Introduction
Surgical skin flaps are often used in plastic and
reconstructive surgery to repair defects resulting from trauma,
congenital defects, cancer excision or additional mechanisms.
Partial or complete skin flap necrosis is a common problem
encountered postoperatively. Inadequate blood perfusion is thought
to be the main factor resulting in flap necrosis. Several methods
have been used in an attempt to increase blood supply and tissue
perfusion in compromised tissues, including therapeutic
angiogenesis using exogenous growth factors such as vascular
endothelial growth factor (VEGF) and fibroblast growth factor
(1,2), as well as ischemic preconditioning
(3) and surgical delay (4). However, these methods are not widely
used in the clinic due to lack of validation of efficacy and side
effects.
The density of vascular structures affects blood
circulation and perfusion to the skin flap. The skin flap
transplantation process is associated with tissue injury, and the
regeneration of blood vessels (neovascularization) is required to
revascularize the injured tissue. Stromal cell derived factor-1
(SDF-1) is a constitutively expressed and inducible chemokine that
regulates multiple physiological processes including organ repair
and tumor development. The biological effects of SDF-1 are mediated
by the specific receptor CXCR4, which belongs to a family of
G-protein-coupled receptors, and is widely and constitutively
expressed by numerous hematopoietic and endothelial cells (5). It has been demonstrated that SDF-1
and CXCR4 are constitutively expressed in a wide range of tissues,
including brain, lung, liver, skin and bone marrow (6). In addition, recent studies have shown
that the expression of SDF-1 in a large number of tumors and
injured tissues strongly suggests that the activation of CXCR4 is
involved in promoting neovascularization (7,8).
Numerous studies have demonstrated the efficacy of
hyperbaric oxygen (HBO) treatment on enhancing skin flap and skin
graft survival by increasing arterial oxygen tension, reducing
ischemia-reperfusion injury, enhancing host response to local
infections, and stimulating neovascularization and tissue growth
(4,9–11).
The present study aimed to assess whether HBO treatment is able to
improve skin flap survival and neovascularization, whether the
expression of SDF-1 and CXCR4 in skin flaps may be induced by HBO
treatment and whether neovascularization is affected by the
expression of SDF-1 and CXCR4.
Materials and methods
Experimental animals
All the experiments were performed in accordance
with the ethical guidelines determined by the Committee for the
Purpose of Control and Supervision of Experiments on Animals at the
Capital Medical University (Beijing, China). Forty healthy adult
male Sprague Dawley rats (weighing, 250–300 g at the beginning of
the study) were used. Rats were maintained at 25±1.0°C with a
12/12-h light/dark cycle and received food and water ad
libitum.
Experimental groups
Forty rats were randomly assigned to one of the
following groups (n=8 per group): i) sham-operated (SH group); ii)
ischemia followed by reperfusion and analysis on the third and
fifth day postoperatively (IR3d and IR5d groups, respectively); and
iii) ischemia followed by reperfusion and HBO treatment and
analysis on the third and fifth day postoperatively (HBO3d and
HBO5d groups, respectively).
Epigastric pedicle skin flap model
The epigastric pedicle skin flap model used in this
study has been described previously (12), with a modification in flap design.
Procedures were performed aseptically under anesthesia using
intraperitoneal injections of 350 mg/kg 10% chloral hydrate. Rats
were fixed on the operating table, the abdomen was shaved and
washed before the single inferior epigastric vessel pedicle skin
flaps (9×6 cm) were obtained. Skeletonization of the right inferior
epigastric artery and vein pedicle was performed, while the
contralateral inferior epigastric vessel was ligated with sutures
and the feeding vessel was clamped using a microvascular clamp to
achieve ischemia. For reperfusion, the microvascular clamp was
removed 3 h later and blood flow was restored. The skin flaps were
repositioned above a silicone sheet (the same area as the flap)
with continuous 5-0 monofilament nylon sutures to prevent vascular
supply other than that of the pedicle. The sham-operated group
underwent the same surgery but the rats were not exposed to
ischemia. All the rats received a single dose of 0.8 mg/g
intramuscular penicillin sodium postoperatively.
HBO treatment
In the HBO3d and HBO5d groups, the rats were placed
into a custom-made pressure chamber of transparent acrylic plastic
(701 Space Research Institute, Beijing, China) immediately
following surgery and received 1 h of HBO treatment in a 2.0 ATA
chamber in 100% O2 twice per day (8-h intervals) for 3
days and then daily for 2 consecutive days. Compressed air was
administered at a rate of 1 kg/cm2/min into the 2.0 ATA
chamber including 100% O2 and maintained for 60 min. The
chamber was flushed with 100% O2 at a rate of 5 l/min to
avoid CO2 accumulation and decompression was performed
at 0.2 kg/cm2/min. During HBO exposure, O2
and CO2 content were continuously monitored and
maintained at ≥98% and ≤0.03%, respectively, while the chamber
temperature was maintained between 22 and 25°C. To minimize the
effects of diurnal variation, HBO exposure was initiated at
approximately 8 am and 4 pm. Additionally, in the SH, IR3d and IR5d
groups, rats were treated with normobaric air in a 1.0 ATA chamber
with 21% O2 at an ambient temperature of 22–25°C,
postoperatively.
