Introduction
Schistosomiasis is one of the most widespread
parasitic infections worldwide. The three predominant schistosome
species identified to infect humans are Schistosoma
haematobium (endemic in Africa and the eastern Mediterranean),
S. mansoni (endemic in Africa, the Middle East, the
Caribbean and South America) and S. japonicum (endemic
mainly in China, Japan and the Philippines) (1). It is estimated that 843,007
individuals in China are infected with S. japonicum(2). Intestinal schistosomiasis is an acute
or chronic, specific enteropathy caused by the deposition of
schistosome ovum on the colon and rectal walls. S. japonicum
is prevalent in China and, following efforts to control schistosome
infection, the incidence of acute schistosome infection has
decreased to only a few sporadic cases (3–8). The
intestinal and general symptoms of schistosome infection are not
typical (9), thus, the accurate
rate of diagnosis is low and the infection may be easily
misdiagnosed.
Adult S. japonicum worms live in the
mesenteric veins in pairs and lay numerous eggs in the
gastrointestinal mucosa (~3000/day/pair). The eggs are responsible
for inflammatory and immunopathological responses, leading to
erythema, edema, granuloma formation, ulceration, hemorrhage and
fibrosis. Gastrointestinal symptoms may include nausea, meteorism,
abdominal pain, bloody diarrhea, rectal tenesmus and
hepatosplenomegaly (10). In
addition, the clinical manifestations are correlated with the
severity of the infection. In order to prevent the misdiagnosis of
schistosoma infection, a rational approach to the endoscopic
diagnosis is required.
In the present study, we retrospectively analyzed
the clinical, endoscopic and pathological features of 96 patients
with intestinal schistosomiasis, which were diagnosed by a
combination of endoscopic biopsy and histopathological examination
from June 2000 to June 2010, at the Renmin Hospital of Wuhan
University (Wuhan, Hubei, China).
Patients and methods
Patients
We studied 96 patients diagnosed among 37,865
patients with intact endoscopic and histopathology records at the
Department of Gastroenterology (Renmin Hospital of Wuhan
University) between June 2000 and June 2010. There were 72 male and
24 female patients aged 16–79 years (average age, 44.7 years). The
clinical time since the symptoms appeared ranged from 2 months to
25 years. The study was approved by the Ethics Committee of Renmin
Hospital of Wuhan University, Wuhan, China and written informed
consent was obtained from the patients/patients’ families.
Collecting clinical data
The medical history, clinical symptoms, colonoscopy
manifestation and pathological data of the patients with intestinal
schistosomiasis were obtained from the Digestive Endoscopy Center
and Record Room of Renmin Hospital.
Endoscopy
The CF240 (Olympus, Tokyo, Japan) or the CF260
(Fujifilm, Tokyo, Japan) electronic colonoscope was used to examine
the patients. During the colonoscopy examination, at least three
colonic mucosal biopsies were taken, and normal colonic mucosa and
polyps were removed using high frequency electrocoagulation and
electrocision. Pathological tissues from the biopsies were sent for
histopathological examination at the Department of Pathology,
Renmin Hospital of Wuhan University.
Histopathology
The specimens for histopathological examination were
fixed in 10% formaldehyde, dehydrated with various concentrations
of alcohol and xylol, then paraffin-embedded and cut into 4-μm
sections. Hematoxylin and eosin staining was used to observe the
pathological changes under a light microscope (Olympus, Tokyo,
Japan).
Results
Patient history and clinical
symptoms
Among 96 patients with intestinal schistosomiasis,
57 had received anthelmintic treatment, 21 lived in areas without
schistosome infection, and 25 (26%) had no history of schistosome
infection and no apparent contact with infested water. The patients
were mainly hospitalized for abdominal pain, diarrhea, mucus and
bloody purulent stool, with stool passage two to seven times per
day. Of the 96 intestinal schistosomiasis patients, there were 46
with a low fever, emaciation and asthenia; 17 with abdominal
masses; and 61 with a degree of splenomegaly (Table I).
 | Table IClinical symptoms of 96 cases of
intestinal schistosomiasis. |
Table I
Clinical symptoms of 96 cases of
intestinal schistosomiasis.
| Clinical symptom | No. of cases
(n=96) |
|---|
| Diarrhea | 64 |
| Mucous stool | 47 |
| Abdominal
distention | 39 |
| Abdominal pain | 75 |
| Low fever, emaciation
and asthenia | 46 |
| Splenomegaly | 61 |
| Bloody purulent
stool | 21 |
| Constipation | 23 |
| Alternation of
diarrhea and constipation | 9 |
| Abdominal mass | 17 |
| Intestinal
obstruction | 2 |
Position of lesions
Of the 96 patients in this study, 92 were analyzed
by colonoscopy, with the colonoscope inserted to the end of the
ileum, to determine the location of the pathological changes
(Table II).
