Introduction
Breast cancer is one of the most common types of
cancer with >1,300,000 cases and 450,000 mortalities annually
worldwide (1). Although fatalities
due to breast cancer are decreasing, breast cancer remains the
second leading cause of cancer-related mortality among females
(2,3). Regardless of surgical removal of the
primary tumor, relapse may occur within a few months to >40
years following the presentation of the initial symptoms, and ≥15%
of all patients ultimately develop an incurable form of the disease
(3). This highlights the
requirement for therapies that are able to treat the advanced
stages of the disease. Traditional cytotoxic therapy kills
neoplastic cells by multiple mechanisms, which also effect normal
healthy cells. The non-specificity of these agents produces
undesirable side effects, including short term (myelosuppression)
and long term (cardiomyopathy and acute leukemia) toxicities
(4).
Improvement in the understanding of the molecular
mechanisms involved in cancer has led to the identification of
novel targets and the development of specific therapies, which are
referred to as targeted therapies. Targeted therapies have a high
specificity for the molecules involved in the key molecular events
responsible for the cancer phenotype (5). Among which, the cell cycle is a
promising target involved in cancer growth, as a key characteristic
of human breast cancer and other types of cancer is enhanced and
deregulated cell cycle activity leading to unrestricted cell growth
(6,7).
Medicinal plants have been used worldwide and have
been demonstrated to be a source of effective anticancer agents
(8). Euphorbia tirucalli,
belonging to the family Euphorbiaceae, is a subtropical and
tropical ornamental plant. It has a long history of traditional use
as a remedy for a variety of conditions, including rheumatism,
neuralgia, asthma, catarrh, earache, sarcoma and cancer (9–11).
The tetracyclic triterpene alcohol, euphol, is the main constituent
in the sap of Euphorbia tirucalli and the biological
profiles have evoked investigation to elucidate its potential
properties.
Previous studies have demonstrated that euphol
exerts antiviral effects by inhibiting reverse transcriptase in
purified human immunodeficiency virus type 1 (12) and inhibiting the Epstein-Barr virus
early antigen activation induced by the tumor promoter
12-O-tetradecanoylphorbol-13-acetate (TPA) (13). The anti-inflammatory effects of
euphol have been demonstrated to be associated with the inhibition
of the activation of nuclear factor-κB (14), downregulation of tumor necrosis
factor-α and cyclooxygenase-2 (15), inhibition of the T cell-mediated
immune response (15), and the
reduction of protein kinase C/extracellular signal-regulated
protein kinase signaling activation (16). In addition to the antiviral and
anti-inflammatory effects, the antitumor effects of euphol have
also been observed. Topical application of euphol was demonstrated
to suppress tumor promotion by TPA in mouse skin initiated with
7,12-dimethylbenz(a)anthracene (17). However, the underlying mechanisms
involved in the antitumor effect remain to be elucidated.
In the present study, euphol was observed to inhibit
the expression of cyclin D1, cyclin A, cyclin B1 and
cyclin-dependent kinase 2 (CDK2), decrease the phosphorylation of
retinoblastoma (Rb), induce the expression of cyclin-dependent
kinase inhibitors (CKI) p21 and p27, and lead to the G1 arrest and
proliferation inhibition of T47D cells. These data provide an
insight into the anticancer effects of euphol in breast cancer
cells.
Materials and methods
Drugs and antibodies
Euphol was provided by Dr HB Wang (School of Life
Science and Technology, Tongji University, Shanghai, China) and was
stored at 4°C. Stock solutions of euphol were prepared at a
concentration of 100 mM in dimethyl sulfoxide (DMSO). Further
dilutions were immediately prepared prior to each experiment and
not exceeding 0.3% DMSO (v/v) in the culture media. Control cells
were treated with the same quantity of DMSO as used in the
corresponding treatment. The following primary antibodies were used
for western blot analysis: Human cyclin A, CDK2, Rb, phosphorylated
(p)-Rb (Bioworld Technology Co., Ltd., St. Louis Park, MN, USA);
cyclin B1, cyclin D1, cyclin E, p27 (Santa Cruz Biotechnology,
Inc., Santa Cruz, CA, USA); p21 (Abcam, Cambridge, MA, USA) and
glyceraldehyde 3-phosphate dehydrogenase (Santa Cruz Biotechnology,
Inc.).
Cell lines and culture
The T47D breast cancer cell line was purchased from
Cell Bank Type Culture Collection of Chinese Academy of Sciences
(Shanghai, China). Cells were cultured in RPMI-1640 culture medium
(Thermo Fisher Scientific, Shanghai, China) supplemented with 10%
fetal bovine serum (Biochrom, AG, Berlin, Germany), 100 U/ml
penicillin (Gibco-BRL, Grand Island, NY, USA) and 100 μg/ml
streptomycin (Gibco-BRL) in an incubator at 37°C in a 5%
CO2 humidified atmosphere.
Cell proliferation assay
Cell proliferation was determined by a
methyl-thiazol tetrazolium (MTT) assay. Cells were seeded at a
density of 5×103 cells/well in 96-well plates and
treated with euphol at the indicated concentrations. Following 24,
48 and 72 h incubation, 20 μl sterile MTT (5 mg/ml, Sigma-Aldrich,
St. Louis, MO, USA) were added to each well. Subsequent to
incubation at 37°C for 4 h, 150 μl DMSO was added to solubilize the
MTT-formazan product and mixed thoroughly. The spectrometric
absorbance at 490 nm was measured with an enzyme immunoassay
analyzer (FlexStation 3TM; Molecular Devices, Sunnyvale, CA, USA).
