Introduction
Retinal hypoxia and oxidative stress lead to retinal
ganglion cell (RGC) apoptosis, which triggers irreversible neuronal
injury and visual impairment in a number of sight-threatening
disorders, including central retinal vessel occlusion, ischemic
central retinal vein thrombosis, diabetic retinopathy (DR) and a
number of types of glaucoma. The retinal ganglion cell line (RGC-5)
is a widely used cell line which has been characterized as
expressing RGC markers and exhibiting ganglion cell-like behavior
in culture (1). RGC-5 cells were
employed in the current study in an effort to understand the role
of ginsenoside Rb1 (Rb1) in apoptosis induced by hypoxia and
oxidative stress. During hypoxia, production of vascular
endothelial growth factor, nitrogen monoxidum, glutamate
excitotoxicity, reactive oxygen species, inflammatory cytokines and
increased accumulation of intracellular Ca2+ eventually
lead to RGC death (2). Oxidative
stress also plays a role in RGC damage (3,4). By
inducing RGC apoptosis, hypoxia and oxidative stress are important
factors involved in DR. Thus, improving the anti-apoptosis ability
of RGC has been significant in the development of therapies aimed
at reducing blindness from retinal visual loss.
Apoptosis or programmed cell death, occurs during
normal development and when cells are exposed to specific
cell-damaging stimuli, including glutamate, serum withdrawal and
hypoxia. The caspase-cascade system plays vital roles in the
induction, transduction and amplification of intracellular
apoptotic signals (5). Markers of
apoptosis include DNA fragmentation and expression of cleaved
caspases. Caspase-3, which is associated with neuronal death, is a
caspase protein that interacts with caspase-8 and −9. Caspase-3 is
activated in the apoptotic cell by the death receptor and
mitochondrial pathways, which is associated with caspase-8 and −9,
respectively (6,7). Caspase-3 is processed following the
activation of caspases-8 and −9. As an executioner caspase, the
caspase-3 zymogen has essentially zero activity until it is cleaved
by an initiator caspase during apoptosis (8). Therefore, caspases exist as inactive
proenzymes and change to cleaved caspase as an active enzyme under
apoptotic stimulus.
Rb1 (C54H92O23,
molecular weight 1,108), which is the main ginsenoside that is
extracted from ginseng, the root of Panax Ginseng C. A.
Meyer, family Araliacea), is used as a traditional medicine in
Asian countries. Rb1 has been observed to show specific biological
activities protecting neuronal or non-neuronal cells from hypoxic
damage. For example, the anti-hypoxic effect of Rb1 on neonatal rat
cardiomyocytes was mediated through the specific activation of
glucose transporter-4 ex vivo(9). It was reported that Rb1-mediated
neuronal recovery following hypoxic damage was involved in
calcium-independent calmodulin-dependent protein kinases II (CaMK
II)activity (10). It was also
shown that Rb1 protected cardiomyocytes against cobalt chloride
(CoCl2)-induced apoptosis in neonatal rats by inhibiting
the mitochondria permeability transition pore opening (11). However, few studies exist with
regard to whether Rb1 prevents the apoptosis of RGC under hypoxia
and oxidative stress. In present study, Rb1 was investigated to
determine whether it relieves RGC apoptosis induced by hypoxia and
oxidative stress in vitro.
Materials and methods
Cell culture
The transformed RGC-5 (PTA6600, ATCC), developed
from postnatal Sprague-Dawley rats, was grown in Dulbecco’s
modified Eagle’s medium F12, supplemented with 10% fetal bovine
serum (HyClone, Logan, UT, USA; SH30070.03 U.S. Origin), 100 U/ml
penicillin and 100 Ag/ml streptomycin. RGC-5 cells were cultured in
an incubator with 5% CO2 at 37°C. Upon confluency,
cultures were passaged by dissociation in 0.05% (w/v) trypsin in
0.01 mol/l PBS. The cells were trypsinized and subcultured in 25 or
100 cm2 culture flasks according to the appropriate
assay conditions. Cells were used at 50% confluency.
Hypoxic and oxidative stress injury to
RGC-5 cells
CoCl2 (Sigma-Aldrich, St. Louis, MO,
USA), a hypoxia mimic and H2O2 (Merck KGaA,
Darmstadt, Germany), which triggered cellular oxidative stress,
were dissolved in distilled H2O and sterilized through a
0.2 μm filter and added in the medium at a series of
concentrations. RGC-5 cells were incubated with different
concentrations of CoCl2 or H2O2
for 24 and 48 h. The apoptotic rates of RGC-5 cells treated with 0,
100, 200, 300, 400, 500, 600 and 700 μmol/l CoCl2 or 0,
100, 200, 300, 400, 500, 600, 700, 800, 900 and 1,000 μmol/l
H2O2 were analyzed by flow cytometry. The
total apoptotic cells were counted to construct a dose response
curve and to identify the adequate concentration to induce
apoptosis. Three independent experiments were performed.
Analysis of effective dose of Rb1 on
RGC-5 cells apoptosis
Rb1 (Shanghai Tauto Biotech Co., Ltd., Shanghai,
China; 41753439, >97%) was dissolved in DMSO and stored at −20°C
until use. The final concentration of DMSO in culture media was
0.1%. To determine the effective dose of Rb1 on RGC-5 cell
apoptosis, cells were cultured with 0.01, 0.1, 1, 10 and 100 μmol/l
Rb1 for 24 h, then cultured with 400 μmol/l CoCl2 for 48
h or 600 μmol/l H2O2 for 24 h and results
were analyzed by flow cytometry. The percentage of apoptotic
inhibition was a ratio of the total apoptotic cells in the Rb1
groups to that in the CoCl2 or
H2O2 group. Three independent experiments
were performed.