Flap measurements
Evaluation of the skin flaps occurred on the third
and fifth day postoperatively. The survival area of the skin flaps
was determined based on appearance, color and texture. Viability of
the flaps was calculated using Image-Pro Plus software (version
6.0, Media Cybernetics LP, Silver Spring, MD, USA). The results
were expressed as percentages relative to the total flap surface
area.
Histological analysis
The skin flaps were evaluated on the third and fifth
day following surgery. For each skin flap, 3–4 mm-sectioned tissue
blocks from viable regions were fixed in 10% formalin and embedded
in paraffin for hematoxylin and eosin staining. Microvessel density
(MVD) was measured by two double-blinded pathologists using the
‘hot spot’ method. Briefly, five areas with the highest
accumulation of small vessels were identified under a low
magnification (x100) in each tissue section. These areas were then
examined under a high magnification (x400) and microvessels were
counted in five high-power fields. Blood vessels with muscular
layers were excluded.
Immunohistochemical staining
Monoclonal antibody analysis was performed on the
third and fifth day following surgery. For each skin flap,
sectioned tissue blocks (3–4 mm) obtained from the viable portion
of the flap were preserved in 10% formalin. Tissue sections (4 μm)
were obtained and embedded in paraffin for immunohistological
analysis. The monoclonal antibodies anti-SDF-1 (1:100) and
anti-CXCR4 (1:500) (both from Santa Cruz Biotechnology, Inc., Santa
Cruz, CA, USA), were used for immunohistochemical staining. The
proportion of positive cells in at least 10 areas in each section
were analyzed under a high magnification (x400) by two
double-blinded pathologists.
Protein preparation
Skin flap tissues were frozen in liquid nitrogen and
then stored at −80°C until analysis. The tissues were homogenized
in ice-cold isolation solution containing 250 mmol/l sucrose, 10
mmol/l triethanolamine, 1 μg/ml leupeptin and 0.1 mg/ml
phenylmethylsulfonyl fluoride. Homogenates were centrifuged at
15,000 × g for 10 min at 4°C to separate the incomplete homogenized
tissue. The supernatants were obtained and the protein
concentrations were measured using a protein assay kit (Beijing
Sunbio Biotech Co., Ltd., Haidian, China). For deglycosylation of
proteins, an N-glycosidase F Deglycosylation Kit (Roche Diagnostics
GmbH, Mannheim, Germany) was used.
Western blot analysis
Total proteins (50 μg/sample) were diluted in 5X
loading buffer (0.25 mol/l Tris HCl, pH 6.8; 10% sodium dodecyl
sulfate; 0.5% bromophenol blue; 50% glycerol; and 0.5 mol/l
dithiothreitol) and then heated to boiling point for 5 min. Sodium
dodecyl sulfate-polyacrylamide gel electrophoresis was carried out
on 12% gradient gels. The proteins were electrophoretically
transferred to polyvinylidene difluoride (PVDF) membranes that had
been previously treated with methanol and blocked for 1 h at room
temperature in TBST (Tris-buffered saline containing 0.1% Tween-20)
containing 5% non-fat dry milk. PVDF membranes were then incubated
overnight at 4°C with anti-SDF-1 (1:100) and anti-CXCR4 antibodies
(1:500) (Santa Cruz Biotechnology, Inc.) in TBST containing 5%
nonfat dry milk. Membranes were washed in TBST and incubated with
horseradish peroxidase-labeled anti-rabbit antibody (1:3,000, Santa
Cruz Biotechnology, Inc.) for 2–3 h at room temperature. Blots were
developed with enhanced chemiluminescence agents (ECL Plus, Beijing
Sunbio Biotech Co. Ltd.) prior to being exposed to X-ray. To
confirm equivalent loading of samples, the same membranes were
incubated with anti-β-actin antibody (1:300, Santa Cruz
Biotechnology, Inc.) and visualized using ECL, as mentioned
earlier. For quantification, western blotting films were scanned
using a Minolta scanner (Konica Minolta, Inc.) and Adobe Photoshop
software. The labeling density was quantitated using LabWorks
software (UVP Inc., Upland, CA, USA). The value of the relative
density of SDF-1 and CXCR4 bands was normalized to the density of
actin to represent the expression of SDF-1 and CXCR4 proteins.
Statistical analysis
Statistical analysis was performed using SPSS
software, version 15.0 (SPSS, Chicago, IL, USA). Quantitative data
were expressed as the mean ± standard deviation. One-way analysis
of variance procedures were used to test the differences in MVD,
SDF-1, CXCR4 and the skin flap survival area. The t-test was used
to determine the differences in skin flap survival between the
HBO3d and HBO5d groups. Relationships between MVD and expression of
SDF-1 and CXCR4 were analyzed by calculating the Pearson
product-moment correlation coefficient. P<0.05 was considered to
indicate a statistically significant difference.
Results
Flap measurements
The regions of survival and necrosis of skin flaps
in different groups were clearly demarcated. The surviving skin
appeared pink to white, was tender, normal in texture and bled when
cut with a scalpel. By contrast, the necrotic skin was black,
rigid, abnormal in texture and did not bleed when cut. Rats in the
SH group showed an average viable area of 77.5%, compared with that
of the IR3d (43.0%) and IR5d (32.2%) groups. The average viable
area was significantly increased in the HBO3d (54.7%) and HBO5d
(64.7%) groups compared with that of the IR groups (P<0.01).