Percentage cell survival was calculated using the optical density
as follows: % cell survival = (absorbance of treated
cells/absorbance of cells with vehicle solvent) × 100. The half
inhibitory concentration (IC50) values, defined as the
concentration of drug that caused 50% inhibition of absorbance
compared with the control cells, were calculated from the
dose-response curve obtained by plotting percentage of cell
survival versus the concentration of euphol.
Flow cytometry analysis for cell cycle
distribution
To determine the effect of euphol on the cell cycle,
fluorescence-activated cell sorting (FACS) analysis was conducted.
Cells (3.5×105, 4.5×105 and
6×105/well) were seeded in 6-well plates and treated
with the indicated euphol concentrations for 24, 48 and 72 h,
respectively. Attached cells were harvested at the indicated time
points, washed in phosphate-buffered saline, fixed in ice-cold
ethanol (75%) and stored at −20°C. For analysis, fixed cells were
stained with propidium iodide (0.05 mg/ml) containing RNase (0.2
mg/ml) for 1 h at room temperature in the dark. FACS analysis was
performed using the Guava EasyCyte™ 8HT (Millipore, Billerica, MA,
USA) and data were analyzed using the FlowJo software program
(Treestar Inc., Ashland, OR, USA). Percentages of cell populations
distributed in the various phases of the cell cycle (sub-G1, G0/G1,
S and G2/M) were calculated.
qPCR
Total RNA was isolated using the RNAiso Plus (Takara
Bio Inc., Shiga, Japan), and the first strand of cDNA synthesis was
performed using the TianScript RT kit (Tiangen Biotech, Co., Ltd.,
Beijing, China) and the Oligo(dT)15 primer from 2 μg
total RNA, according to the manufacturer’s instructions. The mRNA
levels were observed by quantitative PCR with SYBR®
Premix Ex Taq™ II (Takara Biotechnology Co., Ltd., Dalian, China)
and primers, as listed in Table I,
using the Mx3000P qPCR System (Agilent Technologies, Inc., Santa
Clara, CA, USA). The relative expression level of each candidate
gene was calculated by the 2−ΔΔCt method (18), using β-actin as the internal
normalized control with the same calibrator. Each experiment was
performed independently and in triplicate.
 | Table IPrimer sequences of target genes for
qPCR analysis. |
Table I
Primer sequences of target genes for
qPCR analysis.
| Gene | Forward primer | Reverse primer |
|---|
| cyclin A2 |
AGACCTACCTCAAAGCACCACAG |
GGTTGAGGAGAGAAACACCATGA |
| cyclin B1 |
TGGATAATGGTGAATGGACACCAA |
GCCAGGTGCTGCATAACTGGA |
| cyclin D1 |
CTGTGCATCTACACCGACAACTC |
AGGTTCCACTTGAGCTTGTTCAC |
| cyclin E1 |
GCCAGCCTTGGGACAATAATG |
CTTGCACGTTGAGTTTGGGT |
| CDK1 |
TACATTTCCCAAATGGAAACCAG |
AATTCGTTTGGCTGGATCATAGA |
| CDK2 |
TGAAGATGGACGGAGCTTGTTAT |
CTTGGTCACATCCTGGAAGAAAG |
| CDC25 |
ACAGCTCCTCTCGTCATGAGAAC |
GGTCTCTTCAACACTGACCGAGT |
| p21 |
CGATGGAACTTCGACTTTGTCA |
GCACAAGGGTACAAGACAGTG |
| p27 |
GGTTAGCGGAGCAATGCG |
TCCACAGAACCGGCATTTG |
| β-actin |
GACCTGTACGCCAACACAG |
CTCAGGAGGAGCAATGATC |
Western blot analysis
Cells were homogenized with radio
immunoprecipitation assay (RIPA) lysis buffer (Beyotime Biotech,
Jiangsu, China), followed by sonication on Sonicators (Qsonica,
Newton, CT, USA) at a frequency of 20 kHz and centrifugation at
13,800 × g for 10 min at 4°C. Subsequent to this the supernatant
was harvested and the protein concentration measured using the
Pierce BCA protein assay reagent (Pierce, Rockford, IL, USA).
Following this, 20 μg protein was separated on 12% sodium dodecyl
sulfate-polyacrylamide gel electrophoresis and electrotransferred
to a nitrocellulose membrane (Whatman International Ltd., Germany).
The membrane was blocked by incubation in 3% bovine serum albumin,
fraction V (Life Sciences, Carlsbad, CA, USA) in TBST buffer (10 mM
Tris-HCl, pH 7.6, 150 mM NaCl and 0.1% Tween 20) and incubated with
the primary antibodies at 4°C overnight. The blot was washed with
TBST buffer, incubated with the corresponding horseradish
peroxidase-conjugated secondary antibody for 1 h at room
temperature and visualized by ImageQuant LAS 4000mini (GE
Healthcare Life Sciences, Piscataway, NJ, USA).
Statistical analysis
Data were analyzed by a two-tailed Student’s t-test.
Final values are presented as the mean ± standard error. P<0.05
was considered to indicate a statistically significant difference.