Inverted microscopy study
RGC-5 cells were preincubated with Rb1 for 24 h in
25-cm2 culture dishes and exposed to CoCl2
(400 μmol/l) or H2O2 (600 μmol/l) for a
further 24 or 48 h. The morphological changes were observed under
an inverted microscope (Nikon TS100, Tokyo, Japan). Images were
captured randomly using an Olympus digital camera (Olympus, Tokyo,
Japan). Five non-overlapping fields of view were randomly captured
per dish.
Annexin V-fluorescein isothiocyanate
(FITC)/propidium iodide (PI) assay
Annexin V is a Ca2+-dependent
phospholipid-binding protein with a high affinity for
phosphatidylserine, translocated from the inner leaflet of the
plasma membrane to the outer leaflet in apoptotic cells (12). By staining cells with a combination
of fluorescein Annexin V-FITC and PI, it was possible to
distinguish and quantitatively analyze non-apoptotic cells [Annexin
V-FITC(−)/PI(−)], early apoptotic cells [Annexin V-FITC(+)/PI(−)],
late apoptotic/necrotic cells [Annexin V-FITC(+)/PI(+)] and dead
cells [Annexin V-FITC(−)/PI(+)] using flow cytometry (13). The percentage of cells actively
undergoing apoptosis was determined by flow cytometry using the
Annexin V-FITC apoptosis detection kit (KGA-108; Nanjing KeyGen
Biotech Co., Nanjing, China) according to the manufacturer’s
instructions. Following treatment, the cells were trypsinized and
centrifuged for 5 min at 671 × g. The supernatant was removed and
500 μl of binding buffer, 5 μl Annexin V-FITC and 5 μl PI were
added. Following mixing of the agent, the cells were incubated for
15 min in the dark, at room temperature. The fluorescence of 20,000
events per sample was analyzed using a flow cytometer (BD
FACScalibur; BD Biosciences, San Jose, CA, USA). The percentage of
apoptosis was determined from the number of Annexin V(+)/PI(−)
cells relative to the number of Annexin V(+)/PI(+) cells.
Western blot analysis for caspase protein
expression
For extraction of whole cellular proteins, RGC-5
cells cultured under different conditions were washed twice with
ice-cold PBS and lysed with ice-cold cell lysis buffer (P0013;
Beyotime Institute of Biotechnology, Haimen, China) and
supplemented with a protease inhibitor cocktail (Merck KGaA) on ice
for 30 min. Following cell shaving, the cell homogenates were
collected and centrifuged at 12,000 × g for 15 min at 4°C.
Supernatants were collected and quantified using the BCA protein
assay kit (P0012; Beyotime Institute of Biotechnology). Protein
samples (80 μg) were separated by 10% SDS-PAGE and transferred to
polyvinylidene fluoride membranes (Millipore, Billerica, MA, USA)
and blocked with 5% BSA at room temperature for 2 h. Immunoblots
were incubated at 4°C overnight with primary antibodies specific
for caspase-3 (1:1,000; Cell Signaling Technology, Inc., Danvers,
MA, USA), caspase-8 (1:1,000; Cell Signaling Technology) and
caspase-9 (1:1,000; Cell Signaling Technology). Detection of
β-actin (Boster, China) was used as a loading control.
Statistical analysis
Experiments were repeated at least three times.
Values are expressed as mean ± standard error. Data were evaluated
for statistical significance by analysis using one-way analysis of
variance. P<0.05 was considered to indicate a statistically
significant difference. All tests were performed using the
statistical package SPSS software version 13.0 (SPSS, Inc.,
Chicago, IL, USA).
Results
CoCl2- or
H2O2-induced injury of RGC-5 cells
The apoptotic rate rapidly increased as the
CoCl2 concentrations and incubation time increased
(Fig 1A). Cells incubated with
CoCl2 for 24 h had a decreased effect, as shown by the
persistently low apoptotic rates in the progressive concentrations,
while 48 h of incubation induced higher rates of cellular
apoptosis. When incubated with CoCl2 for 48 h, the cells
showed a large transition in apoptotic rates at 400 μmol/l and the
apoptotic rates increased from 24.5 to 74.8% between 400 and 700
μmol/l. In the case of H2O2, when cells were
incubated with H2O2 for 24 h, a large
transition in apoptotic rates at 600 μmol/l was observed and the
apoptotic rates increased from 21.6 to 62.4% between 600 and 1,000
μmol/l. However, this type of transition was not observed when
cells were incubated for 48 h (Fig.
1B). Thus, 400 μmol/l CoCl2 for 48 h or 600 μmol/l
H2O2 for 24 h treatments were considered as
the early stage of apoptosis.
