Introduction
Osteoclasts are involved in bone resorption by
releasing important cell factors (1–3).
Several important proteins are involved in bone remodeling,
including tartrate-resistant acid phosphatase (TRAP), which is also
a specific marker of osteoclasts. Immunohistochemical staining of
TRAP is one of the most commonly used methods for distinguishing
osteoclasts from other cell types (4,5). In
addition, carbonic anhydrase II (CAII) is involved in the acid
demineralization of osteoclasts (6,7).
CAII is a metal enzyme that possesses one active site containing
Zn2+ and catalyzes the
CO2+H2O↔HCO3−+H+
reaction. CAII synthesizes H2CO3 by combining
CO2 with H2O against the concentration
gradient, which provides an abundance of H+ for the acid
demineralization of bone tissue. When CAII function is inhibited at
the protein level or via gene mutation, osteosclerosis may occur.
Previous studies demonstrated that the osteoclast-associated
receptor (OSCAR) is involved in a series of osteoclast activities
including differentiation, maturation and recruitment (8–11).
Moreover, OSCAR is overexpressed during the acute stage of
arthritis; however, not during the resting stage. The inhibition of
the OSCAR function also results in a reduction of bone loss. As
osteoclasts are a type of terminally differentiated cell, apoptotic
mechanisms are critically important in the regulation of the cell.
The FAS/FASL is an important pathway in apoptosis and is critical
in osteoclast apoptosis (12–14).
The functional expression of TRAP, CAII, OSCAR and FAS/FASL in
osteoclasts is an important focus of current research, and several
studies have investigated potential inhibitors of these factors as
a way of preventing or delaying bone loss.
Alendronate (ALN;
4-amino-hydroxybutylidene-bisphosphonate) is a third generation
bisphosphonate that has been shown to be a potent osteoclast
inhibitor and treatment with ALN results in a reduction of bone
loss (15–20). ALN inhibits bone loss by decreasing
bone turn-over, increasing bone mass and reducing the number of
bone fractures without leading to any type of mineralization
disorder. However, osteoclasts are differentiated cells that cannot
be passaged, and therefore it is difficult to assess the functions
and responses of various cell factors on these cells. Thus, the
effects of ALN on TRAP, CAII, OSCAR and FAS/FASL gene expression in
osteoclasts remain to be elucidated.
Previously we hypothesized that ALN may exhibit an
effect on critical factors involved in osteoclast function based on
the correlation between stress stimuli and osteoclast response
(4,7). In the present study, the mRNA and
protein expression of TRAP, CAII, OSCAR and FAS/FASL were analyzed
in osteoclasts following the treatment of cells with ALN at various
concentrations and incubation times.
Materials and methods
In vitro culture of RAW264.7 cells
RAW264.7 cells (ATCC TIB-71, Chinese Academy of
Medical Sciences, Beijing, China) isolated from the murine
mononuclear/macrophagic system were cultured in Dulbecco’s modified
Eagle’s medium (high glucose, no. 21013024, Gibco BRL, Carlsbad,
CA, USA). When the cells reached 80% confluence, the adherent cells
were scraped away, using a cell scraper, from the 25 cm2
cell culture flask surface (no. 353108, BD Biosciences, Franklin
Lakes, NJ, USA) and a uniform cell suspension was prepared for
osteoclast culture.
In vitro osteoclast culture
The RAW264.7 cell suspension was seeded in 6-well
plates at a density of 1×104 cells/well. The osteoclast
induction culture medium contained 80 μg/ml soluble recombinant
murine soluble receptor activator of NF-Kβ ligand (sRANKL; no.
315-11, Peprotech, Inc., Rocky Hill, NJ, USA), 10% fetal bovine
serum (no. 16000-044, Gibco BRL), 100 U/ml penicillin, 100 μg/ml
streptomycin and 2 mmol/l glutamine. The medium was changed every
three days. On the sixth day of culture, mature osteoclasts were
observed and photographed under an inverted microscope and photo
system (no. IX51-A21PH, Olympus Corp., Tokyo, Japan).
TRAP staining for osteoclast
identification
On the sixth day of culture, mature osteoclasts were
harvested and stained for TRAP according to the manufacturer’s
instructions of the TRAP staining kit (no. 387A, Sigma-Aldrich, St.
Louis, MO, USA). An inverted microscope and photo system were used
to observe the stained mature osteoclasts.
Experimental groups
The induced mature osteoclasts were incubated with 0
M (control group), 10−7 M (low concentration),
10−6 M (medium concentration) and 10−5 M
(high concentration) ALN (no. 126855-100MG, Molar Mass 325.1, Merck
KGaA, Darmstadt, Germany) for 2, 4, 6 and 8 h. The mRNA and protein
expression of TRAP, CAII, OSCAR and FAS/FASL were then detected by
qPCR and western blot analysis for each time point.
qPCR
Primers for the five genes were designed using
Primer Premier 5.0 software (Premier Biosoft, Palo Alto, CA, USA)
and then synthesized in the laboratory. The primer sequences for
the five genes and annealing temperatures are listed in Table I. The reagents included Fermentas
RevertAid™ First-Strand cDNA Synthesis kit (no. K1622, MBI
Fermentas Corp., Hanover, NH, USA), RNA extraction kit (TRIzol, no.
