW, et al:
MicroRNA-30a inhibits cell migration and invasion by downregulating
vimentin expression and is a potential prognostic marker in breast
cancer. Breast Cancer Res Treat. 134:1081–1093. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Yan K, Gao J, Yang T, et al: MicroRNA-34a
inhibits the proliferation and metastasis of osteosarcoma cells
both in vitro and in vivo. PLoS One. 7:e337782012. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Chen J, Chen XR, Zhang R, et al:
MicroRNA-107 inhibits glioma cell migration and invasion by
modulating Notch2 expression. J Neurooncol. 112:59–66. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Wu D, Ding J, Wang L, et al: microRNA-125b
inhibits cell migration and invasion by targeting matrix
metallopeptidase 13 in bladder cancer. Oncol Lett. 5:829–834.
2013.PubMed/NCBI
|
|
10
|
Jurmeister S, Baumann M, Balweierz A, et
al: MicroRNA-200C represses migration and invasion of breast cancer
cells by targeting actin-regulatory proteins FHOD1 and PPM1F. Mol
Cell Biol. 32:633–651. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Takeshita N, Mori M, Kano M, et al:
miR-203 inhibits the migration and invasion of esophageal squamous
cell carcinoma by regulating LASP1. Int J Oncol. 41:1653–1661.
2012.PubMed/NCBI
|
|
12
|
Kinoshita T, Hanazawa T, Nohata N, et al:
Tumor suppressive microRNA-218 inhibits cell migration and invasion
through targeting laminin-332 in head and neck squamous cell
carcinoma. Oncotarget. 3:1386–1400. 2012.PubMed/NCBI
|
|
13
|
Chamorro-Jorganes A, Araldi E, Penalva LO,
et al: MicroRNA-16 and microRNA-424 regulate cell-autonomous
angiogenic functions in endothelial cells via targeting vascular
endothelial growth factor receptor-2 and fibroblast growth
receptor-1. Arterioscler Thromb Vasc Biol. 31:2595–2606. 2011.
View Article : Google Scholar
|
|
14
|
Song J, Kim D and Jin EJ: MicroRNA-488
suppresses cell migration through modulation of the focal adhesion
activity during chondrogenic differentiation of chick limb
mesenchymal cells. Cell Biol Int. 35:179–185. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Tian Y, Luo A, Cai Y, et al: MicroRNA-10b
promotes migration and invasion through KLF4 in human esophageal
cancer cell lines. J Biol Chem. 285:7986–7994. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Fan X, Liu Y, Jiang J, et al: miR-20a
promotes proliferation and invasion by targeting APP in human
ovarian cancer cells. Acta Biochim Biophys Sin (Shanghai).
42:318–324. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Madhyastha R, Madhyastha H, Nakajima Y, et
al: MicroRNA signature in diabetic wound healing: promotive role of
miR-21 in fibroblast migration. Int Wound J. 9:355–361. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Zhang LY, Ho-Fun Lee V, Wong AM, et al:
MicroRNA-144 promotes cell proliferation, migration and invasion in
nasoparyngeal carcinoma through repression PTEN. Carcinogenesis.
34:454–463. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Li QJ, Zhou L, Yang F, et al: MicroRNA-10b
promotes migration and invasion through CADM1 in human
hepatocellular carcinoma cells. Tumour Biol. 33:1455–1465. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Preis M, Gardner TB, Gordon SR, et al:
MicroRNA-10b expression correlates with response to neoadjuvant
therapy and survival in pancreatic ductal adenocarcinoma. Clin
Cancer Res. 17:5812–5821. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Zhang F, Wang C, Jing S, et al:
Lectin-like oxidized LDL receptor-1 expresses in mouse bone
marrow-derived mesenchymal stem cells and stimulates their
proliferation. Exp Cell Res. 319:1054–1059. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Kim YS, Kwon JS, Hong MH, et al:
Promigratory activity of oxytocin on umbilical cord blood-derived
mesenchymal stem cells. Artif Organs. 34:453–461. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Spencer HL, Eastham AM, Merry CL, et al:
E-cadherin inhibits cell surface localization of the pro-migratory
5T4 oncofetal antigen in mouse embryonic stem cells. Mol Biol Cell.
18:2838–2851. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Li L, Hartley R, Reiss B, et al:
E-cadherin plays an essential role in collective directional
migration of large epithelial sheets. Cell Mol Life Sci.
69:2779–2789. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Xiao Y, Xu C, Guan J, et al: Discovering
dysfunction of multiple microRNAs cooperation in disease by a
conserved microRNA co-expression network. PLoS One. 7:e322012012.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Guessous F, Alvarado-Velez M,
Marcinkiewicz L, et al: Oncogenic effects of miR-10b in
glioblastoma stem cells. J Neurooncol. 112:153–163. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Jamora C and Fuchs E: Intercellular
adhesion, signaling and the cytoskeleton. Nat Cell Biol.
4:E101–E108. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Vogelmann R, Nguyen-Tat MD, Giehi K, et
al: TGFbeta-induced downregulation of E-cadherin-based cell-cell
adhesion depends on PI3-kinase and PTEN. J Cell Sci. 118:4901–4912.
2005. View Article : Google Scholar : PubMed/NCBI
|