P<0.05, P<0.01 and P<0.001 are denoted as *,
** or ***, respectively.
Results
Euphol inhibits cell proliferation
To evaluate the effect of euphol on breast cancer
cell proliferation, the MTT assay was performed in T47D cells, an
aggressive triple-positive breast cancer cell line. Briefly,
exponentially growing T47D cells were treated with 0.01, 0.03, 0.1
and 0.3 mM euphol for 24, 48 and 72 h. With an increased
concentration of euphol, the percentage of viable cells was
significantly decreased, particularly following treatment with 0.03
mM euphol (Fig. 1A and B). The
IC50 values of euphol treatment for 24, 48 and 72 h were 0.26, 0.22
and 0.13 mM, respectively (Fig.
1C). These data suggested that euphol markedly inhibited breast
cancer cell proliferation.
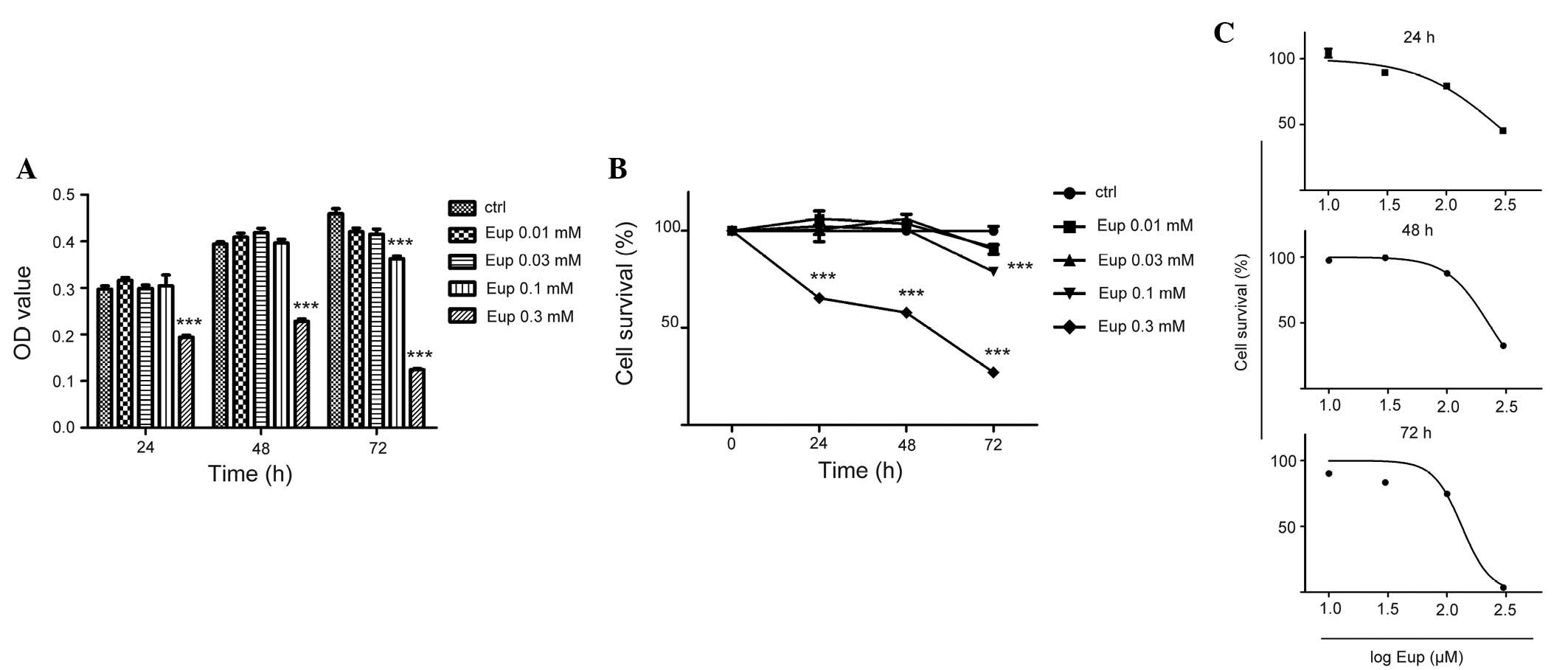 | Figure 1Euphol inhibited the proliferation of
T47D breast cancer cells. T47D cells were treated with euphol at
the indicated concentrations (0.01, 0.03, 0.1 and 0.3 mM) for the
indicated times (24, 48 and 72 h). (A) Cell viability was assessed
by a methylthiazol tetrazolium assay (see Materials and methods).