15596018, Invitrogen Life Sciences, Carlsbad, CA, USA), Taq
DNA polymerase (no. B1263, Promega Corp., Beijing, China), gel
imaging analytical system (no. 2200, Alpha Corp., USA), GeneAmp PCR
system (no. 9700, Applied Biosystems, Foster City, CA, USA) and
ultraviolet spectrophotometer (no. DU800, Beckman Coulter Inc.,
Miami, FL, USA). Routine PCR consisted of RNA extraction,
first-strand cDNA synthesis, PCR amplification of the target gene
fragment, gel electrophoresis and imaging. The integrated optical
density of the electrophoresis strips for the five genes was
recorded for analysis and DNase treatment of the samples was
performed. In addition, the amplification was confirmed to be in
the linear phase when the measurements were taken. The PCR reaction
conditions were as follows: Initial denaturation at 95°C for 2 min,
32 cycles of denaturation at 95°C for 30 sec, renaturation for 30
sec and extension at 72°C for 30 sec.
 | Table IAnnealing temperatures and primer
sequences. |
Table I
Annealing temperatures and primer
sequences.
| Primer name | Sequence
(5′-3′) | Annealing
temperature (°C) | Amplified length
(bp) |
|---|
| Actin |
F-CTAAGGCCAACCGTGAAA
R-TGGAAGGTGGACAGTGAG | 60 | 724 |
| TRAP |
F-CTCCCACCCTGAGATTTG
R-GTTTCCAGCCAGCACATA | 57 | 263 |
| CAII |
F-GATTGGACCTGCCTCACA
R-ACACCTGGGTCTTGCTTT | 54 | 507 |
| OSCAR |
F-TGATTGGCACAGCAGGAG
R-AAGGCACAGGAAGGAAATAGAG | 55 | 272 |
| FAS |
F-AGGAGGGCAAGATAGATG
R-CTGCGACATTCGGCTTTT | 56 | 151 |
| FASL |
F-TGGAGCAGTCAGCGTCAG
R-ACAGGTGGTGGTGGAGGTG | 56 | 245 |
Western blot analysis
The predominant reagents used were as follows: 10X
Ponceau S (no. P0370, BioHao Corp., China), Tris-Glycine buffer
(no. 28380, Thermo Fisher Scientific, Waltham, MA, USA),
Tris-buffered saline (TBS) solution (no. 0788-2PK, Amresco Inc.,
Solon, Ohio, USA), confining liquid (No. PA106-01, Tiangen Corp.,
Beijing, China), 10X TBS with Tween-20 (TBST) solution (GMS12130,
Genmed Corp., Zoetemeer, The Netherlands), polyvinylidene fluoride
(PVDF) membrane (No. IPVH00010, Millipore, Billerica, MA, USA),
electrophoretic apparatus (no. HV164-5056, Bio-Rad Hercules, CA,
USA) and gel imaging system (no. GelDoc2000, Bio-Rad). Osteoclasts
were harvested, lysed in ice-cold cell lysis solution (Western
lysis buffer, no. P0013, Beyotime Biotech, Jiangsu, China) and
centrifuged at 11,279 × g. The proteins encoded by the five genes
were quantified by the bicinchonic acid assay method, resolved by
8–12% sodium dodecyl sulphate-polyacrylamide gel electrophoreses
and transferred onto PVDF membranes. Following this, antibodies
were added and incubated with the proteins, which were then washed
with TBST and analyzed for the detection of the proteins. The
primary antibodies used were: Anti-TRAP (N-17, no. sc-30832) -OSCAR
(M-17, no. sc-34237) -CAII (G-2, no. sc-48351), FAS (5F9, no.
sc-52394, Santa Cruz Biotechnology Inc., Santa Cruz, CA, USA) and
-FASL (101624, no. ab10512 Abcam, Cambridge, UK). The antibodies
were diluted to 1:2000, respectively. The membranes were placed
into western eluant (no. P0023C, Beyotime Biotech) three times for
5 min. Secondary antibodies conjugated to horseradish peroxidase
were diluted to 1:5000 and washed three times for 5 min. Protein
bands were detected with an electrochemiluminesence kit (no.
P0019/P0020, Beyotime Biotech).
Statistical analysis
Experiments were performed in triplicate for each
sample with 3 parallel wells. Data were recorded as the mean ±
standard deviation. In this study, the data were normally
distributed. Analysis of variance was used to assess statistical
differences using SPSS 17.0 software (SPSS, Inc., Chicago, IL,
USA). P<0.05 was considered to indicate a statistically
significant difference.
Results
Observation of RAW264.7 cells in
culture
When the RAW264.7 cells were seeded, the cells with
1–2 nuclei initially displayed a round or oval shape. Subsequent to
2 days of culture in vitro, the RAW264.7 cells grew rapidly
and began to form colonies. The RAW264.7 cells occupied 80–90% of
the flask surface area by the third day of culture in vitro,
the number and properties of the cells were suitable for their use
in osteoclast culture.
Observation of sRANKL-induced
osteoclasts
Several large multinuclear cells were observed by
the third day of culture when RAW264.7 cells had been treated with
sRANKL. By the fifth day, increased numbers of multinuclear cells
were observed and by the sixth day there were large numbers of the
cells present. The cells were large and irregular in shape, and
several nuclei were observed (Fig.
1).
TRAP staining of osteoclasts
The multinuclear cells were confirmed to be
osteoclasts by TRAP staining features, where the cytoplasm was a
rose-pink color and the nuclei appeared light yellow (Fig. 2). The nucleoli were also clearly
observed.
mRNA expression of TRAP, CAII, OSCAR and
FAS/FASL in osteoclasts
The mRNA expression of TRAP, CAII, OSCAR and
FAS/FASL significantly decreased when osteoclasts had been exposed
to ALN in a dose-dependent manner for the indicated incubation
times. In addition, the mRNA expression of TRAP, CAII, OSCAR and
FAS/FASL significantly decreased when osteoclasts had been exposed
to ALN in a time-dependent manner for the indicated dose
concentrations (Table II and
Figs. 3 and 5; P<0.05). No significant difference
in expression was identified between the control groups
(P>0.05).