 | Table IILocation of pathological change. |
Table II
Location of pathological change.
| Location of
pathological change | No. of cases
(n=96) |
|---|
| Rectum | 47 |
| Sigmoid colon | 55 |
| Descending colon | 11 |
| Transverse colon | 8 |
| Ascending colon | 6 |
| Cecum | 9 |
Colonoscopic manifestations
Generally, intestinal schistosomiasis was divided
into three types: Acute colonitis, chronic colonitis and mixed
colonitis (11), as demonstrated
in Table III.
 | Table IIIColonoscopy manifestations of
intestinal schistosomiasis. |
Table III
Colonoscopy manifestations of
intestinal schistosomiasis.
| Colonoscopy
manifestation | Acute colonitis
(n=16) | Chronic colonitis
(n=41) | Mixed colonitis
(n=39) |
|---|
| Hyperaemic edema | 16 | 6 | 10 |
| Mucus exudation | 15 | 5 | 15 |
| Anabiosis and
canker | 10 | 13 | 4 |
| Yellow nodules | 9 | 39 | 28 |
| Disorder of vascular
textures | 0 | 41 | 30 |
| Cicatrice | 0 | 32 | 23 |
| Polypus | 0 | 26 | 14 |
| Neoplasm | 0 | 1 | 1 |
| Stenosis | 0 | 4 | 0 |
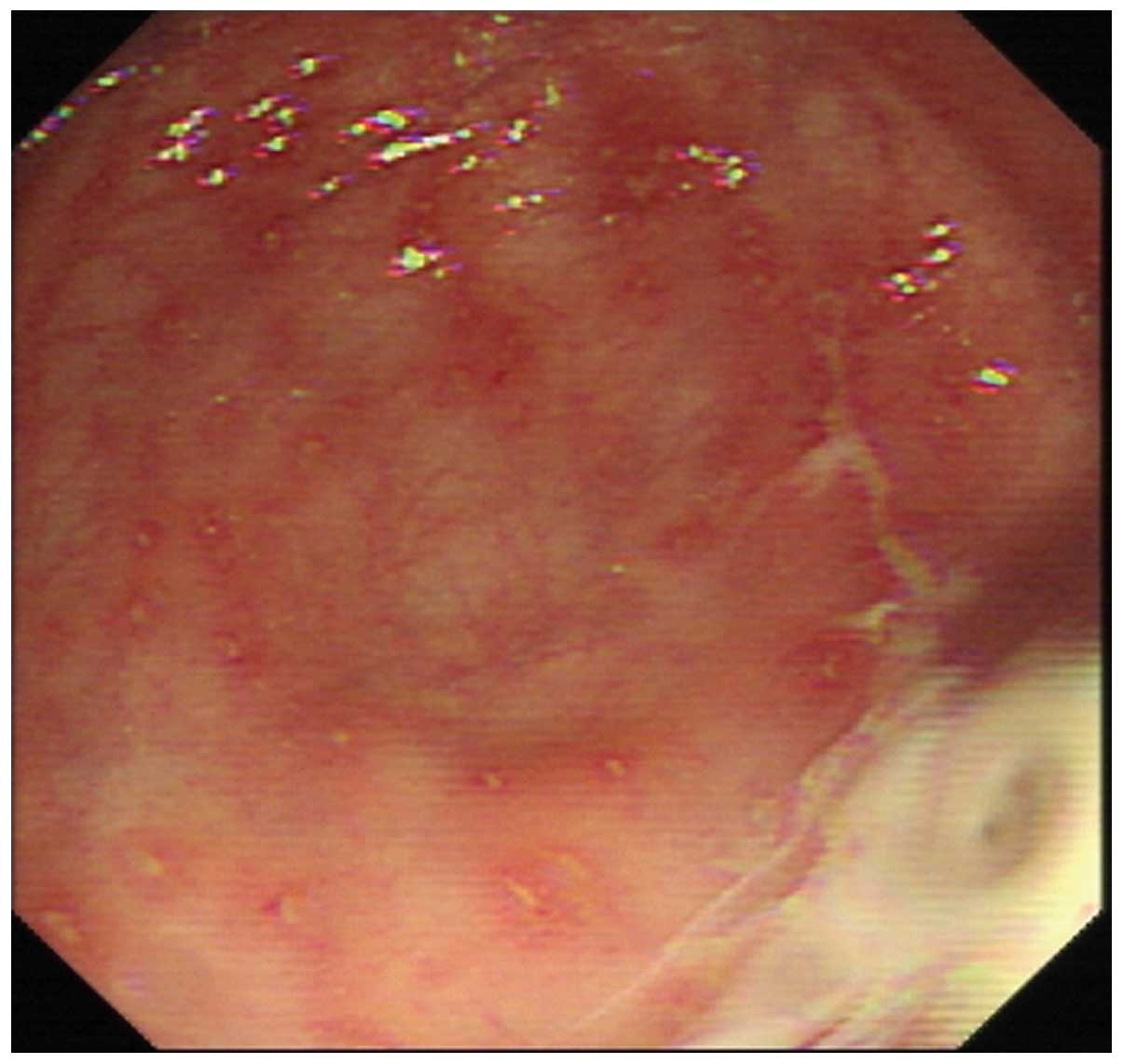
Sixteen cases of acute colonitis were observed with
endoscopy and exhibited hyperaemic edema, mucus exudation, vague
vascular striation and scattered aphtha and punctate hemorrhage of
the intestinal mucosae (Fig. 1).
