Introduction
Mossy fiber sprouting (MFS) has been observed in
experimental models of temporal lobe epilepsy (TLE) (1) and in the epileptic human hippocampus
(2), and the involvement of MFS in
TLE has long been demonstrated. Previous studies have supported the
hypothesis that MFS alters synaptic connectivity and circuit
organization, thus forming recurrent excitatory connections, which
contribute to an enhanced susceptibility to seizures (3). However, other studies have
demonstrated that MFS is a consequence of TLE, an event that is not
correlated with seizures (4).
Therefore, the mechanisms underlying MFS remain unclear and the
sequence of occurrence of MFS and TLE is controversial.
Tau protein is an important microtubule-associated
protein that is localized in neuronal axons and facilitates
microtubule dynamics, axonal transport, neurite outgrowth and
axonal elongation (5). These
functions of tau are modulated by site-specific phosphorylation,
mainly occurring at Ser202/Thr205 (6). Furthermore, various epilepsy models
have confirmed the significantly increased phosphorylation of tau
protein following seizure, and the resulting induced MFS is
important in TLE (7,8). Although different kinases may modify
tau, it has been suggested that glycogen synthase kinase-3β
(GSK-3β) is important in regulating tau phosphorylation, including
at the Ser202/Thr205 residue, under physiological and pathological
conditions (9).
GSK-3β is a constitutively active multi-functional
serine/threonine kinase that is involved in regulating diverse
physiological pathways, including neuronal structure, apoptosis,
axon growth and guidance, cytoskeletal stability and synaptic
plasticity (10). Furthermore,
previous studies have demonstrated that GSK-3β affects axoplasmic
transport and axonal growth through the phosphorylation of tau
protein (11). Therefore, GSK-3β
may also function in epileptogenesis, particularly in MFS by a
mechanism involving tau phosphorylation. In the present study, the
progression of MFS was observed during kindling in association with
the expression levels of GSK-3β mRNA and protein, and with GSK-3β
activity, in order to investigate the correlation between TLE, MFS
and GSK-3β and the involvement of GSK-3β in epileptogenesis.
Materials and methods
Animal groupings
Male Sprague-Dawley (SD) rats (n=180; age, 6–8
weeks; weight, 180–120 g) obtained from the Animal Experimental
Centre (Central South University, Chengsha, China) were divided
into the control and PTZ groups by the random number method. The
study was conducted in accordance with the guidelines of the
Central South University and the Guide for the Care and Use of
Laboratory Animals (12), and was
approved by the Institutional Animal Care and Use Committee of
Central South University. All efforts were made to minimize animal
suffering and to reduce the number of animals used in each group.
The groups were further divided into five subgroups, each
containing 18 rats, which were sacrificed at different time points
(3 days and 1, 2, 4 and 6 weeks) following the initial PTZ
injection (Sigma-Aldrich, St. Louis, MO, USA). Rats of the five
subgroups were sacrificed and perfused for Timm staining and
scoring, immunohistochemistry and in situ hybridization of
GSK-3β and GSK-3β kinase activity.
Animal models and behavior
monitoring
Rats in the PTZ group received one dose of 30 mg/kg
PTZ intraperitoneally (i.p.) daily until they were kindled or
sacrificed. The control rats were injected with an equal dose of
saline. Rats were observed every 2 h daily for the occurrence of
PTZ-induced seizures prior to kindling. Convulsive behavior was
classified into five stages as proposed by Racine (13). Rats were considered to be kindled
when a seizure attack (score, ≥3) occurred following each PTZ
injection for five consecutive days. Seizure activity in kindled
rats without PTZ induction was perceived as spontaneous recurrent
seizures (SRSs). Kindled rats were monitored for seizure occurrence
using a video camera positioned above the cages until they were
sacrificed.
Timm staining
At the aforementioned time points, rats were
sacrificed (n=60). The rats were anesthetized with 10% chloral
hydrate and intracardially perfused with 150 ml saline, followed by
200 ml 0.1 mol/l phosphate buffer (pH 7.2–7.6) containing 0.4%
sodium sulfide and 250 ml 4% paraformaldehyde at 4°C. The brains
were carefully removed and fixed in 4% paraformaldehyde for 24 h.