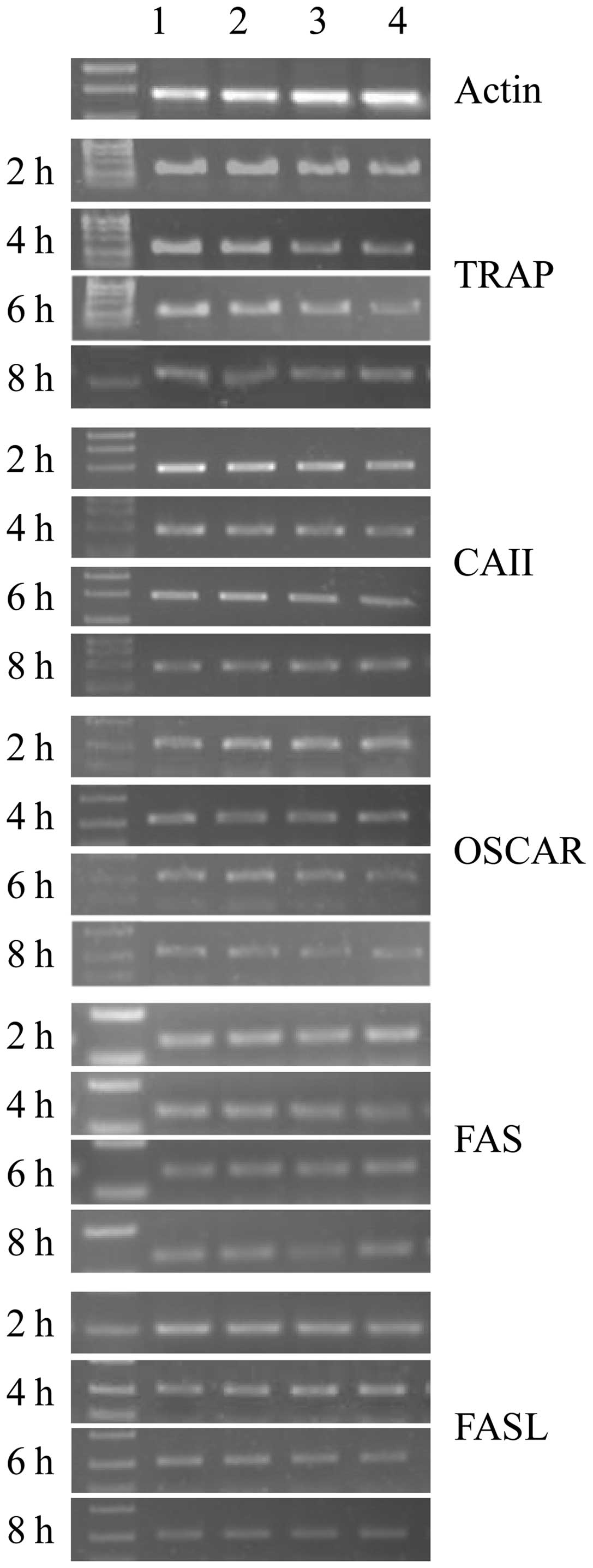 | Figure 3qPCR analysis showing mRNA expression
of tartrate-resistant acid phosphatase (TRAP), carbonic anhydrase
II (CAII), osteoclast-associated receptor (OSCAR), and FAS/FASL
genes in osteoclasts. Lanes 1, 2, 3 and 4 are the control, and low,
medium and high concentration groups, respectively. Cells were
incubated for 2, 4, 6 and 8 h with alendronate. β-actin was used as
an internal control. |
 | Table IIRelative RNA expression of TRAP,
CAII, OSCAR and FAS/FASL genes in osteoclasts at various
concentrations and administration times of ALN. |
Table II
Relative RNA expression of TRAP,
CAII, OSCAR and FAS/FASL genes in osteoclasts at various
concentrations and administration times of ALN.