Among 16 patients, nine had typical yellow nodules that were
commonly distributed around severe small ulcers.
Among the 96 patients, there were 41 chronic
colonitis cases (Figs. 2 and
3), in which the intestinal
mucosae presented with mucosal thickening, loss of vascular
texture, paleness, cicatrization, haustrum blockages and polypoid
protrusion. In addition, in a small number of cases, scattered
cankers were observed. Twenty-six patients demonstrated merged
colon polyps, and 39 patients had typical yellow nodules which were
of a more concentrated distribution compared with those of the
acute type.
There were 39 cases of mixed colonitis, in which the
intestinal mucosae demonstrated the combined manifestations of the
aforementioned two types of colonitis. A combination of acute
(primarily in the left intestines) and chronic (primarily in the
right intestines) inflammation occurred in certain sections. In
addition, the two types of inflammation were sometimes observed
alongside each other, thus rendering it difficult to distinguish
between primary infection and infection recurrence. Twenty-eight
patients had scattered yellow nodules and 14 had merged colon
polyps.
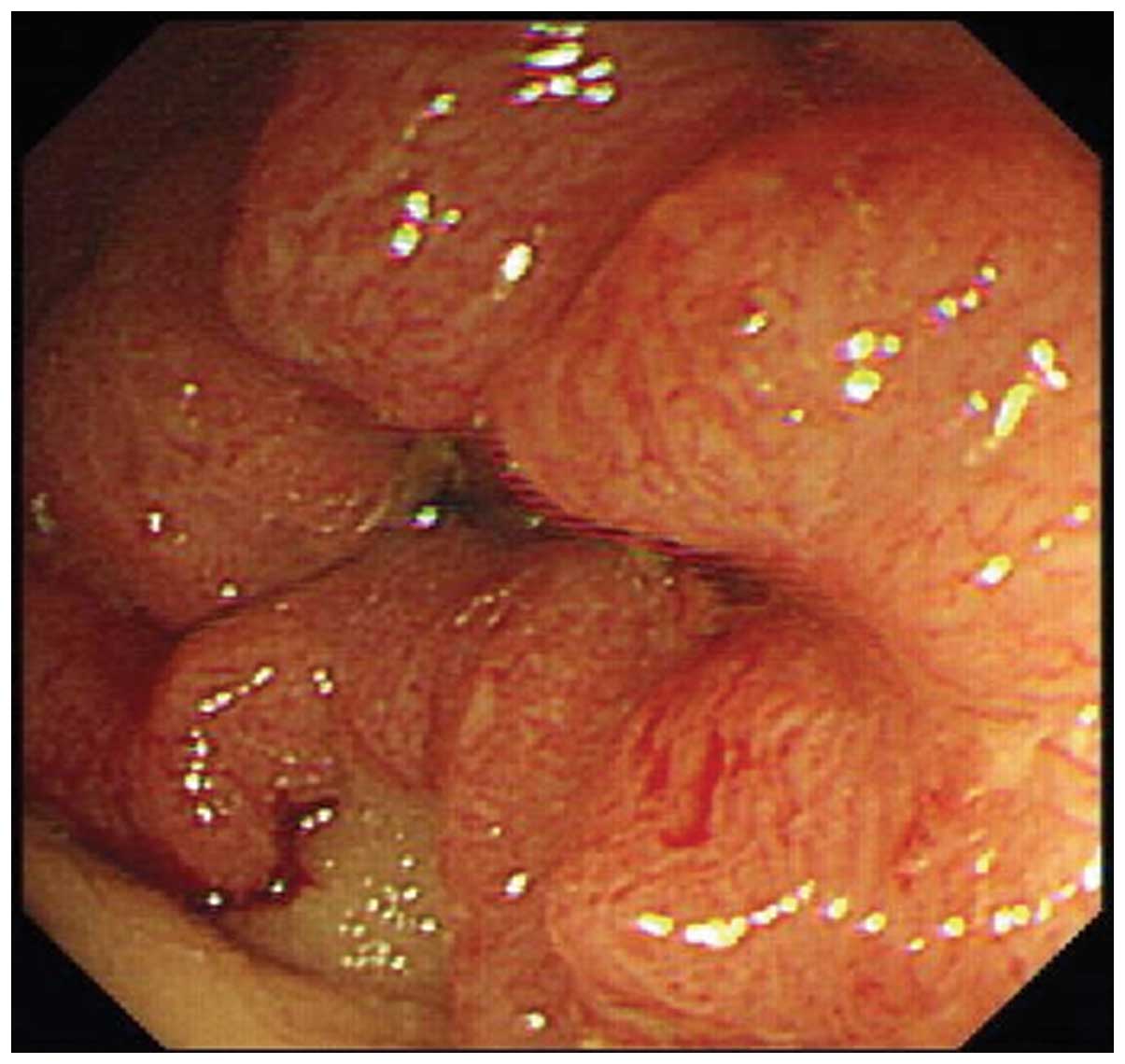
There were six cases of intestinal schistosomiasis
combined with colorectal cancer (Fig.
2), two cases of which were located in the sigmoid colon and
rectum, and four of which were located in the ileocecal valve.
Under endoscopy, two cases were observed with the formation of
neoplasma and four manifested with stenosis.
Histopathological characteristics
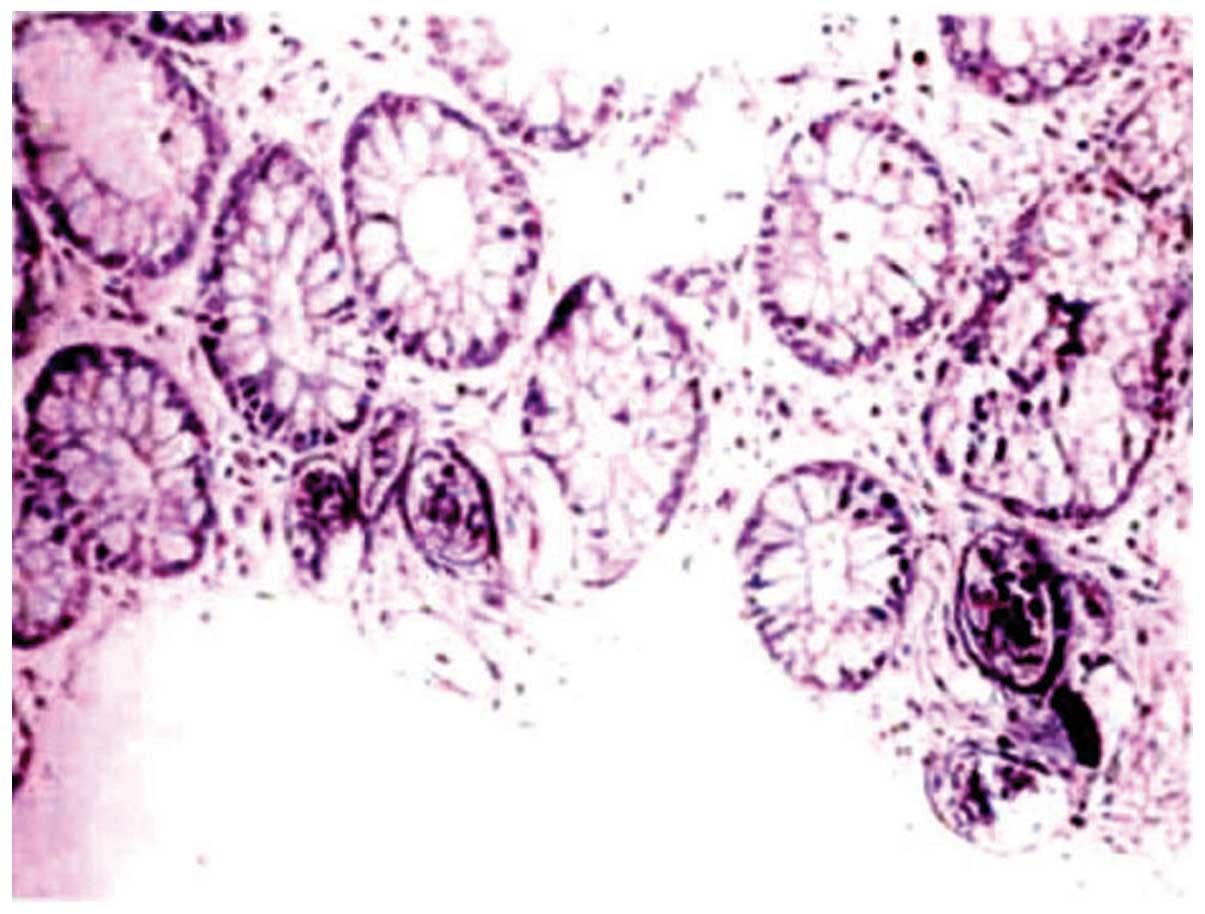
Schistosome ovum, deposited in the lamina propria,
were detected in the endoscopic biopsies of all 96 patients. The 16
patients with acute colonitis pathologically manifested with the
infiltration of numerous interstitial eosinocytes and neutrophilic
granulocytes, and uncalcified schistosome ovum deposition in the
lamina propria under the mucosa (Fig.
