Introduction
Wilms’ tumor is a type of kidney cancer that affects
young children. International trials have been established, which
have collected a number of Wilms’ tumor samples; however, the
mechanisms underlying its pathogenesis and effective treatment
strategies remain to be clearly determined (1). Extensive studies have identified
somatic mutations at several loci in Wilms’ tumorigenesis,
including WT1, CTNNB1, TP53 and Wilms’ tumor gene on the X
chromosome (WTX) (2,3).
WTX has been considered as a key tumor suppressor in
Wilms’ tumor. Inactivation of WTX is the most frequent genetic
event in sporadic Wilms’ tumor, reported in up to 30% of cases
(4). It has been suggested that
WTX interacts with the anaphase-promoting complex and negatively
regulates β-catenin stability (5).
It also modulates the transcriptional activity of WT1, another
Wilms’ tumor suppressor that encodes a zinc finger transcriptional
regulator of cellular differentiation (6). Additionally, previous studies have
also indicated a positive effect on p53 signaling through enhancing
CBP/P300-mediated acetylation of p53 at Lysine 382 (7). Therefore, WTX regulates Wilms’ tumor
initiation and progression through several mechanisms.
Berberine, a well-studied naturally occurring
isoquinoline alkaloid, is an active component of the Ranunculaceae
and Papaveraceae plant families. Recent studies focused on its
antitumor effect have shown that berberine inhibits the growth of
multiple tumor cell types derived from the liver, lung,
gastrointestinal tract, leukocytes, brain, skin, bladder, bone,
breast and prostate (8–13). At the molecular level, several
mechanisms involved in the antitumor activity of berberine have
been identified, including stimulating caspase-dependent apoptosis
and caspase-independent cell death by the activation of
apoptosis-inducing factors, suppressing tumor cell proliferation
and growth by the induction of cell-cycle arrest, and inhibiting
metastasis by downregulating matrix metalloproteinases (14–16).
Numerous signaling pathways, including p53, NF-κB and MAPK pathways
have been identified to be involved in the anticancer effects of
berberine (12,17–19).
Therefore, the results suggesting that the mechanisms underlying
berberine’s anticancer effects are distinct among tumor cell types
suggest a cell-type specific effect of berberine on the inhibition
of tumor progression.
However, it remains unclear whether berberine
directly inhibits proliferation in the G401 human Wilms’ tumor cell
line. Thus, the aim of the present study was to investigate the
effect of berberine on G401 cell proliferation and the underlying
molecular mechanisms, to potentially provide results which may aid
in the development of effective drugs for the clinical treatment of
Wilms’ tumor.
Materials and methods
Cell cultures
The G401 Wilms’ tumor cell line was purchased from
the Cell Bank of Type Culture Collection of Chinese Academy of
Sciences (Shanghai, China), and cultured in RPMI-1640 (Dulbecco’s
modified Eagle’s medium) supplemented with 10% fetal calf serum,
100 IU/ml penicillin and 100 mg/ml streptomycin (all obtained from
Gibco-BRL, Carlsbad, CA, USA). Cultures were maintained at 37ºC in
a humidified 5% CO2 atmosphere.
Cell viability and bromodeoxyuridine
(BrdU) incorporation assays
Cell viability was measured by the
3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT)
assays using kits from Sigma-Aldrich, St. Louis, MO, USA. MTT
assays were performed by incubating the cells with 0.4 mg/ml MTT
for 6 h. The formazan product was dissolved in dimethyl sulfoxide,
and absorbance was read at 490 nm. A cell proliferation
enzyme-linked immunosorbent assay kit (Beyotime, Shanghai, China)
was used to measure the incorporation of BrdU during DNA synthesis
according to the manufacturer’s instructions. All experiments were
repeated at least four times in triplicate.