Columns, mean absorbance at 490 nm of quintuplicate readings from a
representative experiment; bars, ± standard error. (B) Percentage
cell survival was calculated as: % cell survival = (absorbance of
treated cells/absorbance of cells with vehicle solvent) × 100. (C)
The dose-response curve was obtained by plotting the percentage of
cell survival versus the log concentration of euphol used. Points,
mean percentage of cell survival based on quintuplicate assays,
bars, ± SE. ***P<0.001 vs. the control group. OD,
optical density; Eup, euphol; ctrl, control; OD, optical
density. |
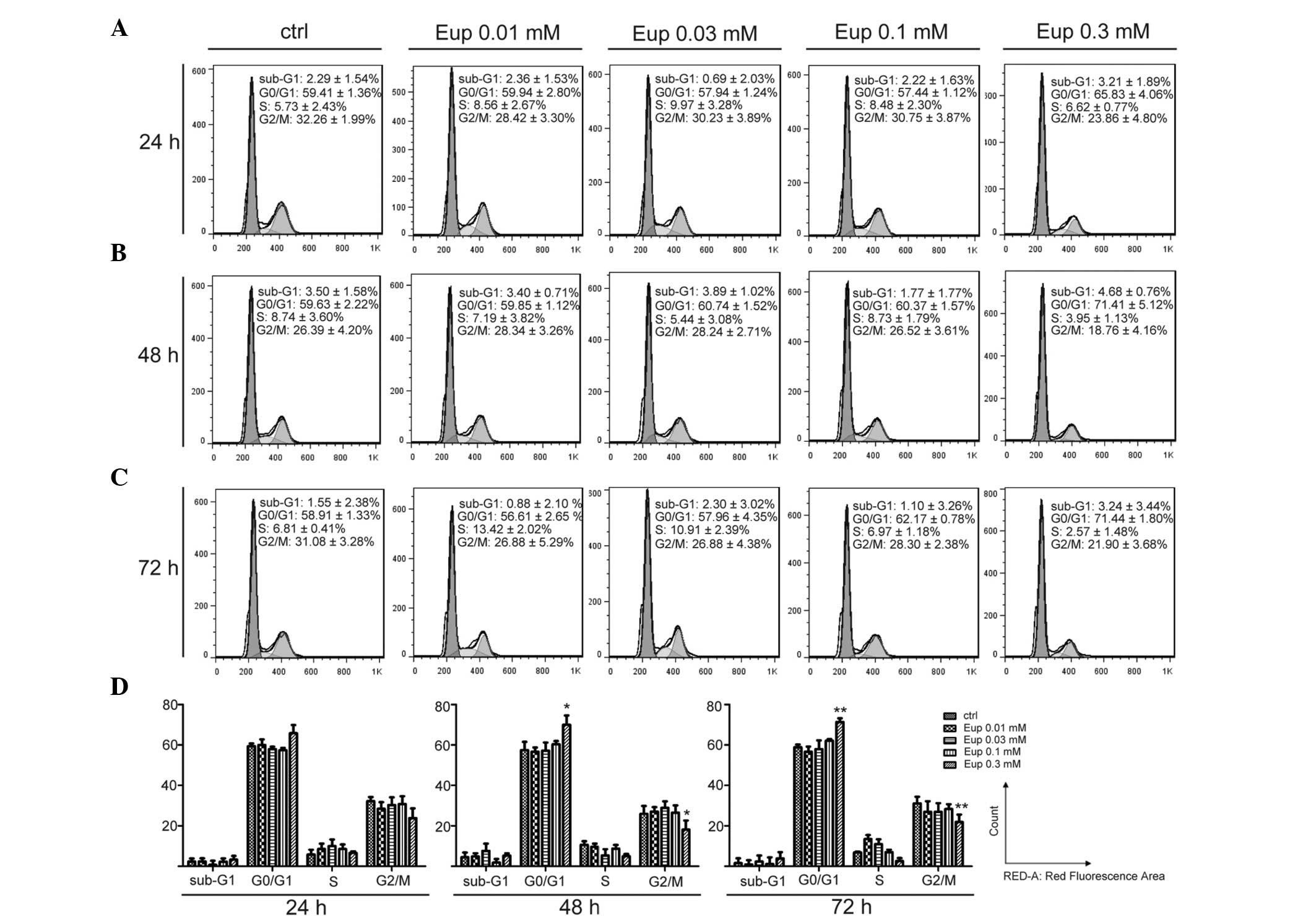
Euphol induces cell cycle arrest
To investigate the functional mechanism of euphol in
inhibiting cell growth, the effect of euphol on the regulation of
the cell cycle in T47D cells was determined. Cells treated with
euphol were stained with PI and analyzed by flow cytometry.
Following 24 h of treatment, stability was observed in all cell
cycle subpopulations, with a slight increase in the G0/G1 and a
decrease in the S and G2/M populations treated with 0.3 mM euphol
(Fig. 2A and D). Following 48 h
incubation, euphol (0.3 mM) caused a redistribution of the cell
cycle resulting in a significant increase of the G0/G1
sub-population from 59.63 to 71.41%, and a concomitant decrease in
the G2/M subpopulation from 26.39 to 18.76% (Fig. 2B and D). At 72 h, a significant
increase remained in the G0/G1 population to the detriment of the S
and G2/M subpopulations in 0.3 mM euphol treatment (G0/G1: Control,
58.91%, 0.3 mM euphol, 71.44%; S: Control, 6.81%, 0.3 mM euphol,
2.57%; G2/M: Control, 31.08%, 0.3 mM euphol, 21.90%) (Fig. 2C and D). Notably, no significant
changes were observed in the sub-G1 subpopulation at any indicated
concentration or time point. The level remained low at 3.21, 4.68,
and 3.24% for 24, 48 and 72 h at 0.3 mM euphol, respectively
(Fig. 2A, 2B and 2C). These
results indicated that euphol may inhibit cell proliferation by
arresting the cells at the G0/G1 phase and preventing them from
entering the S phase.