Subsequent to this, the brains were transferred into 0.1 mol/l
phosphate buffer containing 30% sucrose at 4°C until they sank for
72 h, following which they were embedded in OCT and stored at −70°C
for further analysis. The embedded brains were cut into 25-μm
coronal sections using a vibratome (CM1950; Leica, Wetzlar,
Germany) and the microscope slides with brain slices were placed
into a Timm developer (MaxQ7000; Thermo Scientific, Waltham, MA,
USA) for 60–80 min at 26°C in the dark and then removed for a 5-min
wash in water to terminate the reaction. The slides were
dehydrated, cleaned and mounted with gum consisting of 60 ml 50%
Arabic gum, 10 ml 2.55% citric acid, 10 ml 2.35% sodium citrate, 30
ml 5.67% hydroquinone and 1.5 ml 17% silver nitrate. The
distribution of Timm granules in the dentate gyrus (DG)
supragranular regions and the stratum oriens of CA3 was rated on a
scale of 0 to 5 according to previously described criteria
(14).
Immunohistochemistry and in situ
hybridization
Rats (n=60) were anesthetized with 10% chloral
hydrate (i.p.) and perfused intracardially with saline and 4%
paraformaldehyde. The brains were removed and coronally divided
into two sections, 3.5 mm posterior to the bregma. The anterior
section was placed in 4% paraformaldehyde overnight for
immunohistochemistry, while the posterior section was placed in 4%
paraformaldehyde with 0.1% diethylpyrocarbonate for 30 min, for
in situ hybridization. Subsequent to this, tissues were
sliced, routinely processed and embedded in paraffin wax. Serial
sections were cut at 5-μm thickness. Immunohistochemistry was
performed using the SABC method (polyclonal antibody against
GSK-3β; Boster Biological Tech Ltd., Fremont, CA, USA). In
situ hybridization was conducted using the in situ
hybridization kit (MK1750; Boster, Wuhan, China) according to the
manufacturer’s instructions. The gray value was measured and
analyzed using the image analysis system HPIAS-1000
(Championimages, Wuhan, China).
GSK-3β activity assay
Rabbit monoclonal anti-GSK-3β antibody (2
μg/reaction; Abcam, Cambridge, MA, USA) was added to rat brain
tissue lysate (hippocampus and frontal region) and incubated for 1
h with gentle agitation. Protein G-agarose (40 μl; Sigma-Aldrich)
was added to the lysate and incubated for another 6 h, followed by
high-speed centrifugation (12,000 × g) for 10 min. Immune complexes
were collected with protein G-agarose and washed three times with
cell dissociation buffer. The washed beads were incubated with 15
μl kinase buffer [250 μM ATP, 1.4 μCi (γ-32P) ATP, 20 mM Tris-HCl
pH 7.5, 5 mM MgCl2, 1 mM dithiothreitol and 100 μM
phosphate glycogen synthase peptide 2] at 30°C for 30 min. The
reaction was terminated by the addition of 12.5 μl 300 mmol/l
phosphate. GSK-3β kinase activity was assayed using the Liquid
Scintillation Counter (Beckman Coulter, Miami, FL, USA).
Statistical analysis
Intragroup differences in Timm scores were analyzed
using the Kruskal-Wallis H test and intergroup differences were
analyzed with a Mann-Whitney U test, followed by a Nemenyi test for
pairwise comparison. The remaining indices for the difference
between the PTZ and control group were performed with Student’s
t-test. The means of each group were compared using a one-way
analysis of variance and then Fisher’s least significant difference
test for pairwise comparison. α=0.05 was selected as the level of
significance and P<0.05 was considered to indicate a
statistically significant difference. All statistical analyses were
two-sided and were performed using the Statistical Package for the
Social Sciences (SPSS), version 16.0 (SPSS, Inc., Chicago, IL,
USA).
Results
Behavioral outcomes
In each experimental group, prior to kindling, with
the exception of one fatality in the 1 week group due to persistent
generalized tonic-clonic seizure, seizure activity in the rats was
only observed at grades 0–3. Following kindling in the experimental
group, with the exception of one fatality in the 4-week group due
to frequent seizures, the rats developed seizure activity at grades
3–5 following continuous PTZ injections for 18–22 days. The
PTZ-treated rats were in accordance with the kindling criterion
26.1±1.6 days following PTZ injection. The PTZ-induced seizure
activity usually occurred 5–10 min following injection, with a
duration of 5–30 min. SRSs at grades 2–3 were detected in kindled
rats as early as 23 days following PTZ injection. However, no
epileptiform behavior was observed in the control rats.