| | Expression | |
|---|
| |
| |
|---|
| Gene | Time (h) | Control | Low
concentration | Medium
concentration | High
concentration | |
|---|
| TRAP | 2 | 7.510±0.02 | 7.413±0.03 | 7.313±0.03 | 7.273±0.07 | * |
| 4 | 7.490±0.03 | 6.780±0.02 | 6.693±0.02 | 6.557±0.06 | * |
| 6 | 7.477±0.02 | 6.660±0.06 | 6.587±0.15 | 6.420±0.04 | * |
| 8 | 7.453±0.05 | 6.657±0.06 | 6.523±0.05 | 6.390±0.10 | * |
| | ** | * | * | * | |
| CAII | 2 | 7.557±0.04 | 7.350±0.04 | 7.213±0.03 | 7.133±0.04 | * |
| 4 | 7.487±0.12 | 6.907±0.03 | 6.813±0.03 | 6.760±0.06 | * |
| 6 | 7.460±0.14 | 6.777±0.04 | 6.613±0.10 | 6.590±0.02 | * |
| 8 | 7.430±0.13 | 6.637±0.05 | 6.503±0.03 | 6.357±0.05 | * |
| | ** | * | * | * | |
| OSCAR | 2 | 7.420±0.05 | 7.347±0.03 | 7.290±0.03 | 7.257±0.04 | * |
| 4 | 7.340±0.05 | 7.273±0.03 | 7.140±0.07 | 6.877±0.12 | * |
| 6 | 7.313±0.42 | 7.027±0.03 | 6.843±0.08 | 6.760±0.04 | * |
| 8 | 6.900±0.07 | 6.613±0.11 | 6.520±0.06 | 6.447±0.04 | * |
| | ** | * | * | * | |
| FAS | 2 | 7.533±0.06 | 7.347±0.05 | 7.270±0.04 | 7.223±0.10 | * |
| 4 | 7.523±0.05 | 7.277±0.04 | 7.183±0.01 | 7.083±0.04 | * |
| 6 | 7.490±0.02 | 7.100±0.05 | 6.747±0.06 | 6.183±0.07 | * |
| 8 | 7.443±0.03 | 6.190±0.02 | 6.130±0.02 | 6.000±0.10 | * |
| | ** | * | * | * | |
| FASL | 2 | 7.713±0.12 | 7.583±0.02 | 7.523±0.09 | 7.423±0.07 | * |
| 4 | 7.693±0.12 | 6.300±0.03 | 6.173±0.03 | 6.153±0.02 | * |
| 6 | 7.667±0.13 | 6.280±0.03 | 6.167±0.03 | 6.040±0.03 | * |
| 8 | 7.647±0.16 | 6.067±0.07 | 5.860±0.06 | 5.717±0.09 | * |
| | ** | * | * | * | |
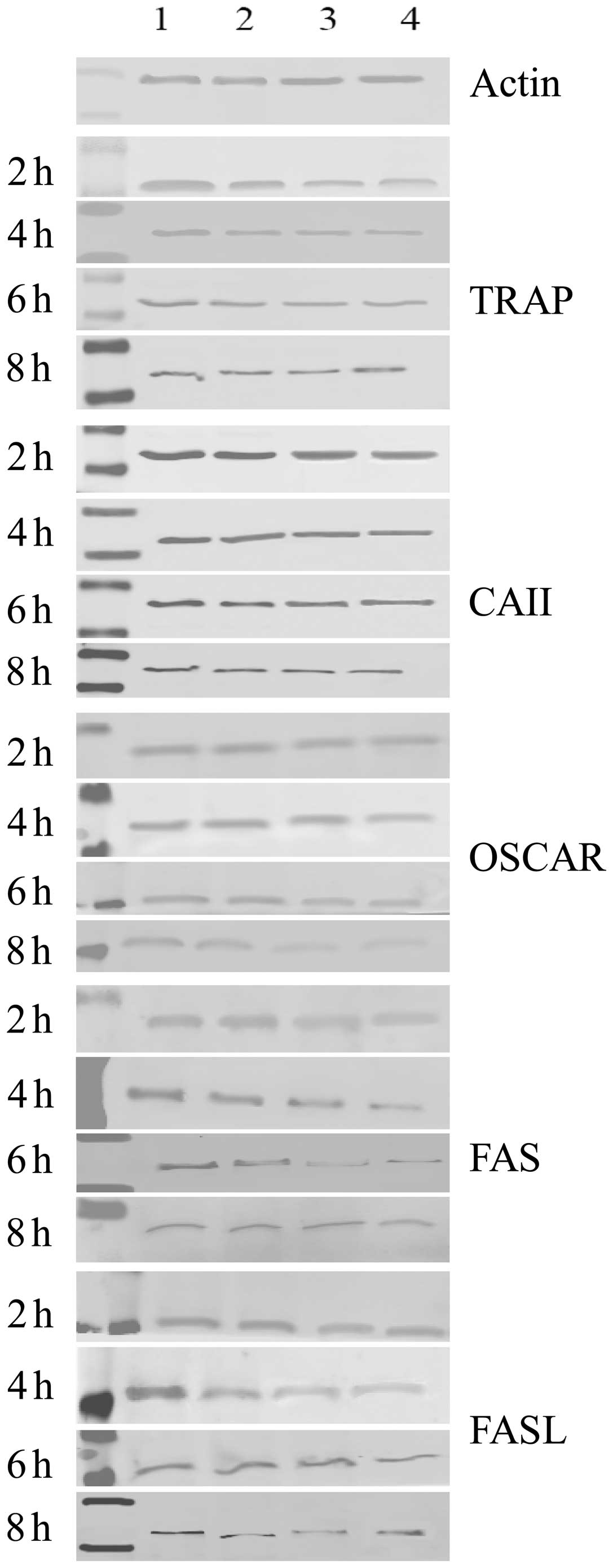
Protein expression of TRAP, CAII, OSCAR
and FAS/FASL in osteoclasts
The protein expression of TRAP, CAII, OSCAR and
FAS/FASL significantly decreased when osteoclasts were exposed to
ALN in a dose-dependent manner for the indicated incubation times.
Furthermore, the protein expression of TRAP, CAII, OSCAR and
FAS/FASL also significantly decreased when osteoclasts were exposed
to ALN in a time-dependent manner for the indicated dose
concentrations (Table III;
Figs. 4 and 6; P<0.05). No significant difference
in expression was observed between the control groups
(P>0.05).