RNA isolation and qPCR
Total RNA was isolated from cells by TRIzol reagent
(Invitrogen Life Technologies, Carlsbad, CA, USA), and reverse
transcription was conducted using a Takara RNA PCR kit (Takara
Biotechnology, Dalian, China), according to the manufacturer’s
instructions. In order to analyze the transcripts of the genes of
interest, qPCR was performed using an SYBR-Green Premix Ex Taq
(Takara Biotechnology) on an ABI 7500 machine (Invitrogen Life
Technologies).
Western blot analysis
Cells were harvested and lysed with ice-cold lysis
buffer (50 mM Tris-HCl, pH 7.4; 100 mM DTT; 2% w/v SDS; and 10%
glycerol). Following centrifugation at 20,000 × g for 10 min at
4ºC, proteins in the supernatants were quantified and separated by
10% sodium dodecyl sulfate-polyacrylamide gel electrophoresis, and
transferred onto nitrocellulose membranes. Subsequent to blocking
with 5% non-fat milk, membranes were immunoblotted with antibodies,
followed by horseradish peroxidase (HRP)-linked secondary
antibodies. The signals were detected by Millipore
SuperSignal® HRP Substrate kit (Millipore, Billerica,
MA, USA) according to the manufacturer’s instructions.
Anti-glyceraldehyde 3-phosphate dehydrogenase (GAPDH),
anti-AMP-activated protein kinase (AMPK), anti-ACC, anti-MAPK,
anti-S6K and anti-WTX antibodies were purchased from Santa Cruz
Biotechnology, Inc. (Santa Cruz, CA, USA) or and anti-p21, anti-p27
and anti-cyclin E antibodies were obtained from Cell Signaling
Technology Inc. (Danvers, MA, USA).
Small interfering RNA (siRNA)
Cells were transfected with siRNA targeting the WTX
or luciferase gene (all siRNA oligos from Qiagen, Valencia, CA,
USA) using Lipofectamine 2000 (Invitrogen Life Technologies)
according to the manufacturer’s instructions. Cell cultures were
incubated for 18 h with 100 nM siRNA prior to berberine
treatment.
Statistical analysis
Statistical analysis was performed with a paired
Student’s t-test or two-way analysis of variance test. Numerical
data are presented as the mean ± SEM. *P<0.05,
**P<0.01 or ***P<0.001 were considered
to indicate a statistically significant difference.
Results
Berberine treatment inhibits cell growth
in a dose-dependent manner
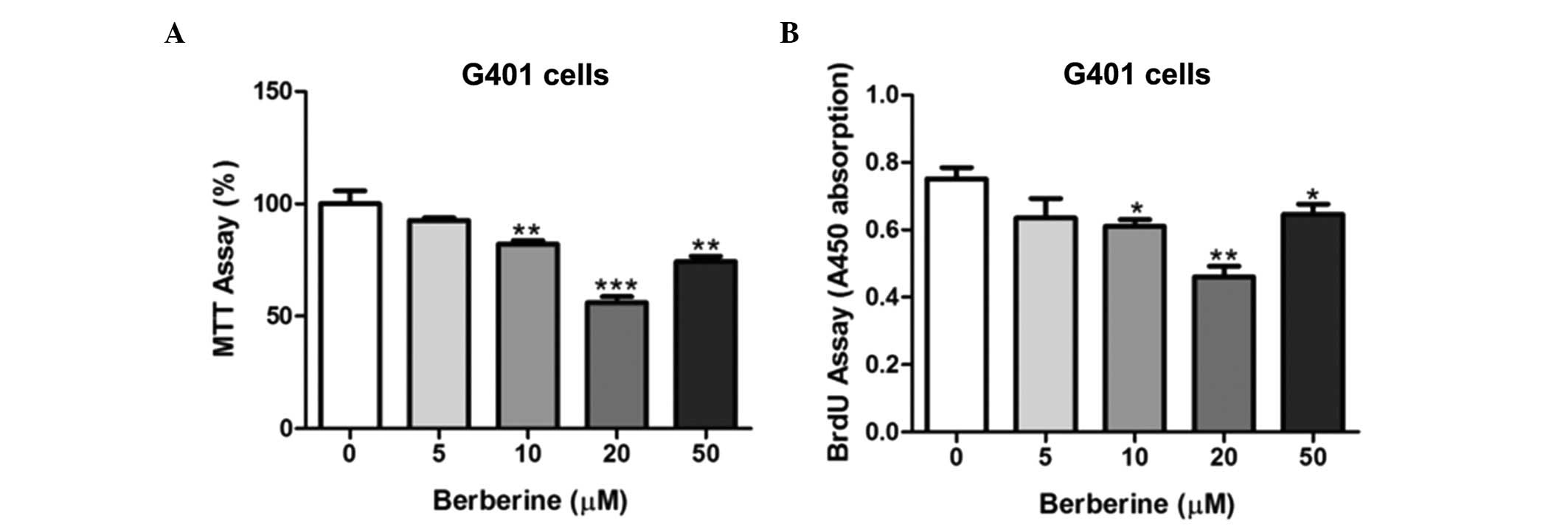
To the best of our knowledge, the effects of
berberine on Wilms’ tumor cells has not been previously analyzed.