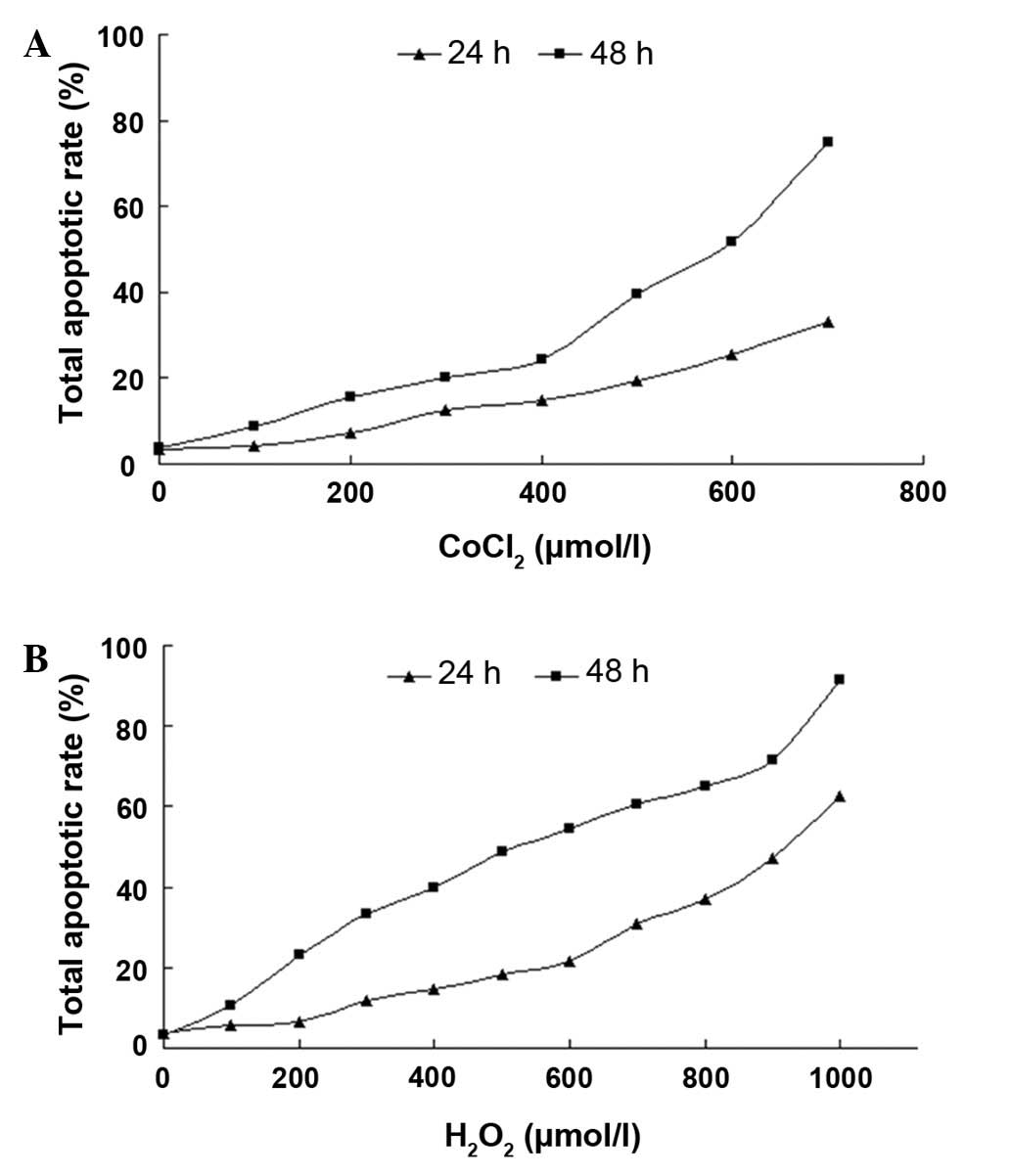 | Figure 1Total apoptotic rate analysis of RGC-5
cells with CoCl2 or H2O2
treatment. Cells were incubated with various concentrations of
CoCl2 (0, 100, 200, 300, 400, 500, 600 and 700 μmol/l)
or H2O2 (0, 100, 200, 300, 400, 500, 600,
700, 800, 900 and 1,000 μmol/l) for 24 and 48 h, respectively. The
cell apoptotic rate at each concentration and time point was
assayed using a flow cytometry assay; n=3. RGC-5, retinal ganglion
cell line; CoCl2, cobalt chloride. |
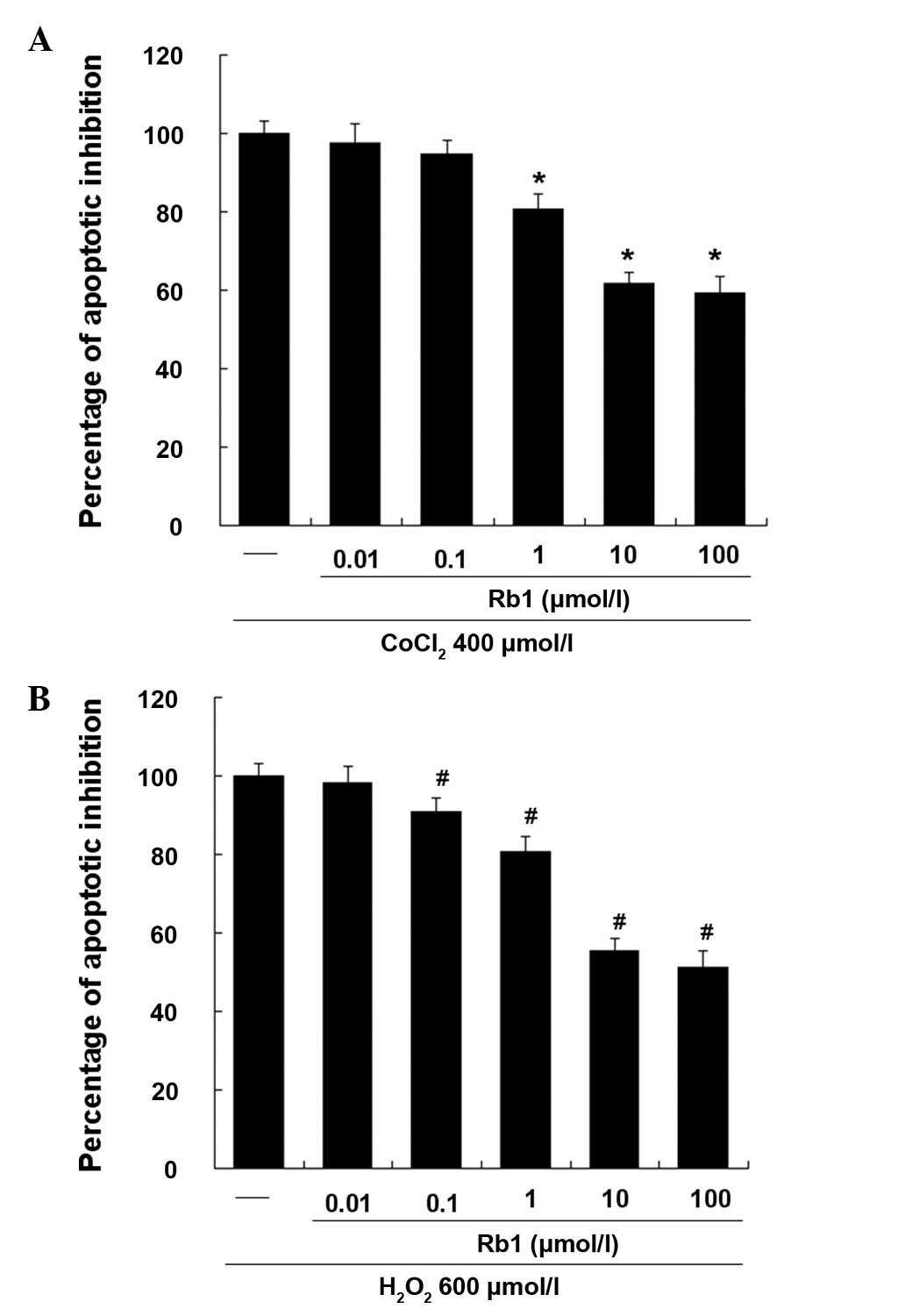
Effective dose of Rb1 on RGC-5 cells
induced by CoCl2 or H2O2
As shown in Fig. 2,
the effect of Rb1 on RGC-5 apoptosis induced by CoCl2 or
H2O2 was investigated by flow cytometry. DMSO
(0.1%), treated as a vehicle group, showed no significant effect on
CoCl2- or H2O2-induced apotosis
(data not shown). Rb1 dose-dependently inhibited the apoptosis of
RGC-5 cells and showed ~50% inhibition of cell apoptosis at 10
μmol/l, while 100 μmol/l showed no significant additive effect.
Therefore, in subsequent experiments, 10 μmol/l of Rb1 was used as
the final effective dose.
Effect of Rb1 on CoCl2-induced
apoptosis in RGC-5 cells
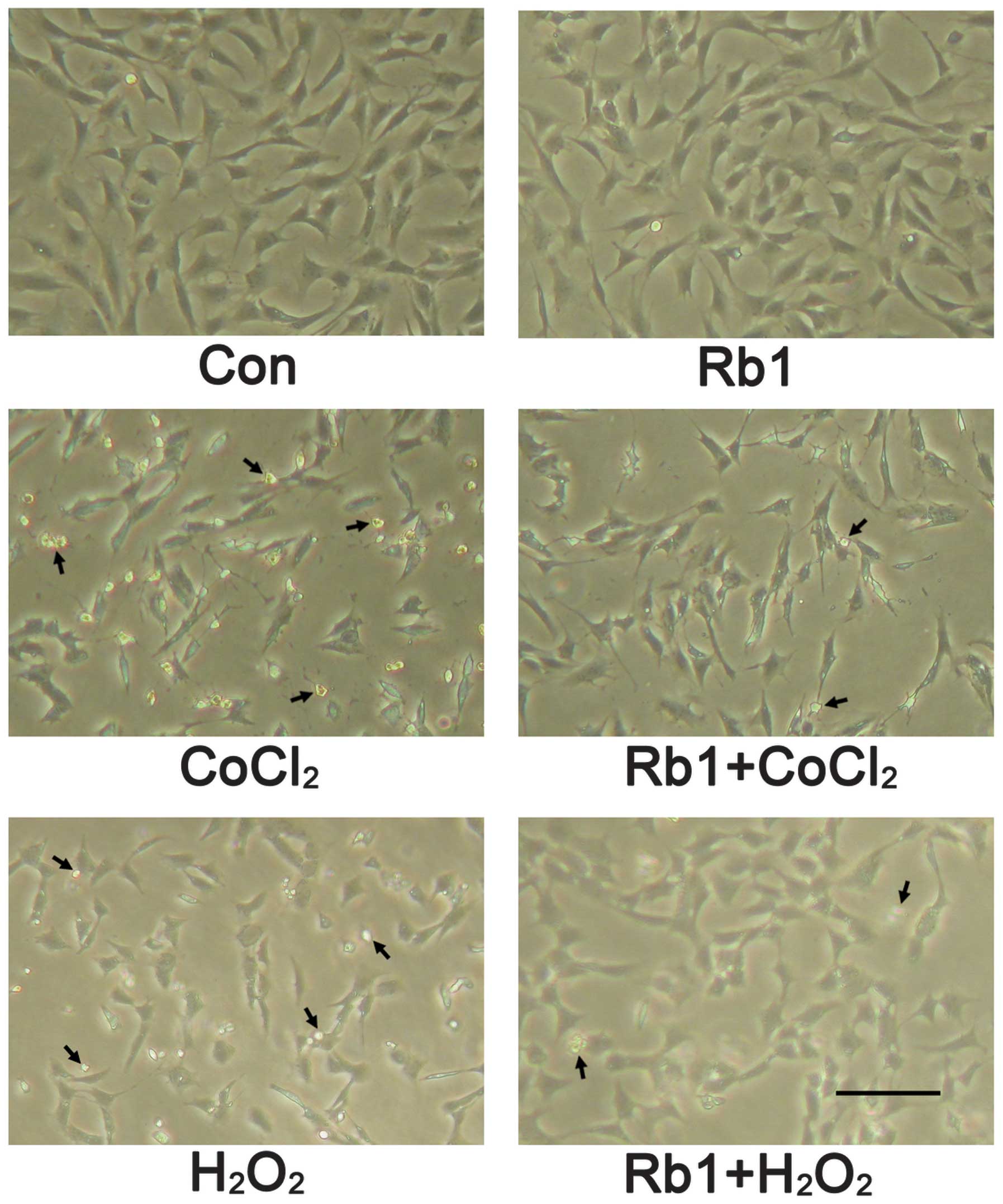