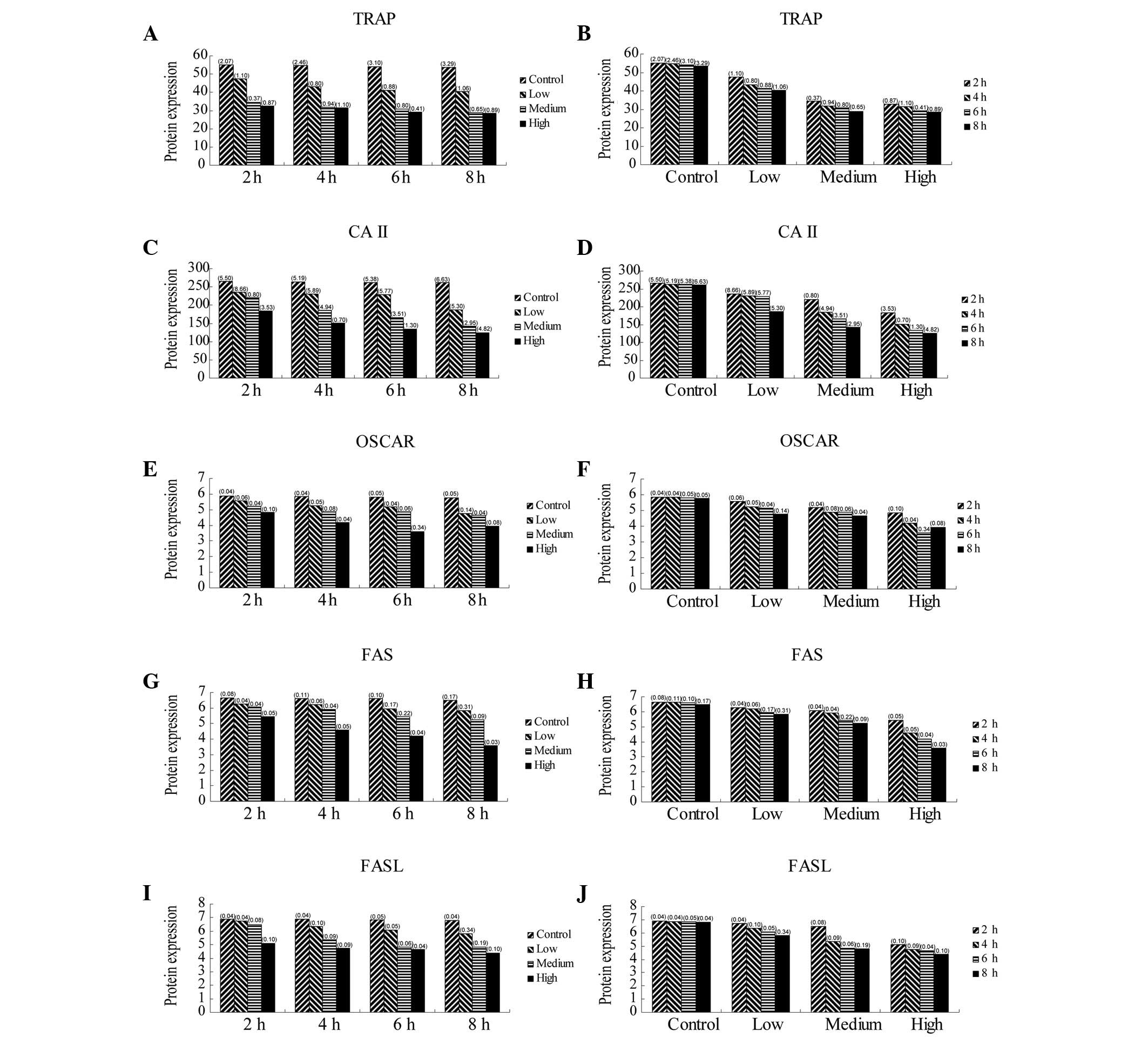
 | Table IIIProtein expression of TRAP, CAII,
OSCAR and FAS/FASL genes in osteoclasts at various concentrations
and administration times of ALN. |
Table III
Protein expression of TRAP, CAII,
OSCAR and FAS/FASL genes in osteoclasts at various concentrations
and administration times of ALN.
| | Expression | |
|---|
| |
| |
|---|
| Gene | Time (h) | Control
concentration | Low
concentration | Medium
concentration | High
concentration | |
|---|
| TRAP | 2 | 55.167±2.07 | 47.433±1.10 | 34.337±0.37 | 32.633±0.87 | * |
| 4 | 54.733±2.46 | 43.250±0.80 | 31.660±0.94 | 31.367±1.10 | * |
| 6 | 53.967±3.10 | 41.193±0.88 | 30.727±0.80 | 29.067±0.41 | * |
| 8 | 53.600±3.29 | 40.440±1.06 | 28.943±0.65 | 28.543±0.89 | * |
| | ** | * | * | * | |
| CAII | 2 | 265.333±5.50 | 235.600±8.66 | 220.040±0.80 | 183.467±3.53 | * |
| 4 | 263.333±5.19 | 230.177±5.89 | 185.173±4.94 | 150.567±0.70 | * |
| 6 | 262.567±5.38 | 229.133±5.77 | 166.143±3.51 | 133.833±1.30 | * |
| 8 | 261.167±6.63 | 186.607±5.30 | 142.633±2.95 | 124.867±4.82 | * |
| | ** | * | * | * | |
| OSCAR | 2 | 5.863±0.04 | 5.567±0.06 | 5.210±0.04 | 4.853±0.10 | * |
| 4 | 5.840±0.04 | 5.250±0.05 | 4.870±0.08 | 4.177±0.04 | * |
| 6 | 5.810±0.05 | 5.170±0.04 | 4.867±0.06 | 3.583±0.34 | * |
| 8 | 5.757±0.05 | 4.753±0.14 | 4.637±0.04 | 3.937±0.08 | * |
| | ** | * | * | * | |
| FAS | 2 | 6.657±0.08 | 6.267±0.04 | 6.077±0.04 | 5.433±0.05 | * |
| 4 | 6.620±0.11 | 6.203±0.06 | 5.903±0.04 | 4.600±0.05 | * |
| 6 | 6.600±0.10 | 5.950±0.17 | 5.430±0.22 | 4.193±0.04 | * |
| 8 | 6.487±0.17 | 5.840±0.31 | 5.233±0.09 | 3.570±0.03 | * |
| | ** | * | * | * | |
| FASL | 2 | 6.900±0.04 | 6.730±0.04 | 6.493±0.08 | 5.107±0.10 | * |
| 4 | 6.863±0.04 | 6.333±0.10 | 5.370±0.09 | 4.750±0.09 | * |
| 6 | 6.837±0.05 | 6.090±0.05 | 4.827±0.06 | 4.663±0.04 | * |
| 8 | 6.797±0.04 | 5.823±0.34 | 4.807±0.19 | 4.383±0.10 | * |
| | ** | * | * | * | |
Discussion
The inhibitory mechanism of ALN occurs in a number
of stages. Initially, ALN is selectively enriched in the bone
tissue due to an infusion of ALN into the cytoplasm, which
interferes with osteoclast differentiation, maturation and
recruitment. Osteoclasts then undergo apoptosis during which the
ALN-mediated inhibition is most prominent. However, it has also
been demonstrated that ALN may be metabolized as a quasi-ATP decoy,
thereby affecting ATPase in osteoclasts. In addition, ALN has been
shown to inhibit the mevalonic acid pathway during cholesterol
metabolism and subsequently inactivate the prenylation of
osteoclasts by affecting GTP-binding proteins (19). Previously, several studies have
demonstrated that ALN may inhibit osteoclast function. The addition
of various concentrations of ALN to calcium phosphate bone cement
was shown to inhibit osteoclastogenesis and osteoclast function
(18). Another study indicated
that bisphosphonate exhibited an inhibitory effect on osteoclast
proliferation (15). Kawata et
al(16) suggested that ALN
inhibited the resorption of ectopic bone grafts when injected at
specific concentrations. Another study demonstrated that ALN
affected the mineral density and remodeling of bone tissue
(20). In addition, RANKL-induced
osteoclast differentiation of RAW264.7 cells was inhibited on
ALN-coated titanium surfaces and the mRNA expression of TRAP was
downregulated (17). Therefore,
based on these results, additional studies were required to