Thus, G401 cells were selected to investigate whether berberine
exhibits potential anti-proliferative functions. G401 cells were
treated with berberine at several concentrations. After 48 h of
treatment, growth was inhibited in a dose-dependent manner as
determined by MTT and BrdU incorporation assays (Fig. 1). Moreover, these results suggested
that the concentration of berberine at 20 μM was optimal in G401
lines. Therefore, 20 μM of berberine was selected for the further
analysis of gene expression in G401 cells.
Expression of p27, p21 and cyclin E in
berberine-treated cells
It was speculated that growth inhibition in G401
cells may be due to cell-cycle arrest following berberine
treatment. To confirm this hypothesis, the expression of p21, p27
and cyclin E was analyzed, which are known to be key molecules in
cell-cycle arrest. Expression levels of p21 and p27 were
significantly increased in G401 cells (Fig. 2). In addition, the contents of
cyclin E were markedly downregulated in berberine-treated cells
(Fig. 2).
Berberine upregulates AMP kinase activity
in Wilms’ tumor cells
Several studies have indicated that the
antiproliferative effects of berberine involve the AMP kinase
pathway (19). In the present
study, the results of the western blot analysis indicated that
berberine stimulated AMPK phosphorylation in G401 cells (Fig. 3A). Phosphorylated ACC, a downstream
target of AMPK, was also enhanced in cells treated with berberine
(Fig. 3A). As AMPK activation
inhibits energy-consuming pathways and protein synthesis, it was
observed that AMPK activation is associated with an increase in the
phosphorylation of mTOR and S6 kinase (Fig. 3B).
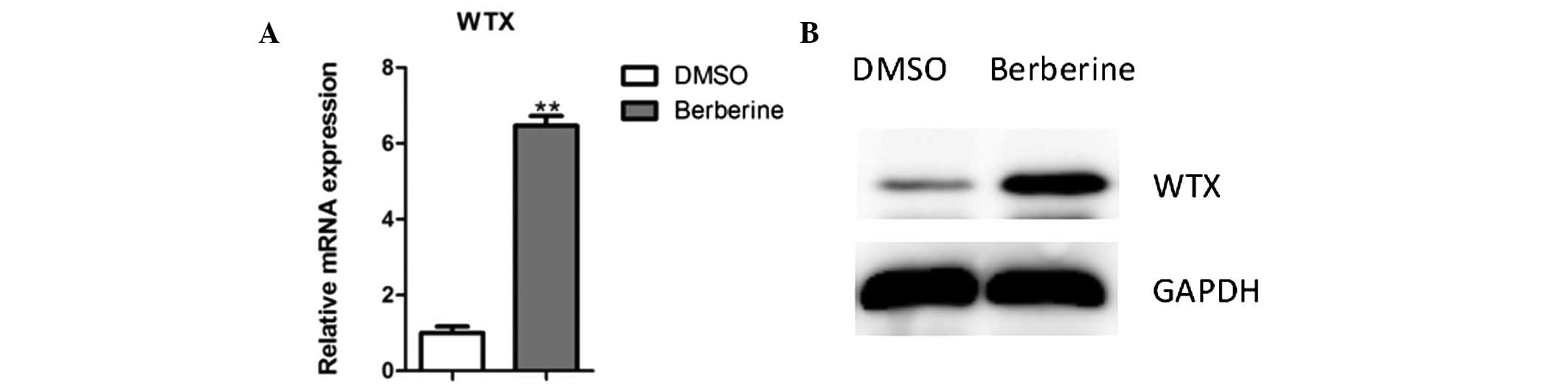
Berberine increases WTX expression in
G401 cells
The expression of several cell-cycle regulators,
including p21, is controlled by tumor suppressor WTX (20). As these genes are regulated by
berberine treatment in G401 cells, WTX abundance was analyzed in
these cells. It was observed that WTX expression was markedly
increased following berberine treatment in G401 cells (Fig. 4).