These results indicate that HBO treatment significantly improved
skin flap survival. Additionally, when the number of HBO treatments
increased from 3 to 5 days, the survival area of skin flaps also
significantly increased (P<0.05) (Fig. 1).
Flap neovascularization measurements
To evaluate whether neovascularization varied among
the different groups, we measured MVD in the skin flaps and found
that MVD was significantly increased in the groups treated with HBO
(HBO3d, 8.5±2.9 and HBO5d, 10.0±2.9), compared with that of the IR
groups (IR3d, 5.5±1.8 and IR5d, 3.8±1.3) (Fig. 2). These results demonstrate that
HBO treatment increased neovascularization in the skin flaps of
rats.
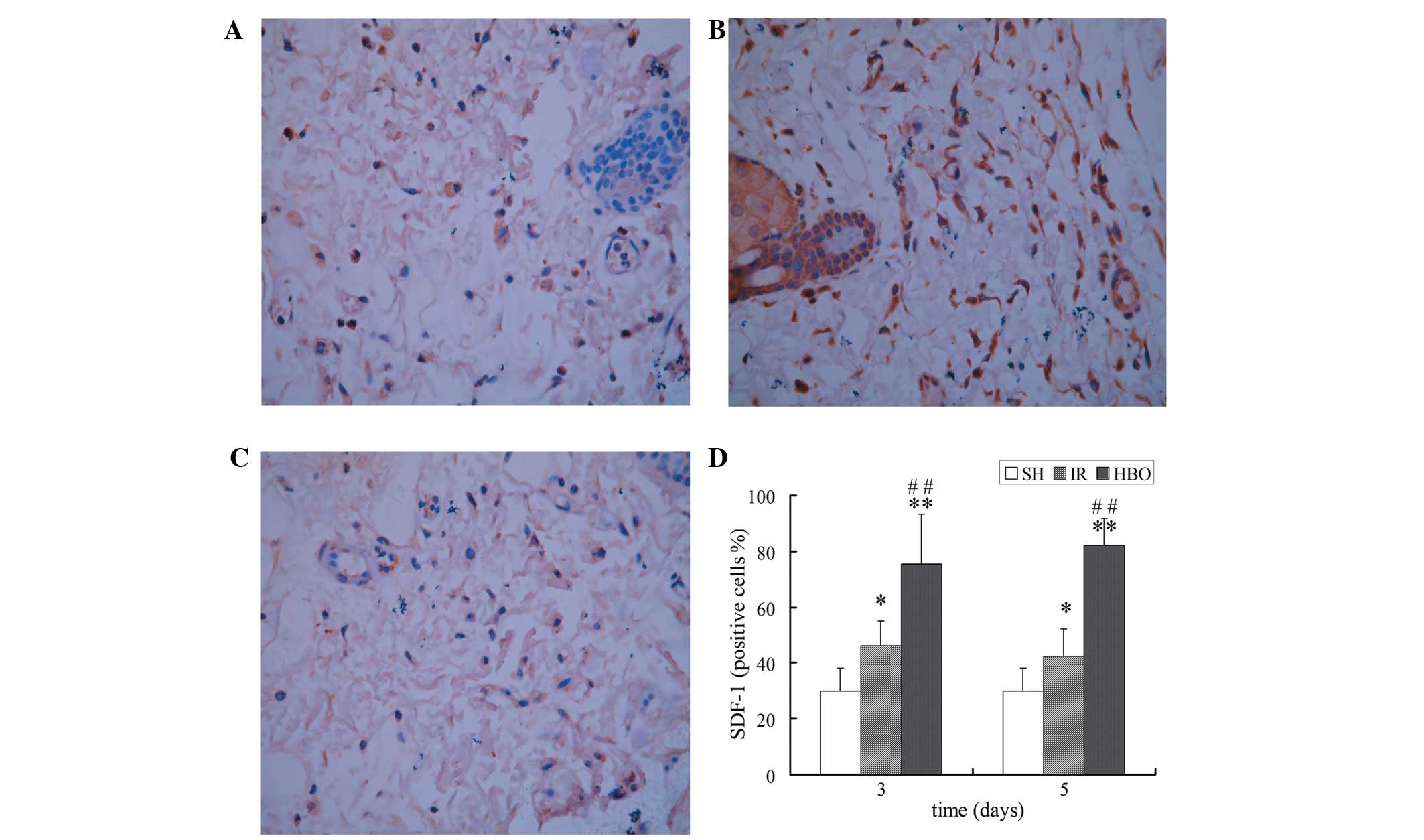
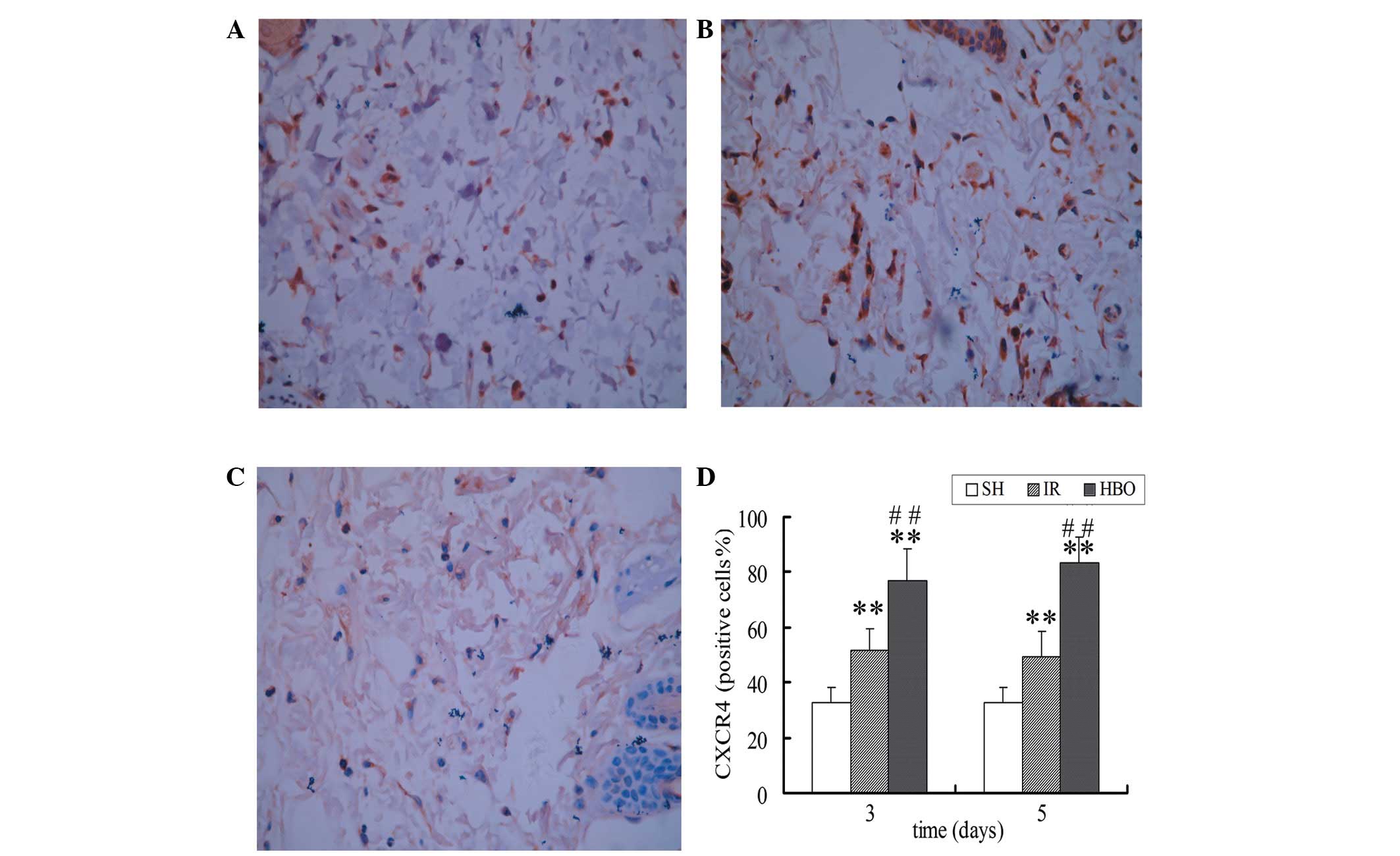
Immunohistochemical staining of SDF-1 and
CXCR4
Immunohistochemical staining showed that cells were
positive for brown particles in the cell membrane, cytoplasm and
nucleus. The expression of SDF-1 and CXCR4 in the IR groups was
higher than that in the SH group (P<0.05 and P<0.01,
respectively). Moreover, the expression of SDF-1 and CXCR4 in the
HBO treatment groups was significantly higher than that in the IR
groups (P<0.01) and the SH group (P<0.01) (Figs. 3 and 4). These results suggest that HBO
treatment significantly increased the expression of SDF-1 and CXCR4
in the skin flaps of rats.