The morphological characterization of RGC-5 cells
showed that cells grew as a monolayer and exhibited axonal
processes (Fig. 3). Incubation
with CoCl2 (400 μmol/l) for 48 h induced morphological
changes of RGC-5 cells as shown by neurite retraction, cell body
area reduction, condensation of the nucleus and cytoplasm and
formation of cell fragments. However, these types of morphological
changes were prevented in the pretreatment of the Rb1 group. In the
case of apoptosis analysis, the effect of Rb1 on RGC-5 cells was
quantified by flow cytometry with Annexin V/PI double staining
(Fig. 4A). The total apoptotic
rate (which was the sum of early and late apoptotic cells) was
significantly increased in the CoCl2-treated group
compared with that of the control (CoCl2, 24.50±1.25%
vs. control, 3.97±0.91%; P<0.01). However, in the Rb1-pretreated
CoCl2 group, the total apoptotic rate was significantly
decreased (Rb1-pretreated CoCl2, 15.12±1.20% vs.
CoCl2, 24.50±1.25%; P<0.05). Treatment of Rb1 alone
did not affect the apoptotic rate compared with that of the control
(Rb1, 3.06±1.34% vs. control, 3.97±0.91%; P>0.05) (Fig. 4B). The percentage of inhibition
ratio by quantitative analysis showed that the inhibition ratio of