4). The 41 patients with chronic colonitis predominantly
demonstrated lymphocyte and plasmocytes at the submucosa and lamina
propria. Atrophy of the intestinal mucosal epithelium, reduction of
the intestinal gland, hyperplasia of partial submucosa tissue
accompanied with fibrosis and chronic ova nodules were also
observed, however several calcified schistosome ovum were hidden
(Fig. 5). There were 39 cases of
mixed colonitis with combined manifestations of acute and chronic
enteritis. Among which, certain mucosal segments demonstrated an
infiltration of lymphocytes while others demonstrated an
infiltration of eosinocytes.
Forty-one cases were complicated with colorectal
polyps, all of which were removed with high frequency
electrocoagulation and electrocision. Patholgical analysis
demonstrated that these were hyperplastic or inflammatory polyps.
In addition, no polyps were observed in acute colorectal enteritis.
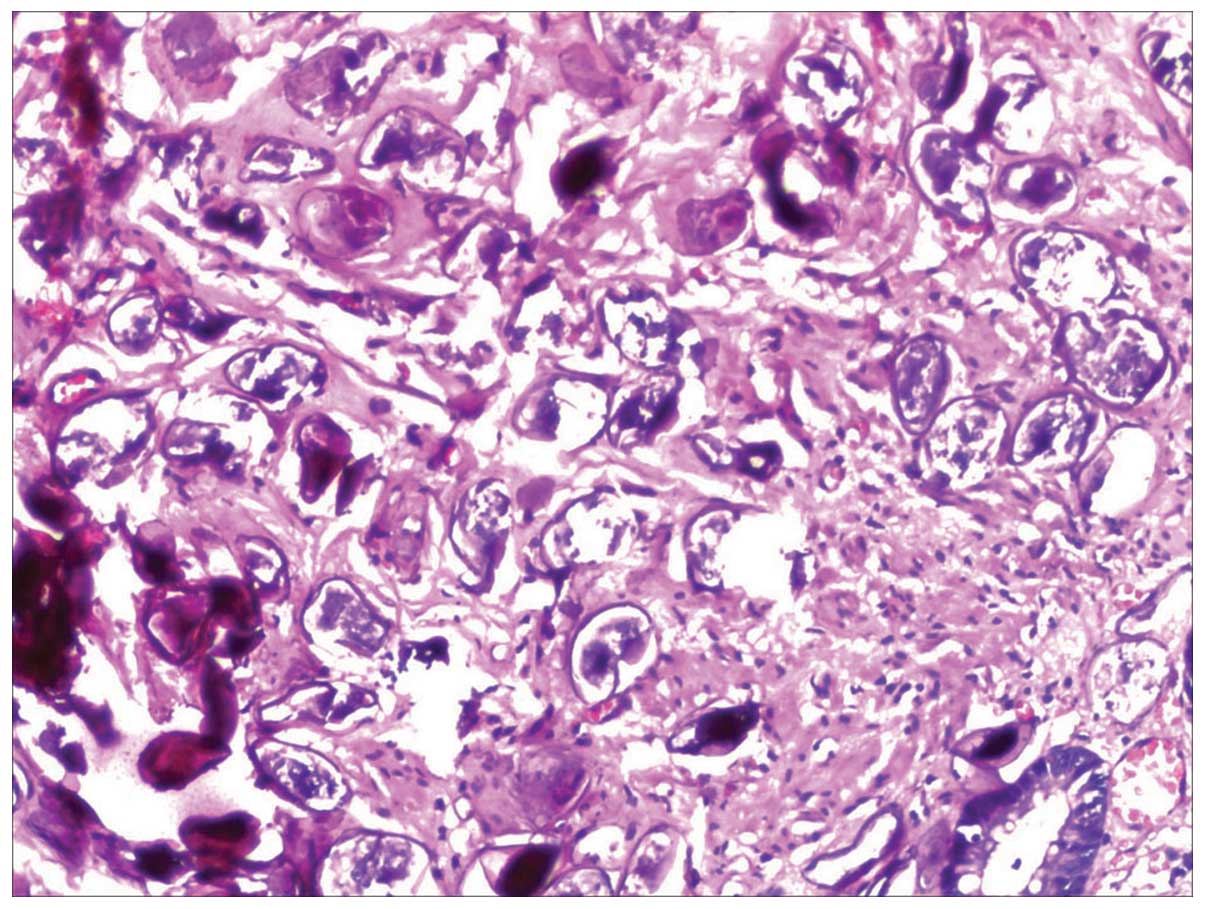
Six out of 96 intestinal schistosomiasis patients also demonstrated
the presence of colorectal cancer. Two cases of mucinous
adenocarcinoma, two mid-differentiated tubular adenocarcinoma, one
papillary adenocarcinoma and one mixed carcinoma were detected. The
deposition of schistosome ovum and tumor cells was examined
simultaneously in the same pathological specimen in six patients
(Fig. 6). A medium or heavy degree
of hyperplasia considered as precancerosis was observed in nine
cases.
Misdiagnosis
In the group of 96 cases, 24 were misdiagnosed
outside the hospital (misdiagnosis rate, 25%). Eight of the 24
cases were misdiagnosed as ulcerative colitis, five as ileocecal
tumor, five as intestinal tuberculosis, four as chronic bacillary
dysentery and two as irritable bowel syndrome.
Endoscopic features of the intestinal mucosa in the
24 misdiagnosed cases were non-typical. Eight cases demonstrated
intensive or scattered ulcer-like changes with varying size and
depth. The majority of the ulcers were congestive and edematous and
attached by secretions to the peripheral mucosa. In addition, the
typical yellow nodules in the intestinal tract were rare; thus, the
cases were misdiagnosed as ulcerative colitis. Five cases exhibited
the deformation of the ileocecal valve, occasionally accompanied by
scattered non-typical yellow nodules and friable mucosa with the
increased likelihood of contact bleeding. As a result, they were
misdiagnosed as ileocecal tumors. Another five cases, with lesions
in the sigmoid colon demonstrated granular protrusions of the