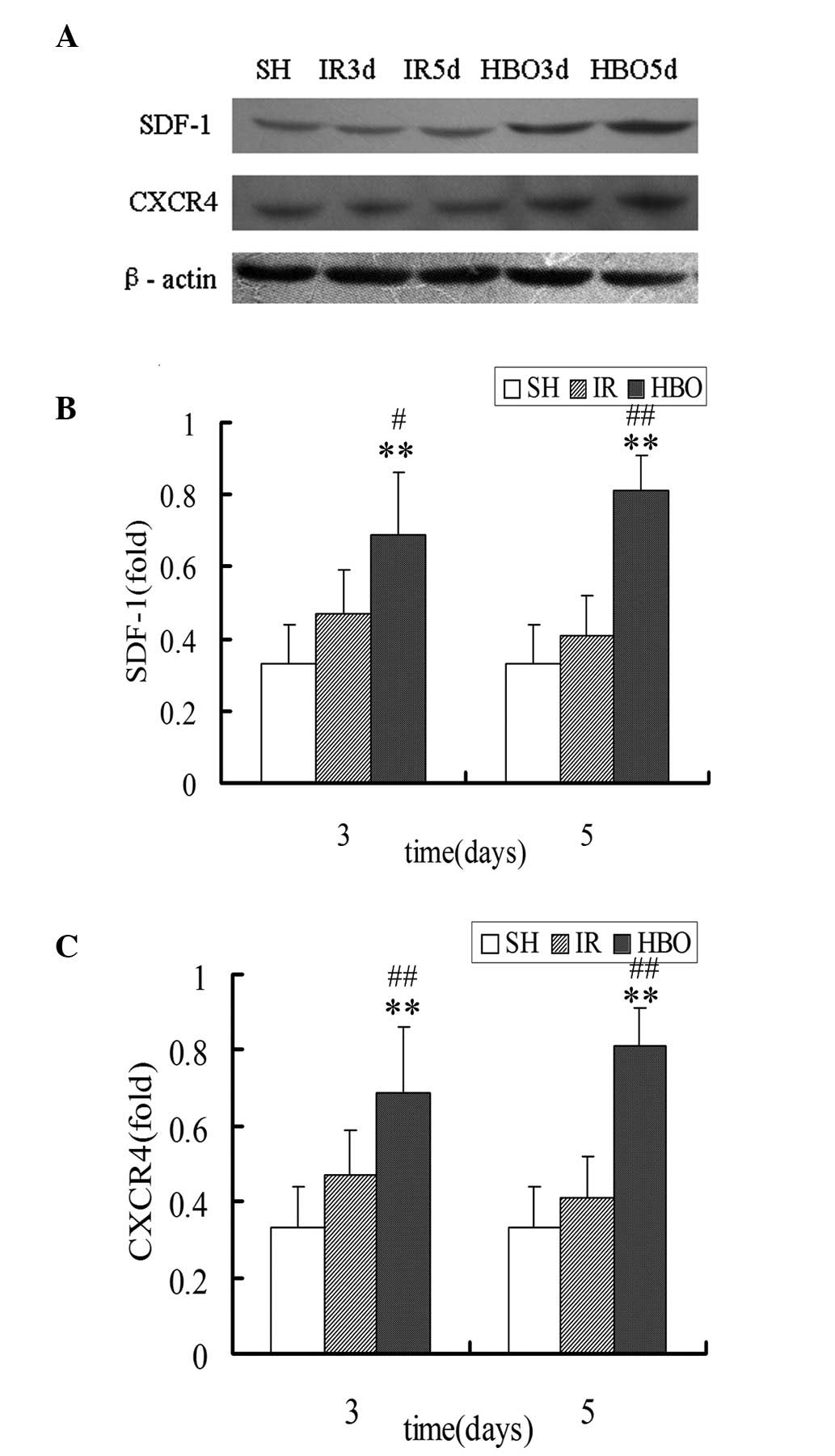
Western blot analysis of SDF-1 and
CXCR4
Western blot analysis identified that the expression
of SDF-1 proteins in the HBO3d (0.69±0.17) and HBO5d groups
(0.81±0.10) was significantly increased compared with that in the
IR3d (0.47±0.12) and IR5d groups (0.41±0.11) (P<0.05 and
P<0.01, respectively). The expression of CXCR4 proteins in the
HBO3d (0.67±0.16) and HBO5d groups (0.84±0.17) was also
significantly increased compared with that in the IR3d (0.41±0.13)
and IR5d groups (0.38±0.09) (P<0.01). The expression of SDF-1
and CXCR4 in the HBO groups was significantly higher than that in
the SH group (SDF-1, 0.33±0.11; CXCR4, 0.31±0.08) (P<0.01)
(Fig. 5). These results support
the previous findings that HBO treatment significantly increased
the expression of SDF-1 and CXCR4 proteins in the skin flaps of
rats.
Positive correlation between
neovascularization and SDF-1 and CXCR4 expression
Pearson’s correlation analysis demonstrated that at
the protein level, there was a positive correlation between
neovascularization (MVD) and the expression of SDF-1 and CXCR4 in
the skin flaps of rats treated with HBO (Table I).
 | Table IPearson’s correlation analysis between
neovascularization (MVD) and SDF-1 and CXCR4 expression in the skin
flap of rats (n=6 per group) treated with HBO. |
Table I
Pearson’s correlation analysis between
neovascularization (MVD) and SDF-1 and CXCR4 expression in the skin
flap of rats (n=6 per group) treated with HBO.
| Factors | Immunohistochemical
staining | Immunoblot
analysis |
|---|
|
|---|
| 3 days | 5 days | 3 days | 5 days |
|---|
|
|
|
|
|
|---|
| MVD | SDF-1 | CXCR4 | SDF-1 | CXCR4 | SDF-1 | CXCR4 | SDF-1 | CXCR4 |
| R | 0.851a | 0.838a | 0.914a | 0.835a | 0.879a | 0.821a | 0.829a | 0.866a |
| P | 0.032 | 0.037 | 0.011 | 0.039 | 0.021 | 0.045 | 0.041 | 0.026 |
Discussion
Improving the survival rate of skin flaps is one of
the main aims in plastic surgery. A number of studies have
demonstrated the beneficial effects of HBO treatment on improving
survival of compromised skin grafts and flaps, including the venous
flap, the tubed pedicle flap, the composite graft and the random
pattern skin flap (10,11,13–15).