Timm scores
Timm staining patterns in PTZ-treated rats differed
significantly from those of the control rats. There was an
increased distribution of Timm granules in the stratum pyramidale
of the CA3 region at each time point in the PTZ group (P<0.05)
(Fig. 1A and C). In addition, the
degree of MFS in the CA3 area progressed with the development of
behavioral kindled seizures and peaked at 6 weeks. However, the
Timm scores for the DG supragranular regions were 0–1 throughout
the experiment in the PTZ group, with no significant difference
compared with those of the control group (P>0.05) (Fig. 1B and D). In addition, no
significant difference was identified in the Timm scores for the
CA3 area and the supragranular regions at each time-point within
the control rats (P>0.05).
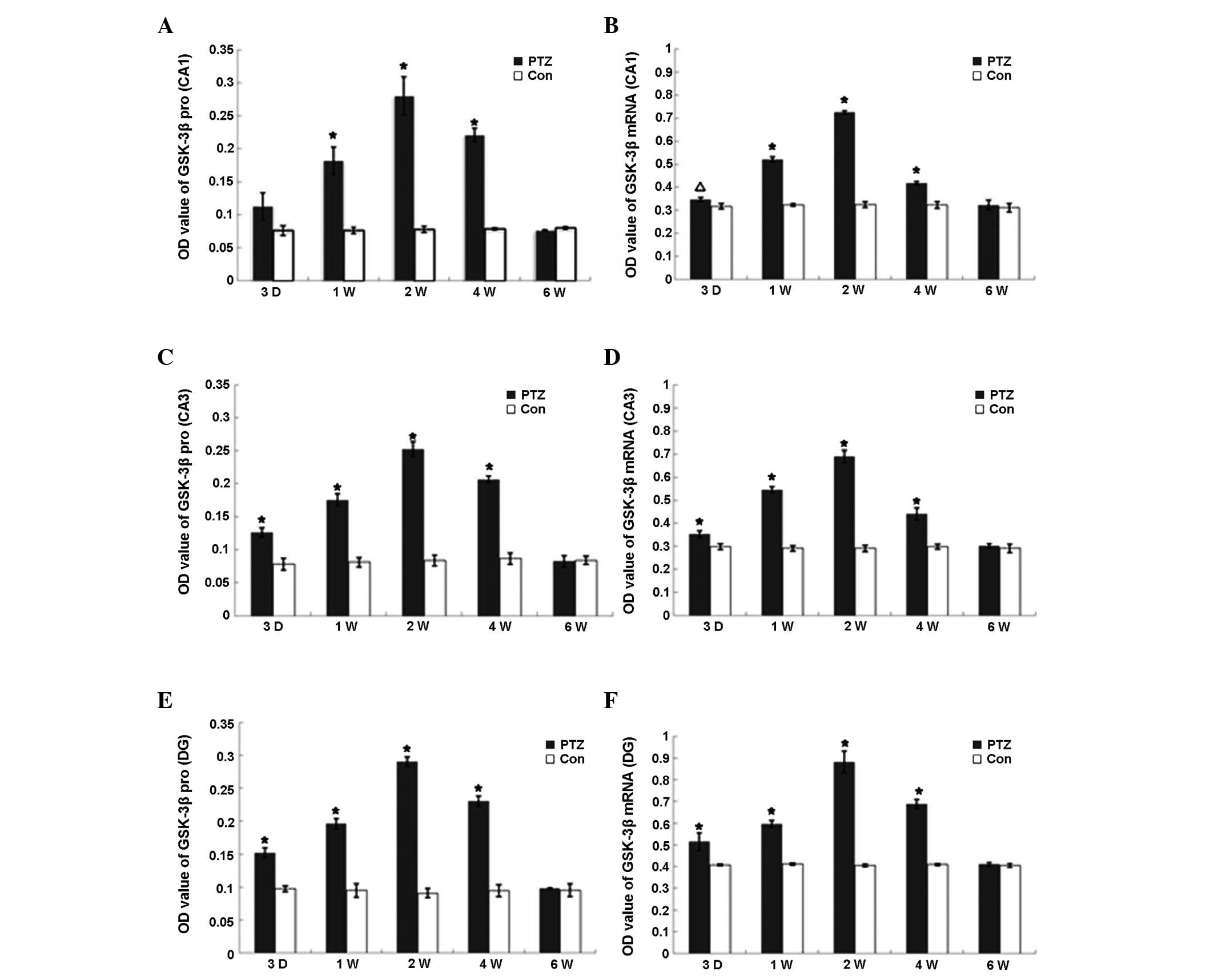
Expression and activity of GSK-3β
The expression of GSK-3β mRNA and protein in the PTZ
group increased markedly from 3 days to 2 weeks (P<0.01) and
declined to control levels at 6 weeks (Fig. 2), whereas the expression of GSK-3β
mRNA and protein was observed at various regions in the hippocampus
of control rats, with no significant difference among the
time-points. GSK-3β kinase activity increased in parallel with
GSK-3β protein expression from day 3 in the PTZ group, reaching a
peak at 2 weeks, declining from 4 weeks and finally returning to
the control levels at 6 weeks. Conversely, there was no significant
difference in GSK-3β activity in the frontal region between the PTZ
and control groups. Furthermore, no significant changes were
identified in the GSK-3β activity in the hippocampi of the control
rats (Table I).