intestinal mucosa, small scattered ulcers, intestinal stiffness,
narrowed lumen and stenosis, combined with a low-level fever in
patients. These cases were misdiagnosed as intestinal tuberculosis.
Furthermore, biopsies were not taken in six out of 24 cases, while
in another 18 cases, an insufficiently sized specimen was
collected; thus, in these cases, the ova nodules were not
located.
Discussion
It is generally acknowledged that the endoscopic
features of intestinal schistosomiasis may be divided into the
acute colitis type and the chronic colitis type (11,12).
The mucosas of the acute colitis type predominantly expressed
congestion and edema. By contrast, the chronic colitis type
expressed chronic inflammation, such as mucosal hypertrophy and
scarring. The ova nodules of the acute colitis type were acute and
consisted of one or multiple central ova surrounded by eosinocytes.
By contrast, in the chronic colitis type, the middle ova of the
nodules had ruptured and calcified, and were surrounded by
lymphocytes and epithelioid cells. This was accompanied by the
proliferation of numerous submucosal fibrous tissues. In the 96
patients, 16 were of the acute colitis type and 41 were of the
chronic colitis type; however, 39 demonstrated a combination of
acute and chronic mucosal inflammation changes and the biopsies
showed the concurrence of 2 types of ova nodules. This is
consistent with the results of a study by Liu et al(13) in which adding the mixed type to the
classification of shistosomiasis was proposed.
Recently, the morbidity of intestinal
schistosomiasis has increased due to inadequate knowledge. Numerous
patients in affected areas suffer from chronic schistosomiasis. Due
to the lack of thorough treatment and repeated infection, these
patients demonstrated a combination of chronically inflamed
pathological manifestations with acute inflammation. As a result of
the multiple and atypical clinical manifestations of these
patients, misdiagnosis was common. For example, patients with acute
inflammatory manifestations, such as fever, abdominal pain,
diarrhea and bloody stools, were often misdiagnosed with bacillary
or amebic dysentery. Additionally, those exhibiting chronic
inflammation, such as abdominal pain and diarrhea, were often
misdiagnosed with ulcerative colitis, intestinal tuberculosis and
colon cancer. Hence, it is essential for clinicians to consider
schistosomiasis when making a diagnosis, particularly in patients
living in or in contact with infected areas.