However, the detailed mechanisms involved are not fully understood.
In the present study, we aimed to characterize the effects of HBO
treatment on neovascularization and skin flap survival, as well as
investigate its mechanism at a molecular level by analyzing the
expression of angiogenic mediators, such as SDF-1 and CXCR4 in the
skin flaps of rats.
Observations by Ju et al(16) showed that HBO treatment may
increase angiogenesis of the expanded skin and subsequently
increase skin flap survival. HBO treatment increases
neovascularization through angiogenic stimulation, resulting in
blood vessel formation from local endothelial cells, and by
stimulating systemic stem/progenitor cells to differentiate into
blood vessels (17). At present,
MVD assessment is one of the most reliable methods of measuring
angiogenic activity (18).
Consistent with previous studies, the present study measured MVD in
skin flap biopsies and findings indicated that MVD was
significantly increased in the HBO treatment groups compared with
that of the IR groups. These findings demonstrate that HBO
treatment increased neovascularization in the skin flaps. To the
best of our knowledge, this study analyzed for the first time the
concentration of SDF-1 and CXCR4 in the skin flaps of rats, as well
as the effects of HBO treatment on the expression of SDF-1 and
CXCR4. We found that postoperative administration of HBO resulted
in a high expression of SDF-1 and CXCR4 in skin flaps. In addition,
Pearson’s correlation analysis indicated a positive correlation
between neovascularization and the expression of SDF-1 and CXCR4 in
the skin flaps of rats treated with HBO. The results of this study
provide a theoretical basis for broadening the clinical application
of HBO treatment.
In the present study, the expression of SDF-1 and
CXCR4 in the IR groups was higher than that in the SH group, and
compared with that of the IR groups, the expression of SDF-1 and
CXCR4 was much higher in the HBO groups. Hypoxia initiates
neovascularization by inducing angiogenic factor expression, but
cannot sustain it. A threshold level of oxygenation is required to
support the metabolic needs of tissue remodeling. Acute hypoxia
facilitates the angiogenic process (19), while chronic hypoxia impairs wound
angiogenesis (20). Sustained
hypoxia results in the dysfunction of tissues and cell death. SDF-1
is upregulated in hypoxic or ischemic tissues, such as arteries
(21) and brain (22). Moreover, hyperoxia induces the
expression of SDF-1 and CXCR4 via the upregulation of VEGF
expression (23,24). In addition, Salvucci et
al(25) demonstrated that
endothelial expression of SDF-1 and CXCR4 may be reduced by
inflammatory cytokines, such as tumor necrosis factor-α and
interferon-γ. As HBO treatment inhibits these inflammatory
cytokines, the expression of SDF-1 and CXCR4 may be upregulated by
HBO through the inhibition of inflammatory cytokines. It is well
known that bone marrow (BM)-derived endothelial progenitor cell
(EPC) is a key cell involved in neovascularization and homes to
peripheral tissue in response to ischemia (26). Endothelial nitric oxide synthase
(eNOS) is essential in the BM microenvironment and increased BM NO
levels results in the mobilization of EPCs from BM niches into
circulation, ultimately allowing their participation in
tissue-level vasculogenesis and wound healing (27,28).
Induction of hyperoxia via HBO treatment has been shown to increase
NO levels in perivascular tissues and BM via a NOS-mediated
mechanism, resulting in EPC release from the BM and an increase in
EPCs in tissues (29–31). EPCs themselves express and secrete
SDF-1 and CXCR4 (32), thus
following HBO treatment, the expression of SDF-1 and CXCR4 may be
increased. The effects of HBO treatment on the expression of SDF-1
and CXCR4 were supported in the present study.
Ghadge et al(33) indicated that manipulating SDF-1 and
its receptor CXCR4 in ischemic cardiac disease is beneficial for
neovascularization and tissue repair. Li et al(34) indicated that SDF-1 may induce
neovascularization potentially via the SDF-1/CXCR4 axis following
traumatic brain injury. Blocking the SDF-1/CXCR4 axis resulted in
reduced homing of cells to lesions and decreased MVD after ischemic
heart disease (35). These
findings suggest that the SDF-1/CXCR4 axis is important in blood
vessel growth and development. In the present study, the Pearson’s
correlation analysis demonstrated a positive correlation between
neovascularization and the expression of SDF-1 and CXCR4 in the
skin flaps of rats. There may be various mechanisms involved in the
SDF-1/CXCR4 axis governing neovascularization. In addition to its
role in recruiting EPCs to ischemic sites (36), SDF-1 directly participates in blood
vessel formation. SDF-1/CXCR4 has an angiogenic effect on
endothelial cells by inducing cell proliferation, differentiation,
sprouting and tube formation in vitro and by preventing
apoptosis of EPCs (37). SDF-1
also modulates vascularization of ischemic tissues and tumors by
improving the secretion of other angiogenic factors, such as
VEGF-A, interleukin-6 (IL-6), IL-8, tissue inhibitor of
metalloproteinase-2, and decreasing the production of the
anti-angiogenic molecules such as angiostatin (38,39).