siRNA against WTX rescues cells from
berberine-induced growth inhibition
To determine whether the induction of WTX by
berberine is required for the anti-proliferative effect of the
drug, WTX knockdown experiments using siRNA oligos were
conducted(Fig. 5A and B). As a
result, the siRNA rescued G401 cells from the inhibitory effect of
berberine (Fig. 5C and D).
Consistently, the inhibitory functions of berberine on the
expression levels of cell-cycle regulators were also reversed by
WTX siRNA oligos (Fig. 5E).
Therefore, the results indicate WTX as a potential novel molecular
target in anticancer therapy, which is upregulated by berberine
treatment.
Discussion
In the present study, the involvement of berberine
and its molecular mechanism in Wilms’ tumor cells was investigated.
Berberine was shown to inhibit cell proliferation in G401 cells as
demonstrated by MTT and BrdU incorporation assays. Moreover,
berberine treatment induced p21 and p27 expression while repressing
cyclin E expression. At the molecular level, the results
demonstrated that berberine activated AMP kinase activation and
inhibited mTOR signaling. In addition, WTX was identified to be a
novel molecular target of berberine. WTX invalidation, using siRNA
oligos, abrogated berberine inhibition in G401 tumor cells. In
conclusion, the data suggested that berberine may be beneficial in
the treatment of Wilms’ tumor.
Previous studies have suggested that the
antiproliferative effects of berberine involved the AMP kinase
pathway. In the present study, inhibition of AMPK signaling
reversed the anticancer roles of berberine. AMPK is a highly
conserved Ser/Thr protein kinase complex that is central in the
regulation of cellular energy homeostasis (21). AMPK is activated in response to
decreased fuel supply and functions in the allocation of nutrients
toward catabolic/energy-producing or anabolic/growth-promoting
metabolic pathways (22). From a
metabolic standpoint, AMPK promotes ATP conservation under
conditions of metabolic stress by activating pathways of catabolic
metabolism (such as autophagy) and inhibiting anabolic processes
(including lipid biosynthesis, mTOR-dependent protein synthesis,
cell growth and proliferation) (23,24).
Thus, AMPK activity has been associated with stress resistance and
survival in tumor cells. Moreover, AMPK activation was shown to be
associated with certain tumor suppressors, including the p53
pathway. AMPK activation induces phosphorylation of p53 on serine
15, and this phosphorylation is suggested to be required to
initiate AMPK-dependent cell-cycle arrest (25). Consistently, AMPK-induced p53
activation promotes cellular survival in response to glucose
deprivation, and cells that have undergone a p53-dependent
metabolic arrest rapidly re-enter the cell cycle upon glucose
restoration. Moreover, persistent activation of AMPK leads to
accelerated p53-dependent cellular senescence (25). Notably, this study demonstrated
that berberine activates AMPK signaling and simultaneously induces
WTX expression in G401 cells. Therefore, further investigation is
required to determine whether AMPK regulates WTX at the
transcriptional or post-transcriptional levels.