Euphol regulates transcription levels of
cell cycle-associated genes
To elucidate whether the genes involved in the cell
cycle redistribution were induced by euphol, the mRNA levels of
cell cycle-associated genes were observed following treatment with
euphol. Genes selected for analysis included cyclin A2, B1, D1 and
E, cyclin-dependent kinase 1 (CDK1), CDK2, cell division cycle 25
and p21 and p27 (CKIs) The results of the qPCR analysis showed that
incubation with euphol (0.3 mM), for 24, 48 and 72 h, significantly
downregulated the mRNA levels of cyclin A2 and B1 (Fig. 3A). Furthermore, the mRNA levels of
CDK1 and CDK2 were significantly reduced following treatment with
euphol (0.03 mM) for 48 h (Fig.
3B). The mRNA levels of p21 were also significantly increased
following treatment with euphol (0.03 mM) for 48 h (Fig. 3C).
 | Figure 3Downregulation of the expression of
cell cycle genes in T47D cells. T47D cells were treated with euphol
at the indicated concentrations (0.01, 0.03, 0.1 and 0.3 mM) for
the indicated times (24, 48, and 72 h). The mRNA levels of (A)
cyclins, (B) CDK and (C) CKIs were examined by qPCR. β-actin was
used as an internal normalization control. Columns, mean of
triplicate experiments; bars, ± SE. CDK, cyclin-dependent kinase;
CKI, CDK inhibitor; ctrl, control; Eup, euphol. |
Euphol regulates expression of cell
cycle-associated proteins
To identify the proteins involved in euphol-induced
cell cycle arrest, the effect of euphol on the protein levels
involved in the cell cycle redistribution were determined.
Whole-cell lysates were prepared following treatment with euphol
for 48 or 72 h, and the expression levels of cyclin A, B1, D1 and
E, as well as CDK2, p21, p27, Rb and p-Rb, were detected by western
blot analysis (Fig. 4). Incubation
with euphol (0.03 mM) for 48 h or 72 h reduced the expression of
cyclin A, B1 and D; however, it increased the expression of p21 and
p27. The expression of CDK2 decreased following treatment with
euphol (0.03 mM) for 48 h. Furthermore, the level of p-Rb was
decreased in response to euphol (0.03 mM) compared with that of the
control cells despite the fact that the expression level of Rb was
not significantly different between the two. These results
suggested that the transcriptional downregulation of certain
cyclins and the upregulation of p21 and p27 by euphol may have led
to cell cycle arrest, and ultimately resulted in growth
inhibition.
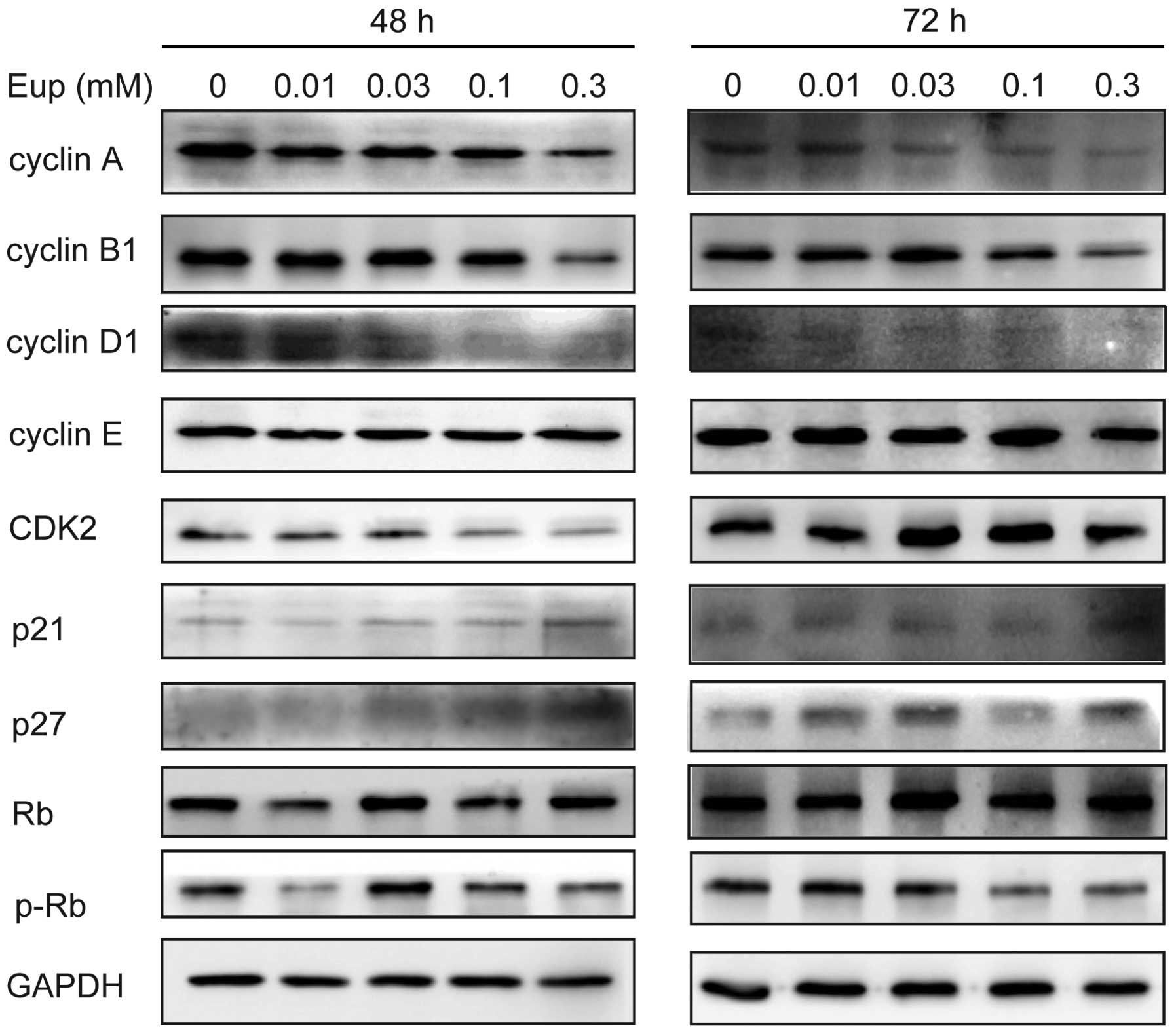 | Figure 4Euphol regulated the expression of
cell cycle proteins in T47D cells. Cell lysates were analyzed by
western blotting using specific antibodies to cyclin A, B1, D1 and
E, as well as to CDK2, p21, p27, Rb and p-Rb protein. Protein
loading was normalized based on GAPDH. Eup, euphol; CDK,
cyclin-dependent kinase; p-Rb, phosphorylated-Rb protein; GAPDH,
glyceraldehyde 3-phosphate dehydrogenase. |
Discussion
Euphol is a tetracyclic triterpene alcohol isolated
from the sap of Euphorbia tirucalli. It has been
demonstrated to possess a wide variety of biological properties,
including antimicrobial, anti-inflammatory and immunosuppressive
actions. Yasukawa et al also demonstrated the involvement of
euphol in the suppression of skin tumor formation (17). However, the exact mechanisms
underlying the antitumor effects of euphol have not been fully
elucidated. This study investigated the cytostatic effects of
euphol on T47D breast cancer cells. According to the results,
euphol induced cell cycle arrest at the G0/G1 phase, which resulted
in growth inhibition. The arrest may have been mediated by the
reduction of cyclin D1 and CDK2, as well as the induction of the