Rb1 against CoCl2-induced apoptosis was 58.49±3.11% in
the early apoptotic cells, 20.79±3.38% in the late apoptotic cells
and 38.29±3.45% in the total apoptotic cells (Fig. 4C). The protective effect of Rb1 in
RGC-5 cell injury induced by CoCl2 was exerted primarily
against early apoptotic cells.
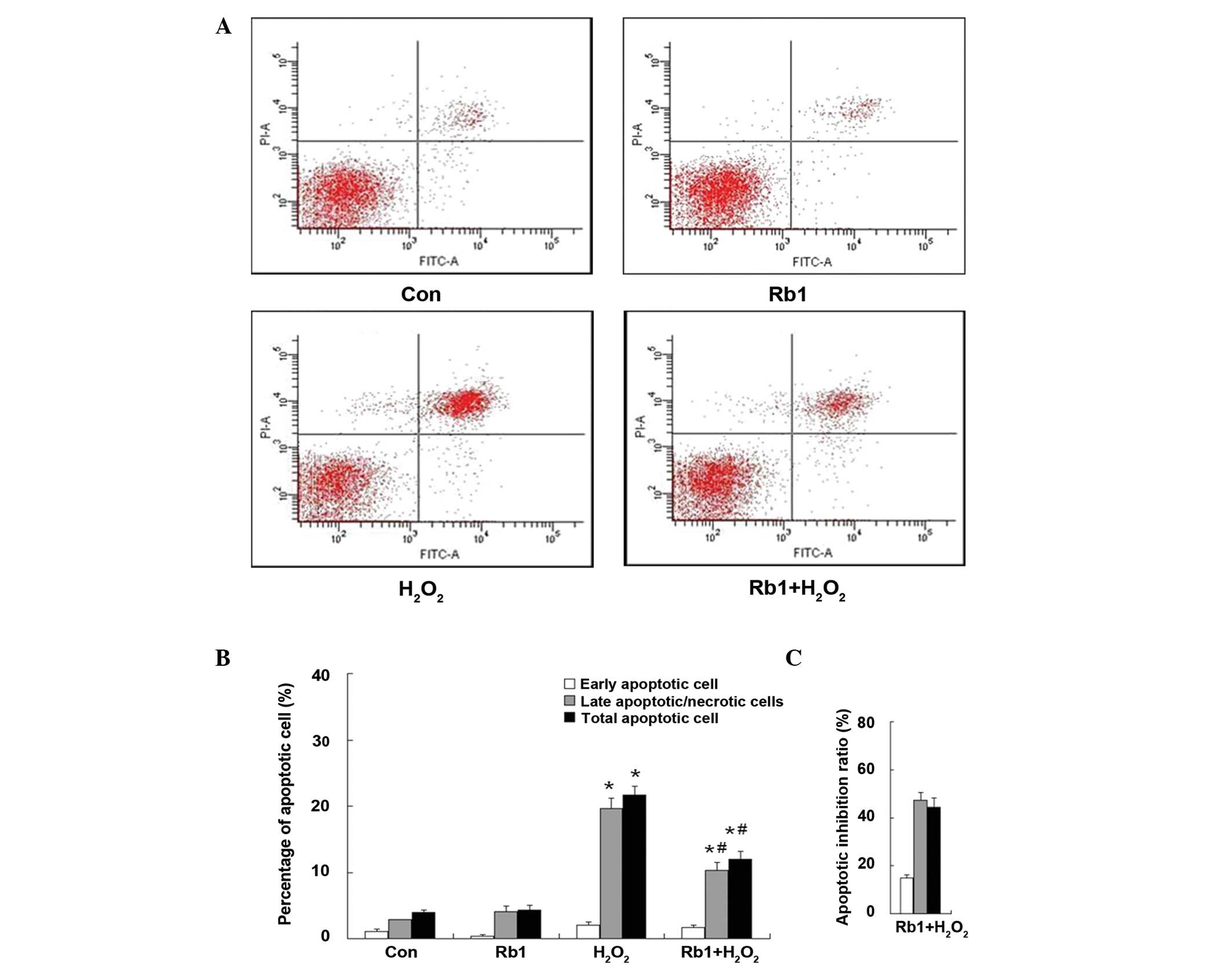
Effect of Rb1 on
H2O2-induced apoptosis in RGC-5 cells
The morphological characterization of RGC-5 cells
showed that incubation with H2O2 (600 μmol/l)
for 24 h induced morphological changes of RGC-5 cells as shown by
cell body shrinkage, neurite number decrease and the formation of
apoptotic bodies. However, these types of morphological changes
were also prevented in the pretreatment of Rb1 group (Fig. 3). In the case of apoptosis
analysis, the representative figures of flow cytometry are shown in
Fig. 5A, the total apoptotic rate
was significantly increased in the
H2O2-treated group compared with the control
(H2O2, 21.63±1.37% vs. control, 3.97±0.91%;
P<0.01). However, in the Rb1-pretreated
H2O2 group, the total apoptotic rate was
significantly decreased (Rb1-pretreated H2O2,
12.03±1.10% vs. H2O2, 21.63±1.37%;