Among 96 cases of intestinal schistosomiasis, 24
cases were misdiagnosed outside the hospital and were examined at
the Renmin Hospital due to the lack of effective treatment. The
causes of misdiagnosis were demonstrated to be partly due to the
diverse and complex endoscopic features of these patients and also
to the mucosal biopsies. In certain patients, this was due to the
fact that biopsies had not been conducted; however, for the
majority, the biopsies were derived from inaccurate positions or
samples were insufficient in size. The lack of effective biopsies
meant that the egg depositions had not been located and thus
resulted in misdiagnosis.
The endoscopic manifestations of intestinal
schistosomiasis are prone to be confused with those of ulcerative
colitis, Crohn’s disease, intestinal tuberculosis and colon cancer
without careful identification. For example, the intestinal mucosas
of the acute colitis type of intestinal schistosomiasis manifest as
congestion, edema and ulcers; while those of the chronic colitis
type exhibit mucosal hypertrophy, granuloma or polyp formation and
a narrowed, hardened lumen, which may be misdiagnosed as active or
recovery ulcerative colitis, or intestinal tuberculosis. The
presence of granular hyperplasia, multiple nodules or large
granuloma and large ulcers in the intestine lumen may lead to a
misdiagnosis of colon cancer.
Although the incidence of intestinal schistosomiasis
has decreased compared with that in the 1970s and 1980s, it has not
been completely eliminated. Therefore, it is important to determine
patients’ history of exposure to infected water. Moreover, it is
necessary to be aware of the endoscopic features of intestinal
schistosomiasis and to carefully distinguish these from those of
ulcerative colitis, intestinal tuberculosis, intestinal cancer and
other diseases. In addition, it is also necessary to analyze
biopsies, particularly the multi-site, multi-block biopsies for
suspected cases. This is due to the fact that, when the typical
histopathological features of intestinal schistosomiasis were
observed, it was diagnosed. The results were concordant with a
study by Ohmae et al in which the schistosome egg nodules
observed in histological specimens were proposed to be the gold
standard for the diagnosis of this disease (14).
It is suggested that intestinal schistosomiasis is a
risk factor for colorectal cancer; however, this remains
controversial. Clinical pathological studies have demonstrated that
the concurrent incidence of intestinal schistosomiasis and
colorectal cancer was higher than that of the control group
(15,16). In the present study, of the 96
patients with intestinal schistosomiasis, six cases were
accompanied by colorectal cancer and nine with a medium or heavy
degree of hyperplasia considered to be precancerosis. According to
the results, it was determined that intestinal schistosomiasis may
be correlated with the occurrence of colorectal cancer.
A possible mechanism for the induction of cancer by
intestinal schistosomiasis may be the deposition of schistosome
eggs in the digestive tract and the accumulation of a large number
of eggs under the intestinal mucosa, which may lead to chronic
mucosal inflammation, thus resulting in polypoid hyperplasia and
canceration. Li et al(17)
observed that deposited schistosome ova are important in the
carcinogenesis of colorectal cancer. It was also demonstrated that
colorectal cancer foci were often located in significant sections
of intestinal schistosomiasis; the possible factors were the
presence of endogenously produced carcinogens (18), chronic immunomodulation resulting
in impairment of immunological surveillance (19), symbiotic action of other infective
agents (20) and the presence of
schistosomal toxins (21). It is
possible that the intestinal mucosa is constantly stimulated by
schistosome eggs, which leads to mutations in genes of the mucosal
gland epithelial cells (15).
Thus, investigation at the molecular level is required to determine
the precise correlation between intestinal schistosomiasis and
colorectal cancer.
In conclusion, intestinal schistosomiasis is risk
factor for colorectal cancer and the lesions of the disease may be
considered as precancerous. Therefore, histopathological analysis
of periodic endoscopic follow-up and multi-block and multi-site
endoscopic biopsy are important. To reduce the incidence of
colorectal cancer, early endoscopic or surgical treatment for
patients with schistosome infection combined with colon polyps is
required.
Acknowledgements
This study was supported by the Health Department
Foundation of Hubei Province, China (grant no. XF2010-21).