In conclusion, the present study established a
modified epigastric pedicle skin flap model in rats. Data of this
study indicate that HBO treatment promoted neovascularization and
increased the survival of skin flaps. In addition, the expression
of SDF-1 and CXCR4 was upregulated in the skin flaps of rats
treated with HBO. Pearson’s correlation analysis demonstrated a
positive correlation between neovascularization and the high
expression of SDF-1 and CXCR4. These results suggest that the
beneficial effects of HBO treatment in promoting neovascularization
may be explained by the upregulation of SDF-1 and CXCR4 expression
in the skin flaps of rats.
References
|
1
|
Kryger Z, Zhang F, Dogan T, Cheng C,
Lineaweaver WC and Buncke HJ: The effects of VEGF on survival of a
random flap in the rat: examination of various routes of
administration. Br J Plast Surg. 53:234–239. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Lu WW, Ip WY, Jing WM, Holmes AD and Chow
SP: Biomechanical properties of thin skin flap after basic
fibroblast growth factor (bFGF) administration. Br J Plast Surg.
53:225–229. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
de Souza Filho MV, Loiola RT, Rocha EL,
Simão AFL and Ribeiro RA: Remote ischemic preconditioning improves
the survival of rat random-pattern skin flaps. Eur J Plast Surg.
33:147–152. 2010.(In Spanish).
|
|
4
|
Ulkür E, Karagoz H, Ergun O, Celikoz B,
Yildiz S and Yildirim S: The effect of hyperbaric oxygen therapy on
the delay procedure. Plast Reconstr Surg. 119:86–94.
2007.PubMed/NCBI
|
|
5
|
Hamon M, Mbemba E, Charnaux N, et al: A
syndecan-4/CXCR4 complex expressed on human primary lymphocytes and
macrophages and HeLa cell line binds the CXC chemokine stromal
cell-derived factor-1 (SDF-1). Glycobiology. 14:311–323. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Ratajczak MZ, Zuba-Surma E, Kucia M, Reca
R, Wojakowski W and Ratajczak J: The pleiotropic effects of the
SDF-1-CXCR4 axis in organogenesis, regeneration and tumorigenesis.
Leukemia. 20:1915–1924. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Hassan S, Ferrario C, Saragovi U, et al:
The influence of tumor-host interactions in the stromal
cell-derived factor-1/CXCR4 ligand/receptor axis in determining
metastatic risk in breast cancer. Am J Pathol. 175:66–73. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Tysseling VM, Mithal D, Sahni V, et al:
SDF1 in the dorsal corticospinal tract promotes CXCR4+ cell
migration after spinal cord injury. J Neuroinflammation.
8:162011.PubMed/NCBI
|
|
9
|
Jones SR, Carpin KM, Woodward SM, Khiabani
KT, Stephenson LL, Wang WZ and Zamboni WA: Hyperbaric oxygen
inhibits ischemia-reperfusion-induced neutrophil CD18 polarization
by a nitric oxide mechanism. Plast Reconstr Surg. 126:403–411.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Richards L, Lineaweaver WC, Stile F, Zhang
J and Zhang F: Effect of hyperbaric oxygen therapy on the tubed
pedicle flap survival in a rat model. Ann Plast Surg. 50:51–56.
2003. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Zhang T, Gong W, Li Z, et al: Efficacy of
hyperbaric oxygen on survival of random pattern skin flap in
diabetic rats. Undersea Hyperb Med. 34:335–339. 2007.PubMed/NCBI
|
|
12
|
Lubiatowski P, Goldman CK, Gurunluoglu R,
Carnevale K and Siemionow M: Enhancement of epigastric skin flap
survival by adenovirus-mediated VEGF gene therapy. Plast Reconstr
Surg. 109:1986–1993. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Yücel A and Bayramiçli M: Effects of
hyperbaric oxygen treatment and heparin on the survival of
unipedicled venous flaps: an experimental study in rats. Ann Plast
Surg. 44:295–303. 2000.PubMed/NCBI
|
|
14
|
Fodor L, Ramon Y, Meilik B, Carmi N,
Shoshani O and Ullmann Y: Effect of hyperbaric oxygen on survival
of composite grafts in rats. Scand J Plast Reconstr Surg Hand Surg.
40:257–260. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Metselaar M, Dumans AG, van der Huls MP,
Sterk W and Feenstra L: Osteoradionecrosis of tympanic bone:
reconstruction of outer ear canal with pedicled skin flap, combined
with hyperbaric oxygen therapy, in five patients. J Laryngol Otol.