P<0.05). Treatment of Rb1 alone did not affect the apoptotic
rate compared with the control (Rb1, 4.33±0.73% vs. control,
3.97±0.91%; P>0.05) (Fig. 5B).
The percentage of inhibition ratio by quantitative analysis showed
that the inhibition ratio of Rb1 against
H2O2-induced apoptosis was 15.00±1.12% in the
early apoptotic cells, 47.38±3.08% in the late apoptotic cells and
44.38±3.96% in the total apoptotic cells (Fig. 5C). The protective effect of Rb1 in
RGC-5 cells injury induced by H2O2 was
exerted primarily against the late apoptotic cells.
Effect of Rb1 on caspase activation by
CoCl2 in RGC-5 cells
Caspase-3, −9 and −8 were detected by western
immunoblot analysis and measured by densitometric analysis. There
was no statistical difference in the expression of procaspase-3, −9
and −8 among the four groups (P>0.05). There was little
expression of cleaved caspase-3, −9 and −8 in the Rb1 and control
groups (P>0.05). However, the expression of cleaved caspase-3,
−9 and −8 was significantly increased in the
CoCl2-treated group compared with that of the control.
Pretreatment of Rb1 significantly decreased the expression of
cleaved caspase-3 and −9, but not cleaved caspase-8 in the
CoCl2-treated group (Fig.
6).