 | Table IQuantitative analyses of GSK-3β
activity (P-32 counts per minute value). |
Table I
Quantitative analyses of GSK-3β
activity (P-32 counts per minute value).
| GSK-3β activity |
|---|
|
|
|---|
| Hippocampus | Frontal region |
|---|
|
|
|
|---|
| Group | PTZ | Control | PTZ | Control |
|---|
| 3 day |
5886.47±252.71a | 2013.40±130.31 | 2074.90±214.80 | 2073.55±158.26 |
| 1 week |
7789.87±334.51a | 2011.53±163.37 | 2158.38±196.83 | 2015.13±156.15 |
| 2 weeks |
11503.55±1527.55a | 2061.42±203.47 | 2250.27±266.08 | 2018.53±156.66 |
| 4 weeks |
5822.15±488.93a | 2065.76±191.51 | 2115.87±250.96 | 1967.23±136.75 |
| 6 weeks | 2142.85±129.34 | 2101.73±195.04 | 2078.28±221.22 | 2014.38±105.88 |
Subcellular localization of GSK-3β
The subcellular localization of GSK-3β changed
markedly during the kindling process. The expression of GSK-3β mRNA
and protein was observed in pyramidal cells in the CA3 area, CA1
area and in the granular cells in the DG of the control rat
hippocampus, predominantly in the neuronal membranes and processes.
However, in the PTZ group, GSK-3β translocated to the soma
throughout kindling development, which was particularly notable 2
weeks following the initial PTZ injection, and the expression
decreased to a low level in the soma at 6 weeks (Fig. 3).
Discussion
The present study demonstrated that as the degree of
aberrant MFS increased, the severity of seizures and the amplitude
and frequency of excitatory postsynaptic currents increased. MFS
was observed prior to the occurrence of TLE and was increased
following kindling, which suggested that repeated seizures
aggravated MFS; however, it did not indicate that MFS was a
consequence of SRS. Furthermore, MFS may not be the sole
determinant of the onset of TLE, and other mechanisms, such as
hilar ectopic dentate granule cells, may also be involved in
epileptogenesis (15). An
increased distribution of Timm granules was observed at the CA3
region, but not at the supragranular regions, which differs from
that in the pilocarpine and kainic acid (KA) kindling models
(16,17) and is consistent with the results of
a previous study (18). The
difference in the distribution of Timm granules among the
PTZ-kindling, pilocarpine and KA models may be attributed to the
differences in the type and dose of the different convulsants and
the severity of the induced seizures. Pilocarpine and KA may induce
status epilepticus (SE), which leads to hippocampal neuronal death,
particularly in the CA3 area. This study used moderate doses of PTZ
to establish a kindling model in which SE rarely occurred. The
extent of neuronal injuries caused by generalized tonic-clonic
convulsions and SRS was less severe than that by SE. Regardless of
the marginal injuries in the observed CA3 region and the hilar
neurons, sprouted mossy fibers were able to locate sufficient
post-synaptic target cells without returning to the DG
supragranular regions.