investigate the mechanism of the ALN-mediated inhibition of
osteoclasts.
The ALN concentration and duration of incubation are
critical parameters when assessing the effects of this
bisphosphonate on osteoclasts. ALN, similar to numerous other
inhibitors, is administrated based on a strict dose and time
schedule. The results of this study suggested that ALN affected
osteoclasts in a dose-dependent manner and it has been shown that
high doses of ALN exhibited deleterious effects on osteoclastic
function (21). Koshihara et
al(22) demonstrated that
treatment of osteoclasts with 10−4 M ALN for 6 h
resulted in a breakdown of the actin ring and that cell mobility
was decreased compared with that of cells treated with
10−5 M ALN. In addition, only a partial recovery from
contraction was observed when the cells were treated with
10−4 M ALN compared with that of the other dose groups.
However, the removal of ALN treatment completely restored the
function and appearance of the cells. Therefore, the effects of ALN
on osteoclast motility and morphology were reversible at a
concentration of 10−5 M, suggesting that ALN did not
lead to permanent cell damage in osteoclasts at this dose, and
therefore it was an appropriate and effective dose for bone
resorption (22). In another
study, ALN inhibited bone resorption at concentrations
≤10−7 M. At the highest concentration of 10−5
M, the osteoclast number and resorption were markedly reduced
(23).
In our previous study, it was demonstrated that
osteoclasts possessed a time rhythm, which was not optimal for the
detection of molecular changes when the cells were cultured for
<24 h. The mRNA expression, osteoclastic apoptosis and other
changes were only observed when the cells were cultured for >48
h (24). Based on these results,
we previously concluded that osteoclast mRNA expression reached a
peak level following 24 h of culture and exhibited higher
sensitivity to the surrounding stimuli at that time point (24). The results from the present study
demonstrated that osteoclasts reached peak mRNA expression
subsequent to 6 days of culture. In addition, the effects of ALN on
the mRNA and protein expression of TRAP, CAII, OSCAR and FAS/FASL
at specific concentrations (10−5 M, 10−6 M
and 10−7 M) and duration of incubation (0, 2, 4, 6 and 8
h) were determined. It was demonstrated that that the expression of
these genes decreased marginally over the indicated incubation
time; however, the differences between time points were not
significant. Therefore, osteoclast apoptosis did not occur
following 8 h of treatment with ALN.
The mechanism of action of ALN on osteoclasts has
been the focus of previous studies. D’Amelio et al(25) suggested that ALN predominantly
acted on mature bone resorbing osteoclasts during short-term
treatment, but decreased osteoclast precursors and serum RANKL
levels after long-term treatment. Fisher et al(26) also concluded that ALN acted
directly on osteoclasts and inhibited a rate-limiting step in the
cholesterol biosynthesis pathway, which was essential for
osteoclast function. In addition, other studies have determined
that ALN bound to resorption surfaces and was released locally
during acidification. In addition, an increasing concentration of
ALN prevented osteoclastic resorption and membrane ruffling
(27). However, Owens et
al(28) hypothesized that the
inhibitory mechanism of ALN was not due to resorption.