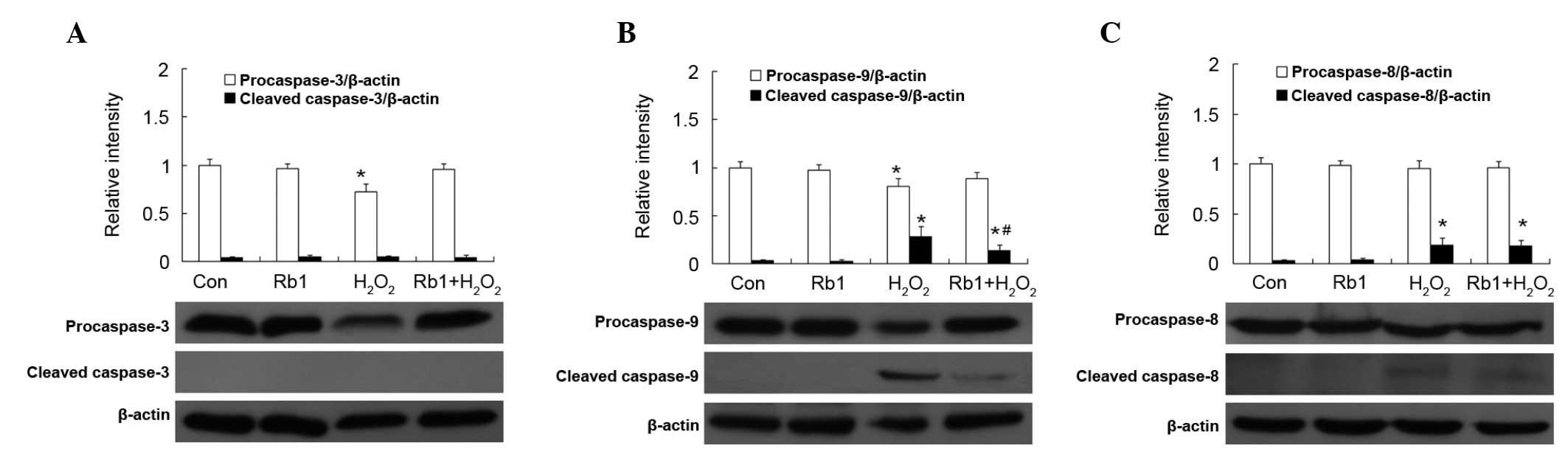
Effect of Rb1 on caspase activation by
H2O2 in RGC-5 cells
The expression of caspase-3, −9 and −8 is shown in
Fig. 7. There was no statistical
difference in the expression of procaspase of cleaved caspase-3, −9
and −8 in the Rb1 and control groups (P>0.05). In the
H2O2 group, the expression of procaspase-3
and −9, but not −8 was decreased compared with the control group
(P<0.05). However, pretreatment of Rb1 prevented this type of
decrease. The expression of cleaved caspase-3 was not detected in
all the groups (Fig. 7A). The
expression of cleaved caspase-9 was increased in the
H2O2-treated group (P<0.05; Fig. 7B). There was no statistical
difference in the expression of procaspase-8 among all groups
(P>0.05). The expression of cleaved caspase-8 was increased in
the H2O2-treated group compared with that of
the control group (P<0.05) and the treatment of Rb1 showed no
effect (Fig. 7C).
Discussion
Retinal hypoxia and oxidative stress are important
causes of a number of ocular pathologies, including glaucoma, DR
and retinal vessel occlusion (14,15).
Apoptosis is the primary consequence of hypoxia and oxidative
stress. A previous study showed that Rb1 had a number of
anti-apoptotic effects on neonatal rat cardiomyocytes (9). However, the protection of Rb1 on
RGC-5 cells from hypoxia- and oxidative stress-induced apoptosis
was determined, for the first time, in the current study. Rb1 may
be a new effective drug for protecting rat RGC. The flow cytometry
results were closely associated with the number of early and late
apoptotic cells. In an effort to explore the potential mechanism of
action behind hypoxia- and oxidative stress-induced cell apoptosis,
as well as the protection offered by Rb1, the protein expression of
caspases were monitored. Thus, caspases may be identified as a
further criterion to elucidate the protection of Rb1.
In the current study, Rb1 was observed to
significantly protect against hypoxia-induced apoptosis of RGC-5
cells in vitro. Two previous studies demonstrated a
neuroprotective effect of Rb1 on neonatal rat cardiomyocytes in
apoptosis induced by CoCl2(9,11).
The current results further showed that the protective effect of
Rb1 in RGC-5 cell injury induced by CoCl2 was exerted
primarily against early apoptotic cells. The recent studies showed
that CoCl2 induced apoptosis through the activation of
caspase-3 in in vitro studies (16,17).
The study by Kong et al revealed that Rb1 decreased the
expression of caspase-3 (11). In
the current study, caspase-3, −8 and −9 were investigated.
CoCl2 was observed to induce apoptosis through the
activation of caspase-3, −9 and −8. In addition, the anti-apoptotic
effect of Rb1 was demonstrated through inhibition of the activation
of caspase-3 and −9, but not caspase-8. The study revealed that Rb1
may prevent RGC-5 cell apoptosis induced by CoCl2 via
the mitochondrial pathways.
The results of the present study also showed that
Rb1 exhibited significant protection against oxidative stress
induced apoptosis of RGC-5 cells in vitro. Oxidative stress
was induced by treatment with H2O2, which
caused a loss of RGC-5 cells. The results showed that the
protective effect of Rb1 in RGC-5 cell injury induced by
H2O2 was exerted primarily against late
apoptotic cells. The expression of procaspase-3 and −9, but not −8,
was decreased in the H2O2 group compared with
the control group, pretreatment of Rb1 prevented this type of
decrease. H2O2 was observed to induce
apoptosis through the activation of caspase-9 and −8, but not
caspase-3. The anti-apoptotic effect of Rb1 in the oxidative stress
model was executed through the inhibition of the activation of
caspase-9 only. These results showed that
H2O2-induced apoptosis did not result in the
activation of caspase-3. Inactivation of caspase-3 in
H2O2-induced apoptosis was due to a cleaved
form of poly (ADP-ribose) polymerase 1, which is known to act as a
substrate for activated caspase-3 and such a product was not
detected in RGC-5 cell extracts that were exposed to
H2O2(18).
In the current study, caspases-3, −9 and −8 were investigated.