GSK-3β is involved in numerous neuronal functions
and affects neuronal polarity through undermining the structural
stability of axons, regulating microtubule dynamics in growing
axons, which leads to synaptic reorganization and the formation of
recurrent excitatory circuits (19). Moreover, a study demonstrated that
GSK-3β was able to hyperphosphorylate tau protein and may
contribute to the pathogenesis of focal cortical dysplasia (FCD) in
older patients. FCD is an important neurodevelopmental cause of
refractory human epilepsy. However, thus far there have been no
studies regarding a correlation between GSK-3β and MFS (20). In the present study, the expression
of GSK-3β mRNA and protein was increased in the DG, however, no MFS
was observed in this area. This does not indicate that GSK-3β does
not exhibit a correlation with MFS, as a previous study determined
that in the PTZ-kindling model, hippocampal neuronal death in the
DG area was more severe than in the CA3 area (13), therefore sprouted mossy fibers were
not able to locate sufficient post-synaptic target cells and turned
back to the inner molecular layer of the DG. In a previous study,
it was demonstrated that tau protein and its phosphorylation at
Ser202 increased significantly in the PTZ group (21). Furthermore, in the present study,
the expression of GSK-3β mRNA and protein, as well as its activity,
also increased significantly from 3 days to 4 weeks in the PTZ
group, which was correlated with an increase in phosphorylated tau
protein. These results suggested that GSK-3β may be involved in MFS
in the kindling rat hippocampus through the phosphorylation of
tau.
The mechanisms underlying the correlation between
GSK-3β and MFS remain unknown. A study demonstrated that GSK-3β is
abundant in soma when its activity is high, whereas it is expressed
in axons when the activity is low (19). Results also suggest that in the PTZ
group, GSK-3β translocated from the axon to soma during the
progression of MFS. Tau is synthesized and subsequently
phosphorylated within the cell body; however, in the proximal
axonal process, such as within growing neurons, it is
dephosphorylated by phosphatases during axonal transport, resulting
in the stabilization of the microtubule cytoskeleton. Furthermore,
excessive aggregation of GSK-3β and phosphorylated tau in soma
exhibited high cytotoxicity and resulted in apoptosis and necrosis
(22,23). This may be due to an alternate
mechanism of MFS caused by GSK-3β. Increased expression and
activity of GSK-3β led to the phosphorylation of tau protein, which
subsequently induced MFS through reducing its microtubule binding
ability and promoting microtubule assembly function.
In conclusion, the present study demonstrated a
correlation between MFS and GSK-3β. GSK-3β may be involved in the
progression of MFS through abnormal phosphorylation of tau protein.
However, the importance and specific mechanisms of GSK in the
formation of MFS were not determined. GSK is not the only enzyme
that regulates tau protein phosphorylation and MFS may not be the
sole determinant of the onset of TLE. Further studies are required
that modulate the expression and activity of GSK-3β in mossy fiber
sprouting in the PTZ-kindling model, and assess whether there are
corresponding changes in the level of tau protein phosphorylation,
MFS formation and the severity of epilepsy. Understanding the
involvement of GSK-3β in MFS may lead to novel therapeutic
interventions, and therefore ameliorate epileptogenic network
dysfunction and associated morbidities.
References
|
1
|
Kuo LW, Lee CY, Chen JH, Wedeen VJ, Chen
CC, Liou HH and Tseng WY: Mossy fiber sprouting in
pilocarpine-induced status epilepticus rat hippocampus: a
correlative study of diffusion spectrum imaging and histology.
NeuroImage. 41:789–800. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Andrade-Valença LP, Valença MM, Velasco
TR, Carlotti CG Jr, Assirati JA, Galvis-Alonso OY, Neder L, Cendes
F and Leite JP: Mesial temporal lobe epilepsy clinical and
neuropathologic findings of familial and sporadic forms. Epilepsia.
6:1046–1054. 2008.PubMed/NCBI
|
|
3
|
Sutula TP and Dudek FE: Unmasking
recurrent excitation generated by mossy fiber sprouting in the
epileptic dentate gyrus: an emergent property of a complex system.
Prog Brain Res. 163:541–563. 2007. View Article : Google Scholar
|
|
4
|
Longo BM and Mello LE: Effect of long-term
spontaneous recurrent seizures or reinduction of status epilepticus
on the development of supragranular mossy fiber sprouting. Epilepsy
Res. 36:233–241. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Johnson GV and Stoothoff WH: Tau
phosphorylation in neuronal cell function and dysfunction. J Cell
Sci. 117:5721–5729. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Merrick SE, Demoise DC and Lee VM:
Site-specific dephosphorylation of tau protein at Ser202/Thr205 in
response to microtubule depolymerization in cultured human neurons
involves protein phosphatase 2A. J Biol Chem. 271:5589–5594. 1996.