CDK inhibitors p21 and p27, followed by the hypophosphorylation of
Rb. Decreased cyclin A and B1 levels correlated with the lower cell
counts in the S and G2/M phases.
Cyclin D1 is a member of a family of three closely
associated D-type cyclins, D1, D2 and D3, which phosphorylate and
inactivate Rb protein and promote progression through the G1-S
phase of the cell cycle (19).
High levels of cyclin D1 have been detected in numerous breast
cancer cell lines through DNA amplification and/or cyclin D1
overexpression (20). This
abnormality has been demonstrated to be important in tumorigenesis
and to confer a poor prognosis in breast cancer (21,22).
In contrast to cyclin D1, cyclin D2 and D3 are not associated with
breast tumor formation (4).
Activation of the cyclin E-CDK2 complex is another essential
requirement for the G1/S transition (23). As observed in this study, euphol
exerted proliferation inhibitory effects on T47D cells by leading
to their arrest at the G0/G1 phase. When the cell cycle phase
distributions are compared with alterations in the cell cycle
regulatory molecules, a decrease in Cyclin D1 levels may be
attributed as a cause of euphol-induced G0/G1 arrest as well as the
inhibition of CDK2. Although the cyclin E level was unchanged,
decreased CDK2 may also imply an inhibition of cyclin E and cyclin
E-CDK2, as the decrease of cyclins or CDKs constrains the formation
of active cyclin-CDK complexes. Consistent with the decreased level
of cyclin D1 and CDK2 expression induced by euphol, the p-Rb level
also decreased, which is probably due to the deficiency of
cyclin-CDK complexes in the euphol treated T47D cells. However, the
possibility that the two reductions are attributed to the
euphol-induced upregulation of CKIs was not excluded.
The predominant CKIs include p21 and p27 of the
Cip/Kip family, which inactivate CDK-cyclin complexes (4,23).
The increased expression of p21 and p27 by euphol is noteworthy as
the development of breast cancer has been associated with a
decrease in these CKIs (4). The
abundance of p27 is regulated primarily at the post-translational
level as demonstrated in the growth factor-mediated reduction in
p27 protein levels, primarily through enhanced ubiquitin-mediated
degradation (24). Consistent with
this study, the transcriptional level of p27 was relatively
unchanged based on the qPCR analysis; however, a significant
increase was observed in its expression level following euphol
treatment. The mechanism of this post-transcriptional regulation of
p27 may involve reduced ubiquitination, but this requires further
validation. Euphol marginally increased p21 at the transcriptional
and post-transcriptional levels compared with that of the untreated
control cells. This upregulation may involve a p53-independent
pathway, as point mutations in the core DNA binding region and
transactivation deficient p53 in the T47D cell line have been
demonstrated (25).
Cyclin A is required for the onset of DNA
replication in mammalian cells (26) and is rate limiting for the G1/S
transition (27). Cylin B is a
mitotic cyclin that accumulates at the G2/M transition and acts
synergistically with cyclin A during the initiation of mitosis
(28). In correlation with the
lower abundance of cells in the S and G2/M subpopulations, mRNA and
protein levels of cyclin A and B1 were downregulated by euphol
treatment. This is similar to a study demonstrating that decreased
levels of cyclin A and B correlated with the decreased levels of
cells in the S and G2 phases, and increased levels of cells in the
G1 phase, in non-small cell lung cancer (29).