123:1114–1119. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Ju Z, Wei J, Guan H, Zhang J, Liu Y and
Feng X: Effects of hyperbaric oxygen therapy on rapid tissue
expansion in rabbits. J Plast Reconstr Aesthet Surg. 65:1252–1258.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Thom SR: Hyperbaric oxygen: its mechanisms
and efficacy. Plast Reconstr Surg. 127:S131–S141. 2011. View Article : Google Scholar
|
|
18
|
Zhang Y, Zhao H, Zhao D, et al:
SDF-1/CXCR4 axis in myelodysplastic syndromes: correlation with
angiogenesis and apoptosis. Leuk Res. 36:281–286. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Semenza GL: HIF-1: using two hands to flip
the angiogenic switch. Cancer Metastasis Rev. 19:59–65. 2000.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Allen DB, Maguire JJ, Mahdavian M, et al:
Wound hypoxia and acidosis limit neutrophil bacterial killing
mechanisms. Arch Surg. 132:991–996. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Shiba Y, Takahashi M, Yoshioka T, et al:
M-CSF accelerates neointimal formation in the early phase after
vascular injury in mice: the critical role of the SDF-1-CXCR4
system. Arterioscler Thromb Vasc Biol. 27:283–289. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Schönemeier B, Schulz S, Hoellt V and
Stumm R: Enhanced expression of the CXCI12/SDF-1 chemokine receptor
CXCR7 after cerebral ischemia in the rat brain. J Neuroimmunol.
198:39–45. 2008.PubMed/NCBI
|
|
23
|
Sheikh AY, Gibson JJ, Rollins MD, Hopf HW,
Hussain Z and Hunt TK: Effect of hyperoxia on vascular endothelial
growth factor levels in a wound model. Arch Surg. 135:1293–1297.
2000. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Hong X, Jiang F, Kalkanis SN, et al: SDF-1
and CXCR4 are up-regulated by VEGF and contribute to glioma cell
invasion. Cancer Lett. 236:39–45. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Salvucci O, Basik M, Yao L, Bianchi R and
Tosato G: Evidence for the involvement of SDF-1 and CXCR4 in the
disruption of endothelial cell-branching morphogenesis and
angiogenesis by TNF-alpha and IFN-gamma. J Leukoc Biol. 76:217–226.
2004. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Yamaguchi J, Kusano KF, Masuo O, et al:
Stromal cell-derived factor-1 effects on ex vivo expanded
endothelial progenitor cell recruitment for ischemic
neovascularization. Circulation. 107:1322–1328. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Aicher A, Heeschen C, Mildner-Rihm C, et
al: Essential role of endothelial nitric oxide synthasefor
mobilization of stem and progenitor cells. Nat Med. 9:1370–1376.
2003. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Murohara T, Asahara T, Silver M, et al:
Nitric oxide synthase modulates angiogenesis in response to tissue
ischemia. J Clin Invest. 101:2567–2578. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Thom SR, Fisher D, Zhang J, et al:
Stimulation of perivascular nitric oxide synthesis by oxygen. Am J
Physiol Heart Circ Physiol. 284:H1230–H1239. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Goldstein LJ, Gallagher KA, Bauer SM, et
al: Endothelial progenitor cell release into circulation is
triggered by hyperoxia-induced increases in bone marrow nitric
oxide. Stem Cells. 24:2309–2318. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Gallagher KA, Liu ZJ, Xiao M, et al:
Diabetic impairments in NO-mediated endothelial progenitor cell
mobilization and homing are reversed by hyperoxia and SDF-1 alpha.
J Clin Invest. 117:1249–1259. 2007. View
Article : Google Scholar : PubMed/NCBI
|
|
32
|
Urbich C, Aicher A, Heeschen C, Dernbach
E, Hofmann WK, Zeiher AM and Dimmeler S: Soluble factors released
by endothelial progenitor cells promote migration of endothelial
cells and cardiac resident progenitor cells. J Mol Cell Cardiol.
39:733–742. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Ghadge SK, Mühlstedt S, Ozcelik C and
Bader M: SDF-1α as a therapeutic stem cell homing factor in
myocardial infarction. Pharmacol Ther. 129:97–108. 2011.
|
|
34
|
Li S, Wei M, Zhou Z, Wang B, Zhao X and
Zhang J: SDF-1α induces angiogenesis after traumatic brain injury.
Brain Res. 1444:76–86. 2012.
|
|
35
|
Dai S, Yuan F, Mu J, et al: Chronic
AMD3100 antagonism of SDF-1alpha-CXCR4 exacerbates cardiac
dysfunction and remodeling after myocardial infarction. J Mol Cell
Cardiol. 49:587–597. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Rosenkranz K, Kumbruch S, Lebermann K,
Marschner K, Jensen A, Dermietzel R and Meier C: The chemokine
SDF-1/CXCL12 contributes to the ‘homing’ of umbilical cord blood
cells to a hypoxic-ischemic lesion in the rat brain. J Neurosci
Res. 88:1223–1233. 2010.
|
|
37
|
Zheng H, Dai T, Zhou B, Zhu J, Huang H,
Wang M and Fu G: SDF-1alpha/CXCR4 decreases endothelial progenitor
cells apoptosis under serum deprivation by PI3K/Akt/eNOS pathway.
Atherosclerosis. 201:36–42. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Wang J, Wang J, Sun Y, Song W, Nor JE,
Wang CY and Taichman RS: Diverse signaling pathways through the
SDF-1/CXCR4 chemokine axis in prostate cancer cell lines leads to
altered patterns of cytokine secretion and angiogenesis. Cell
Signal. 17:1578–1592. 2005. View Article : Google Scholar
|
|
39
|
Wang J, Wang J, Dai J, et al: A glycolytic
mechanism regulating an angiogenic switch in prostate cancer.
Cancer Res. 67:149–159. 2007. View Article : Google Scholar : PubMed/NCBI
|