References
|
1
|
Gryseels B, Polman K, Clerinx J and
Kestens L: Human schistosomiasis. Lancet. 368:1106–1118. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Zhou XN, Wang TP, Wang LY, et al: The
current status of schistosomiasis epidemics in China. Zhonghua Liu
Xing Bing Xue Za Zhi. 25:555–558. 2004.(In Chinese).
|
|
3
|
Wang W, Dai JR, Liang YS, Huang YX and
Coles GC: Impact of the South-to-North Water Diversion Project on
the transmission of Schistosoma japonicum in China. Ann Trop
Med Parasitol. 103:17–29. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Wu GY and Halim MH: Schistosomiasis:
progress and problems. World J Gastroenterol. 6:12–19. 2000.
|
|
5
|
Zhou X: Distribution and control of the
schistosomiasis in China. Journal of Anhui Agri Sci. 12:3766–3768.
2007.(In Chinese).
|
|
6
|
Zhang LJ, Zhu R, Dang H, Xu J, Li SZ and
Guo JG: Analysis of surveillance of schistosomiasis in China in
2011. Zhongguo Xue Xi Chong Bing Fang Zhi Za Zhi. 24:627–631.
2012.(In Chinese).
|
|
7
|
Wang Y, Wang YR, Ying XJ and Zhu XC:
Epidemic situation of schistosomiasis in a provincial surveillance
site of Shengzhou City, 2008–2011. Zhongguo Xue Xi Chong Bing Fang
Zhi Za Zhi. 25:104–105. 2013.(In Chinese).
|
|
8
|
Sun LP, Tian ZX, Yang K, Huang YX, et al:
Effect evaluation of transmission control of schistosomiasis in 14
counties (cities, districts) of Jiangsu Province. Zhongguo Xue Xi
Chong Bing Fang Zhi Za Zhi. 24:26–31. 2012.(In Chinese).
|
|
9
|
Rizzo M, Mansueto P, Cabibi D, et al: A
case of bowel schistosomiasis not adhering to endoscopic findings.
World J Gastroenterol. 11:7044–7047. 2005.PubMed/NCBI
|
|
10
|
Schafer TW and Hale BR: Gastrointestinal
complications of schistosomiasis. Curr Gastroenterol Rep.
3:293–303. 2001. View Article : Google Scholar
|
|
11
|
Anthony BJ, Ramm GA and McManus DP: Role
of resident liver cells in the pathogenesis of schistosomiasis.
Trends Parasitol. 28:572–579. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Larkin BM, Smith PM, Ponichtera HE,
Shainheit MG, Rutitzky LI and Stadecker MJ: Induction and
regulation of pathogenic Th17 cell responses in schistosomiasis.
Semin Immunopathol. 34:873–888. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Liu H, Liu L and Lu J: Diagnosis and
Classification of Schistosomiasis in Children (report of 25 cases).
Zhonghua xiao hua nei jing za zhi. 16:216–217. 1999.(In
Chinese).
|
|
14
|
Ohmae H, Sy OS, Chigusa Y and Portillo GP:
Imaging diagnosis of Schistosomiasis japonica - the use in
Japan and application for field study in the present endemic area.
Parasitol Int. 52:385–393. 2003.
|
|
15
|
Mei J, Hong H, Ding Y and Fang Y: Clinic
pathological characteristics of chronic schistosomiasis concurrent
colorectal Cancer. Chin J Dig Endosc. 1:49–50. 2004.(In
Chinese).
|
|
16
|
Matsuda K, Masaki T, Ishii S, et al:
Possible associations of rectal carcinoma with Schistosoma
japonicum infection and membranous nephropathy: a case report
with a review. Jpn J Clin Oncol. 29:576–581. 1999.PubMed/NCBI
|
|
17
|
Li WC, Pan ZG and Sun YH: Sigmoid colonic
carcinoma associated with deposited ova of Schistosoma
japonicum: a case report. World J Gastroenterol. 12:6077–6079.
2006.PubMed/NCBI
|
|
18
|
Rosin MP, Anwar WA and Ward AJ:
Inflammation, chromosomal instability, and cancer: the
schistosomiasis model. Cancer Res. 54(7 Suppl): 1929s–1933s.
1994.PubMed/NCBI
|
|
19
|
van Riet E, Hartgers FC and Yazdanbakhsh
M: Chronic helminth infections induce immunomodulation:
consequences and mechanisms. Immunobiology. 212:475–490.
2007.PubMed/NCBI
|
|
20
|
Shindo K: Significance of schistosomiasis
japonica in the development of cancer of the large intestine:
Report of a case and review of the literature. Dis Colon Rectum.
19:460–469. 1976. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Long XC, Bahgat M, Chlichlia K, Ruppel A
and Li YL: Detection of inducible nitric oxide synthase in
Schistosoma japonicum and S. mansoni. J Helminthol.
78:47–50. 2004. View Article : Google Scholar : PubMed/NCBI
|