View Article : Google Scholar
|
|
7
|
Pollard H, Khrestchatisky M, Moreau J,
Ben-Ari Y and Represa A: Correlation between reactive sprouting and
microtubule protein expression in epileptic hippocampus.
Neuroscience. 61:773–787. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Palmio J, Suhonen J, Keränen T, Hulkkonen
J, Peltola J and Pirttilä T: Cerebrospinal fluid tau as a marker of
neuronal damage after epileptic seizure. Seizure. 18:474–477. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Plattner F, Angelo M and Giese KP: The
roles of cyclin-dependent kinase 5 and glycogen synthase kinase 3
in tau hyperphosphorylation. J Biol Chem. 281:25457–25465. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Yeste-Velasco M, Folch J, Trullàs R, Abad
MA, Enguita M, Pallàs M and Camins A: Glycogen synthase kinase-3 is
involved in the regulation of the cell cycle in cerebellar granule
cells. Neuropharmacology. 53:295–307. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Zhou FQ and Snider WD: Cell biology.
GSK-3β and microtubule assembly in axons. Science. 308:211–214.
2005.
|
|
12
|
National Institues of Health. Guide for
the care and use of laboratory animals. The National Academies
Press; Washington, D.C: 1996
|
|
13
|
Racine RJ: Modification of seizure
activity by electrical stimulation. II Motor seizure
Electroencephalogr. Clin Neurophysiol. 32:281–294. 1974. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Sogawa Y, Monokoshi M, Silveira DC, Cha
BH, Cilio MR, McCabe BK, Liu X, Hu Y and Holmes GL: Timing of
cognitive deficits following neonatal seizures: relationship to
histological changes in the hippocampus. Brain Res Dev Brain Res.
131:73–83. 2001. View Article : Google Scholar
|
|
15
|
Pierce JP, Melton J, Punsoni M, McCloskey
DP and Scharfman HE: Mossy fibers are the primary source of
afferent input to ectopic granule cells that are born after
pilocarpine-induced seizures. Exp Neurol. 196:316–331.
2005.PubMed/NCBI
|
|
16
|
Holmes GL, Sarkisian M, Ben-Ari Y and
Chevassus-Au-Louis N: Mossy fiber sprouting after recurrent
seizures during early development in rats. J Comp Neurol.
404:537–553. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Cross DJ and Cavazos JE: Synaptic
reorganization in subiculum and CA3 after early-life status
epilepticus in the kainic acid rat model. Epilepsy Res. 73:156–165.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Tian FF, Zeng C, Guo TH, Chen Y, Chen JM,
Ma YF, Fang J, Cai XF, Li FR, Wang XH, et al: Mossy fiber
sprouting, hippocampal damage and spontaneous recurrent seizures in
pentylenetetrazole kindling rat model. Acta Neurol Belg.
109:298–304. 2009.PubMed/NCBI
|
|
19
|
Jiang H, Guo W, Liang X and Rao Y: Both
the establishment and the maintenance of neuronal polarity require
active mechanisms: critical roles of GSK-3β and its upstream
regulators. Cell. 120:123–135. 2005.PubMed/NCBI
|
|
20
|
Sen A, Thom M, Martinian L, Harding B,
Cross JH, Nikolic M and Sisodiya SM: Pathological tau tangles
localize to focal cortical dysplasia in older patients. Epilepsia.
48:1447–1454. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Tian FF, Zeng C, Ma YF, Guo TH, Chen JM,
Chen Y, Cai XF, Li FR, Wang XH, Huang WJ and Wang YZ: Potential
roles of Cdk5/p35 and tau protein in hippocampal mossy fiber
sprouting in the PTZ kindling model. Clin Lab. 56:127–136.
2010.PubMed/NCBI
|
|
22
|
Linseman DA, Butts BD, Precht TA, Phelps
RA, Le SS, Laessig TA, Bouchard RJ, Florez-McClure ML and
Heidenreich KA: Glycogen synthase kinase-3beta phosphorylates Bax
and promotes its mitochondrial localization during neuronal
apoptosis. J Neurosci. 24:9993–10002. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Chen L, Wei Y, Wang X and He R:
D-Ribosylated Tau forms globular aggregates with high cytotoxicity.
Cell Mol Life Sci. 66:2559–2571. 2009. View Article : Google Scholar : PubMed/NCBI
|