The sub-G1 peak observed by FACS analysis was due to
the deficient DNA content in apoptotic cells. Identification of the
sub-G1 subpopulation may be used as an index of apoptotic cells in
the cell cycle analysis (30),
although the lack of changes in the sub-G1 population may also be
due to the loss of severely damaged cells during the washing
process. In this study, the sub-G1 subpopulation remained at a low
level, indicating almost negligible apoptosis in T47D cells treated
with euphol. This is concordant with a study demonstrating that
T47D cells among 8 human breast cancer cell lines of the National
Cancer Institute anticancer drug screen were resistant to
apoptosis, and in which no apoptosis was observed with
7-hydroxystaurosporine (UCN-01) and minimal apoptosis was
identified with camptothecin. This resistance to apoptosis was
considered to be determined by the MDM-2/p53 ratio and to be
p53-independent (31). The
detected minimal apoptosis induced by euphol in T47D cells may be
explained by this mechanism. In addition, the extent to which the
MDM2/p53 ratio contributed to apoptosis resistance requires further
elucidation.
The current study indicated important features of
euphol concerning its capacity to target the cell cycle, thus,
euphol may be a potential cancer chemotherapy agent. The underlying
mechanism of action involved the reduction of cyclin D1, A and B1
levels, downregulation of CDK2, hypophosphorylation of Rb and the
induction of CKIs p21 and p27. Although this study has demonstrated
the euphol-induced regulation of the cell cycle molecules in T47D
cells, further investigation regarding combination therapy leading
to lower doses of therapeutic agents and observations of other
cancer cell lines are required.
Acknowledgements
This study was supported by funding from the
Ministry of Science and Technology (grant nos. 2011CB965100,
2011CBA01100, 2011DFA30480, 2010CB944900, 2010CB945000 and
2012CB966603); the National Natural Science Foundation of China
(grant nos. 91219305, 31101061, 31210103905, 31071306, 31000378,
31171432 and 81170499); the Science and Technology Commission of
Shanghai Municipality (grant nos. 11ZR1438500 and 11XD1405300); and
the Ministry of Education (grant nos. IRT1168 and 20110072110039).
The study was also supported by the Chen Guang project supported by
Shanghai Municipal Education Commission, Shanghai Education
Development Foundation (grant no. 12CG19) and the Fundamental
Research Funds for the Central Universities.
References
|
1
|
Cancer Genome Atlas Network. Comprehensive
molecular portraits of human breast tumours. Nature. 490:61–70.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
DeSantis C, Siegel R, Bandi P and Jemal A:
Breast cancer statistics, 2011. CA Cancer J Clin. 61:409–418. 2011.
View Article : Google Scholar
|
|
3
|
de Bono JS, Tolcher AW and Rowinsky EK:
The future of cytotoxic therapy: selective cytotoxicity based on
biology is the key. Breast Cancer Res. 5:154–159. 2003.PubMed/NCBI
|
|
4
|
Zafonte BT, Hulit J, Amanatullah DF,
Albanese C, Wang C, Rosen E, Reutens A, Sparano JA, Lisanti MP and
Pestell RG: Cell-cycle dysregulation in breast cancer: breast
cancer therapies targeting the cell cycle. Front Biosci.
5:D938–D961. 2000. View
Article : Google Scholar : PubMed/NCBI
|
|
5
|
Munagala R, Aqil F and Gupta RC: Promising
molecular targeted therapies in breast cancer. Indian J Pharmacol.
43:236–245. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Malumbres M and Barbacid M: Cell cycle,
CDKs and cancer: a changing paradigm. Nat Rev Cancer. 9:153–166.
2009. View
Article : Google Scholar : PubMed/NCBI
|
|
7
|
Schwartz GK and Shah MA: Targeting the
cell cycle: a new approach to cancer therapy. J Clin Oncol.
23:9408–9421. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Chin YW, Balunas MJ, Chai HB and Kinghorn
AD: Drug discovery from natural sources. AAPS J. 8:E239–E253.
2006.PubMed/NCBI
|
|
9
|
Rizk AM, Hammouda FM, El-Missiry MM,
Radwan HM and Evans FJ: Biologically active diterpene esters from
euphorbia peplus. Phytochemistry. 24:1605–1606. 1985. View Article : Google Scholar
|
|
10
|
Rasool N, Khan AQ and Malik A: A
taraxerane type triterpene from Euphorbia tirucalli.
Phytochemistry. 28:1193–1195. 1989. View Article : Google Scholar
|
|
11
|
de Melo JG, Santos AG, de Amorim EL, do
Nascimento SC and de Albuquerque UP: Medicinal plants used as
antitumor agents in Brazil: an ethnobotanical approach. Evid Based
Complement Alternat Med. 2011:3653592011.PubMed/NCBI
|
|
12
|
Akihisa T, Ogihara J, Kato J, Yasukawa K,
Ukiya M, Yamanouchi S and Oishi K: Inhibitory effects of
triterpenoids and sterols on human immunodeficiency virus-1 reverse
transcriptase. Lipids. 36:507–512. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Akihisa T, Kithsiri Wijeratne EM, Tokuda
H, Enjo F, Toriumi M, Kimura Y, Koike K, Nikaido T, Tezuka Y and
Nishino H: Eupha-7,9(11),24-trien-3beta-ol (‘antiquol C’) and other
triterpenes from Euphorbia antiquorum latex and their inhibitory
effects on Epstein-Barr virus activation. J Nat Prod. 65:158–162.