Thus, it was observed that Rb1 may prevent RGC-5 cell apoptosis
induced by H2O2 via the mitochondrial
pathways.
In conclusion, to the best of our knowledge, the
present study, using RGC-5 cells, demonstrated that Rb1 inhibited
hypoxia- and oxidative stress-induced apoptosis for the first time.
The study showed that inhibition of the activation of associated
caspase proteins may highlight the beneficial effects of Rb1.
Furthermore, Rb1 exerted a protective effect in RGC-5 cell
apoptosis induced by CoCl2 and
H2O2 through mitochondrial pathways, but not
the death receptor pathways. Rb1 may offer a useful therapeutic
choice in the treatment of neurodegenerative disorders caused by
hypoxia and oxidative stress. However, further studies are required
to determine the mechanisms involved in the action of Rb1 to gain
an improved understanding of its therapeutic value.
Acknowledgements
This study was supported by grants from the Joint
Project of National Education Ministry and Guangdong province (no.
2007B090400089) and (no. 2007A032702001).
References
|
1
|
Krishnamoorthy RR, Agarwal P, Prasanna G,
Vopat K, Lambert W, Sheedlo HJ, et al: Characterization of a
transformed rat retinal ganglion cell line. Brain Res Mol Brain
Res. 86:1–12. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Kaur C, Foulds WS and Ling EA:
Hypoxia-ischemia and retinal ganglion cell damage. Clin Ophthalmol.
2:879–889. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Nakajima Y, Inokuchi Y, Nishi M, Shimazawa
M, Otsubo K and Hara H: Coenzyme Q10 protects retinal cells against
oxidative stress in vitro and in vivo. Brain Res. 1226:226–233.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Nakajima Y, Inokuchi Y, Shimazawa M,
Otsubo K, Ishibashi T and Hara H: Astaxanthin, a dietary
carotenoid, protects retinal cells against oxidative stress
in-vitro and in mice in-vivo. J Pharm Pharmacol. 60:1365–1374.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Fan TJ, Han LH, Cong RS and Liang J:
Caspase family proteases and apoptosis. Acta Biochim Biophys Sin
(Shanghai). 37:719–727. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Salvesen GS: Caspases: opening the boxes
and interpreting the arrows. Cell Death Differ. 9:3–5. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Ghavami S, Hashemi M, Ande SR, Yeganeh B,
Xiao W, Eshraghi M, et al: Apoptosis and cancer: mutations within
caspase genes. J Med Genet. 46:497–510. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Walters J, Pop C, Scott FL, Drag M, Swartz
P, Mattos C, et al: A constitutively active and uninhibitable
caspase-3 zymogen efficiently induces apoptosis. Biochem J.
424:335–345. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Kong HL, Wang JP, Li ZQ, Zhao SM, Dong J
and Zhang WW: Anti-hypoxic effect of ginsenoside Rb1 on neonatal
rat cardiomyocytes is mediated through the specific activation of
glucose transporter-4 ex vivo. Acta Pharmacol Sin. 30:396–403.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Park JK, Namgung U, Lee CJ, Park JO, Jin
SH, Kwon OB, et al: Calcium-independent CaMKII activity is involved
in ginsenoside Rb1-mediated neuronal recovery after hypoxic damage.
Life Sci. 76:1013–1025. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Kong HL, Li ZQ, Zhao YJ, Zhao SM, Zhu L,
Li T, et al: Ginsenoside Rb1 protects cardiomyocytes against
CoCl2-induced apoptosis in neonatal rats by inhibiting
mitochondria permeability transition pore opening. Acta Pharmacol
Sin. 31:687–695. 2010.PubMed/NCBI
|
|
12
|
Kocic G, Bjelakovic G, Pavlovic D,
Jevtovic T, Pavlovic V, Sokolovic D, et al: Protective effect of
interferon-alpha on the DNA- and RNA-degrading pathway in
anti-Fas-antibody induced apoptosis. Hepatol Res. 37:637–646. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Chen S, Cheng AC, Wang MS and Peng X:
Detection of apoptosis induced by new type gosling viral enteritis
virus in vitro through fluorescein Annexin V-FITC/PI double
labeling. World J Gastroenterol. 14:2174–2178. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Osborne NN, Casson RJ, Wood JP, Chidlow G,
Graham M and Melena J: Retinal ischemia: mechanisms of damage and
potential therapeutic strategies. Prog Retin Eye Res. 23:91–147.
2004. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Tezel G: Oxidative stress in glaucomatous
neurodegeneration: mechanisms and consequences. Prog Retin Eye Res.
25:490–513. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Wang Y, Sun A, Xue J, Feng C, Li J and Wu
J: Bone marrow derived stromal cells modified by
adenovirus-mediated HIF-1alpha double mutant protect cardiac
myocytes against CoCl2-induced apoptosis. Toxicol In
Vitro. 23:1069–1075. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Zeng KW, Wang XM, Ko H and Yang HO:
Neuroprotective effect of modified Wu-Zi-Yan-Zong granule, a
traditional Chinese herbal medicine, on CoCl2-induced
PC12 cells. J Ethnopharmacol. 130:13–18. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Li GY and Osborne NN: Oxidative-induced
apoptosis to an immortalized ganglion cell line is caspase
independent but involves the activation of poly (ADP-ribose)
polymerase and apoptosis-inducing factor. Brain Res. 1188:35–43.
2008. View Article : Google Scholar
|