Findings of another study showed that the reduced
efficacy of ALN to prevent bone erosion may be due to locally
increased levels of tumor necrosis factor (TNF), which resulted in
the upregulation of ETS-2 expression in osteoclasts. Moreover,
BCL-XL expression was stimulated, which reduced cell susceptibility
to ALN-induced apoptosis (29).
Wang et al(30) also
demonstrated that ALN promoted the apoptosis of osteoclasts, which
was related to expression of the FAS gene. However, another
hypothesized mechanism suggested that ALN suppression of bone
resorption was independent of its effect on apoptosis (31). In addition, ALN impaired
intracellular vesicle transport in osteoclasts, which suggested
that the effects on apoptosis may be a secondary mechanism
(32).
Kum et al(33) hypothesized that the ALN inhibitory
mechanism was not related to the inhibition of IL-6 production.
Colucci et al(34)
indicated that the inhibitory effect of ALN was due to the
interference with receptors and bone matrix proteins, such as α5β3
integrins. Furthermore, ALN induced Ca2+-mediated
intracellular signaling in osteoclasts and triggered a 2-fold
increase in the intracellular calcium concentration. Moreover, the
study by Schmidt et al(35)
suggested that a tyrosine phosphatase may also be a putative
molecular target of ALN.
The results of this study have shown that ALN
inhibited osteoclasts partly by decreasing the mRNA and protein
expression of important cell molecules, including TRAP, CAII, OSCAR
and FAS/FASL. The expression of these proteins decreased in an ALN
concentration- and time-dependent manner. In addition, the results
were concordant with previous studies. In conclusion, osteoclasts
negatively responded to ALN treatment. In addition, ALN activated
several signaling pathways in osteoclasts, such as
Ca2+-mediated intracellular signaling, tyrosine
phosphatase activity and cholesterol biosynthesis pathways and ALN
induced apoptosis in osteoclasts.
Acknowledgements
This study was supported by the Department of
Education Foundation of the Zhejiang Province of China (grant nos.
N20080180, Y201018976, Y201329982 and N20110143), the Department of
health Foundation of the Zhejiang Province of China (grant no.
2013KYB166), the Zhejiang Provincial Natural Science Foundation of
China (grant nos. Y2080340 and LY12H14004) and the Zhejiang
Provincial and Ministry joint Project (grant no.
WKJ2011-2-009).
Abbreviations:
|
ALN
|
alendronate
|
|
TRAP
|
tartrate-tesistant acid
phosphatase
|
|
OSCAR
|
osteoclast-associated receptor
|
|
CAII
|
carbonic anhydrase II
|
|
sRANKL
|
soluble recombinant murine soluble
receptor activator of NF-κB ligand
|
References
|
1
|
Doody KM, Bussières-Marmen S, Li A, et al:
T cell protein tyrosine phosphatase deficiency results in
spontaneous synovitis and subchondral bone resorption in mice.
Arthritis Rheum. 64:752–761. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Klijn RJ, van den Beucken JJ, Bronkhorst
EM, et al: Predictive value of ridge dimensions on autologous bone
graft resorption in staged maxillary sinus augmentation surgery
using Cone-Beam CT. Clin Oral Implants Res. 23:409–415. 2012.
View Article : Google Scholar
|
|
3
|
Nonaka CF, Cavalcante RB, Nogueira RL, et
al: Immunohistochemical analysis of bone resorption regulators
(RANKL and OPG), angiogenic index, and myofibroblasts in syndrome
and non-syndrome odontogenic keratocysts. Arch Oral Biol.
57:230–237. 2012. View Article : Google Scholar
|
|
4
|
Hong ZQ, Tao LM and Li L: Effect of stress
on mRNA expression of H+-ATPase in osteoclasts. Mol Cell
Biochem. 343:183–190. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Nakayama T, Mizoguchi T, Uehara S, et al:
Polarized osteoclasts put marks of tartrate-resistant acid
phosphatase on dentin slices - a simple method for identifying
polarized osteoclasts. Bone. 49:1331–1339. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Fujisaki K, Tanabe N, Suzuki N, et al:
Receptor activator of NF-kappaB ligand induces the expression of
carbonic anhydrase II, cathepsin K, and matrix metalloproteinase-9
in osteoclast precursor RAW264.7 cells. Life Sci. 80:1311–1318.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Zhang Q, Liang X, Zhu B, et al: Effects of
fluid shear stress on mRNA expression of carbonic anhydrase II in
polarized rat osteoclasts. Cell Biol Int. 30:714–720. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Goettsch C, Rauner M, Sinningen K, et al:
The osteoclast-associated receptor (OSCAR) is a novel receptor
regulated by oxidized low-density lipoprotein in human endothelial
cells. Endocrinology. 152:4915–4926. 2011. View Article : Google Scholar
|
|
9
|
Ishikawa S, Arase N, Suenaga T, et al:
Involvement of FcRgamma in signal transduction of
osteoclast-associated receptor (OSCAR). Int Immunol. 16:1019–1025.
2004. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Kim JH, Kim K, Jin HM, et al: Upstream
stimulatory factors regulate OSCAR gene expression in
RANKL-mediated osteoclast differentiation. J Mol Biol. 383:502–511.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Kim JH, Kim K, Youn BU, et al: MHC class
II transactivator negatively regulates RANKL-mediated osteoclast
differentiation by downregulating NFATc1 and OSCAR. Cell Signal.