2002.PubMed/NCBI
|
|
14
|
Dutra RC, Claudino RF, Bento AF, Marcon R,
Schmidt EC, Bouzon ZL, Pianowski LF and Calixto JB: Preventive and
therapeutic euphol treatment attenuates experimental colitis in
mice. PLoS One. 6:e271222011. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Dutra RC, de Souza PR, Bento AF, Marcon R,
Bicca MA, Pianowski LF and Calixto JB: Euphol prevents experimental
autoimmune encephalomyelitis in mice: evidence for the underlying
mechanisms. Biochem Pharmacol. 83:531–542. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Passos GF, Medeiros R, Marcon R,
Nascimento AF, Calixto JB and Pianowski LF: The role of PKC/ERK1/2
signaling in the anti-inflammatory effect of tetracyclic triterpene
euphol on TPA-induced skin inflammation in mice. Eur J Pharmacol.
698:413–420. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Yasukawa K, Akihisa T, Yoshida ZY and
Takido M: Inhibitory effect of euphol, a triterpene alcohol from
the roots of Euphorbia kansui, on tumour promotion by
12-O-tetradecanoylphorbol-13-acetate in two-stage carcinogenesis in
mouse skin. J Pharm Pharmacol. 52:119–124. 2000. View Article : Google Scholar
|
|
18
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(−Delta Delta C(T)) Method. Methods. 25:402–408. 2001.
|
|
19
|
O’Connor PM, Jackman J, Bae I, Myers TG,
Fan S, Mutoh M, Scudiero DA, Monks A, Sausville EA, Weinstein JN,
et al: Characterization of the p53 tumor suppressor pathway in cell
lines of the National Cancer Institute anticancer drug screen and
correlations with the growth-inhibitory potency of 123 anticancer
agents. Cancer Res. 57:4285–4300. 1997.
|
|
20
|
Gillett C, Fantl V, Smith R, Fisher C,
Bartek J, Dickson C, Barnes D and Peters G: Amplification and
overexpression of cyclin D1 in breast cancer detected by
immunohistochemical staining. Cancer Res. 54:1812–1817.
1994.PubMed/NCBI
|
|
21
|
Zukerberg LR, Yang WI, Gadd M, Thor AD,
Koerner FC, Schmidt EV and Arnold A: Cyclin D1 (PRAD1) protein
expression in breast cancer: approximately one-third of
infiltrating mammary carcinomas show overexpression of the cyclin
D1 oncogene. Mod Pathol. 8:560–567. 1995.
|
|
22
|
Lin SY, Xia W, Wang JC, Kwong KY, Spohn B,
Wen Y, Pestell RG and Hung MC: Beta-catenin, a novel prognostic
marker for breast cancer: its roles in cyclin D1 expression and
cancer progression. Proc Natl Acad Sci USA. 97:4262–4266. 2000.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Vermeulen K, Van Bockstaele DR and
Berneman ZN: The cell cycle: a review of regulation, deregulation
and therapeutic targets in cancer. Cell Prolif. 36:131–149. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Pagano M, Tam SW, Theodoras AM,
Beer-Romero P, Del Sal G, Chau V, Yew PR, Draetta GF and Rolfe M:
Role of the ubiquitin-proteasome pathway in regulating abundance of
the cyclin-dependent kinase inhibitor p27. Science. 269:682–685.
1995. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Nigro JM, Baker SJ, Preisinger AC, Jessup
JM, Hostetter R, Cleary K, Bigner SH, Davidson N, Baylin S, Devilee
P, et al: Mutations in the p53 gene occur in diverse human tumour
types. Nature. 342:705–708. 1989. View
Article : Google Scholar : PubMed/NCBI
|
|
26
|
Girard F, Strausfeld U, Fernandez A and
Lamb NJ: Cyclin A is required for the onset of DNA replication in
mammalian fibroblasts. Cell. 67:1169–1179. 1991. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Resnitzky D, Hengst L and Reed SI: Cyclin
A-associated kinase activity is rate limiting for entrance into S
phase and is negatively regulated in G1 by p27Kip1. Mol Cell Biol.
15:4347–4352. 1995.PubMed/NCBI
|
|
28
|
Knoblich JA and Lehner CF: Synergistic
action of Drosophila cyclins A and B during the G2-M transition.
EMBO J. 12:65–74. 1993.PubMed/NCBI
|
|
29
|
Li H, Sun L, Tang Z, Fu L, Xu Y, Li Z, Luo
W, Qiu X and Wang E: Overexpression of TRIM24 correlates with tumor
progression in non-small cell lung cancer. PLoS One. 7:e376572012.
View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Darzynkiewicz Z, Bruno S, Del Bino G,
Gorczyca W, Hotz MA, Lassota P and Traganos F: Features of
apoptotic cells measured by flow cytometry. Cytometry. 13:795–808.
1992. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Nieves-Neira W and Pommier Y: Apoptotic
response to camptothecin and 7-hydroxystaurosporine (UCN-01) in the
8 human breast cancer cell lines of the NCI Anticancer Drug Screen:
multifactorial relationships with topoisomerase I, protein kinase
C, Bcl-2, p53, MDM-2 and caspase pathways. Int J Cancer.
82:396–404. 1999. View Article : Google Scholar
|