22:1341–1349. 2010. View Article : Google Scholar
|
|
12
|
Kovacić N, Lukić IK, Grcević D, et al: The
Fas/Fas ligand system inhibits differentiation of murine
osteoblasts but has a limited role in osteoblast and osteoclast
apoptosis. J Immunol. 178:3379–3389. 2007.PubMed/NCBI
|
|
13
|
Krum SA, Miranda-Carboni GA, Hauschka PV,
et al: Estrogen protects bone by inducing Fas ligand in osteoblasts
to regulate osteoclast survival. EMBO J. 27:535–545. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Nakamura T, Imai Y, Matsumoto T, et al:
Estrogen prevents bone loss via estrogen receptor alpha and
induction of Fas ligand in osteoclasts. Cell. 130:811–823. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Boanini E, Torricelli P, Gazzano M, et al:
Alendronate-hydroxy- apatite nanocomposites and their interaction
with osteoclasts and osteoblast-like cells. Biomaterials.
29:790–796. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Kawata T, Tenjou K, Tokimasa C, et al:
Effect of alendronate on osteoclast differentiation and bone volume
in transplanted bone. Exp Anim. 53:47–51. 2004.PubMed/NCBI
|
|
17
|
Moon HJ, Yun YP, Han CW, et al: Effect of
heparin and alendronate coating on titanium surfaces on inhibition
of osteoclast and enhancement of osteoblast function. Biochem
Biophys Res Commun. 413:194–200. 2011. View Article : Google Scholar
|
|
18
|
Panzavolta S, Torricelli P, Bracci B, et
al: Alendronate and Pamidronate calcium phosphate bone cements:
setting properties and in vitro response of osteoblast and
osteoclast cells. J Inorg Biochem. 103:101–106. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Rogers MJ, Frith JC, Luckman SP, et al:
Molecular mechanisms of action of bisphosphonates. Bone.
24:73S–79S. 1999. View Article : Google Scholar
|
|
20
|
Sugata Y, Sotome S, Yuasa M, et al:
Effects of the systemic administration of alendronate on bone
formation in a porous hydroxyapatite/collagen composite and
resorption by osteoclasts in a bone defect model in rabbits. J Bone
Joint Surg Br. 93:510–516. 2011. View Article : Google Scholar
|
|
21
|
Sama AA, Khan SN, Myers ER, et al:
High-dose alendronate uncouples osteoclast and osteoblast function:
a study in a rat spine pseudarthrosis model. Clin Orthop Relat Res.
425:135–142. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Koshihara Y, Kodama S, Ishibashi H, et al:
Reversibility of alendronate-induced contraction in human
osteoclast-like cells formed from bone marrow cells in culture. J
Bone Miner Metab. 17:98–107. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Breuil V, Cosman F, Stein L, et al: Human
osteoclast formation and activity in vitro: effects of alendronate.
J Bone Miner Res. 13:1721–1729. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Hong ZQ, Tao LM and Li L: mRNA expression
in osteoclasts: a chronobiological approach. Biological Rhythm
Research. 42:287–298. 2011. View Article : Google Scholar
|
|
25
|
D’Amelio P, Grimaldi A, Cristofaro MA, et
al: Alendronate reduces osteoclast precursors in osteoporosis.
Osteoporos Int. 21:1741–1750. 2010.PubMed/NCBI
|
|
26
|
Fisher JE, Rogers MJ, Halasy JM, et al:
Alendronate mechanism of action: geranylgeraniol, an intermediate
in the mevalonate pathway, prevents inhibition of osteoclast
formation, bone resorption, and kinase activation in vitro. Proc
Natl Acad Sci USA. 96:133–138. 1999. View Article : Google Scholar
|
|
27
|
Sato M, Grasser W, Endo N, et al:
Bisphosphonate action. Alendronate localization in rat bone and
effects on osteoclast ultrastructure. J Clin Invest. 88:2095–2105.
1991. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Owens JM, Fuller K and Chambers TJ:
Osteoclast activation: potent inhibition by the bisphosphonate
alendronate through a nonresorptive mechanism. J Cell Physiol.
172:79–86. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Zhang Q, Badell IR, Schwarz EM, et al:
Tumor necrosis factor prevents alendronate-induced osteoclast
apoptosis in vivo by stimulating Bcl-xL expression through Ets-2.
Arthritis Rheum. 52:2708–2718. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Wang XM, Yu SF and Yang ZP: Apoptosis of
osteoclast-like cells induced by alendronate is related to Fas gene
expression. Chin J Dent Res. 3:26–32. 2000.PubMed/NCBI
|
|
31
|
Halasy-Nagy JM, Rodan GA and Reszka AA:
Inhibition of bone resorption by alendronate and risedronate does
not require osteoclast apoptosis. Bone. 29:553–559. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Alakangas A, Selander K, Mulari M, et al:
Alendronate disturbs vesicular trafficking in osteoclasts. Calcif
Tissue Int. 70:40–47. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Kum KY, Park JH, Yoo YJ, et al: The
inhibitory effect of alendronate and taurine on osteoclast
differentiation mediated by Porphyromonas gingivalis sonicates in
vitro. J Endod. 29:28–30. 2003. View Article : Google Scholar
|
|
34
|
Colucci S, Minielli V, Zambonin G, et al:
Alendronate reduces adhesion of human osteoclast-like cells to bone
and bone protein-coated surfaces. Calcif Tissue Int. 63:230–235.
1998. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Schmidt A, Rutledge SJ, Endo N, et al:
Protein-tyrosine phosphatase activity regulates osteoclast
formation and function: inhibition by alendronate. Proc Natl Acad
Sci USA. 93:3068–3073. 1996. View Article : Google Scholar
|