In conclusion, the results suggest the underlying
mechanisms that may contribute to the antineoplastic effects of
berberine. Further studies are required to investigate the
potential of berberine as a therapy for Wilms’ tumor prevention and
treatment.
References
|
1
|
Scott RH, Stiller CA, Walker L and Rahman
N: Syndromes and constitutional chromosomal abnormalities
associated with Wilms tumour. J Med Genet. 43:705–715. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Malkin D, Sexsmith E, Yeger H, Williams BR
and Coppes MJ: Mutations of the p53 tumor suppressor gene occur
infrequently in Wilms’ tumor. Cancer Res. 54:2077–2079.
1994.PubMed/NCBI
|
|
3
|
Grundy PE, Breslow NE, Li S, et al;
National Wilms Tumor Study Group. Loss of heterozygosity for
chromosomes 1p and 16q is an adverse prognostic factor in
favorable-histology Wilms tumor: a report from the National Wilms
Tumor Study Group. J Clin Oncol. 23:7312–7321. 2005. View Article : Google Scholar
|
|
4
|
Rivera MN, Kim WJ, Wells J, et al: An X
chromosome gene, WTX, is commonly inactivated in Wilms tumor.
Science. 315:642–645. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Major MB, Camp ND, Berndt JD, et al: Wilms
tumor suppressor WTX negatively regulates WNT/beta-catenin
signaling. Science. 316:1043–1046. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Rivera MN and Haber DA: Wilms’ tumour:
connecting tumorigenesis and organ development in the kidney. Nat
Rev Cancer. 5:699–712. 2005.
|
|
7
|
Kim WJ, Rivera MN, Coffman EJ and Haber
DA: The WTX tumor suppressor enhances p53 acetylation 9 by
CBP/p300. Mol Cell. 45:587–597. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Hou Q, Tang X, Liu H, et al: Berberine
induces cell death in human hepatoma cells in vitro by
downregulating CD147. Cancer Sci. 102:1287–1292. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
James MA, Fu H, Liu Y, Chen DR and You M:
Dietary administration of berberine or Phellodendron
amurense extract inhibits cell cycle progression and lung
tumorigenesis. Mol Carcinog. 50:1–7. 2011.
|
|
10
|
Harikumar KB, Kuttan G and Kuttan R:
Inhibition of progression of erythroleukemia induced by Friend
virus in BALB/c mice by natural products - berberine, curcumin and
picroliv. J Exp Ther Oncol. 7:275–284. 2008.PubMed/NCBI
|
|
11
|
Yan K, Zhang C, Feng J, et al: Induction
of G1 cell cycle arrest and apoptosis by berberine in bladder
cancer cells. Eur J Pharmacol. 661:1–7. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Kim S, Han J, Kim NY, et al: Effect of
berberine on p53 expression by TPA in breast cancer cells. Oncol
Rep. 27:210–215. 2012.PubMed/NCBI
|
|
13
|
Meeran SM, Katiyar S and Katiyar SK:
Berberine-induced apoptosis in human prostate cancer cells is
initiated by reactive oxygen species generation. Toxicol Appl
Pharmacol. 229:33–43. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Lv X, Yu X, Wang Y, et al: Berberine
inhibits doxorubicin-triggered cardiomyocyte apoptosis via
attenuating mitochondrial dysfunction and increasing Bcl-2
expression. PLoS One. 7:e473512012. View Article : Google Scholar
|
|
15
|
Tillhon M, Guamán Ortiz LM, Lombardi P and
Scovassi AI: Berberine: new perspectives for old remedies. Biochem
Pharmacol. 84:1260–1267. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Kim S, Han J, Lee SK, et al: Berberine
suppresses the TPA-induced MMP-1 and MMP-9 expressions through the
inhibition of PKC-α in breast cancer cells. J Surg Res.
176:e21–e29. 2012.PubMed/NCBI
|
|
17
|
Chitra P, Saiprasad G, Manikandan R and
Sudhandiran G: Berberine attenuates bleomycin induced pulmonary
toxicity and fibrosis via suppressing NF-κB dependent TGF-β
activation: a biphasic experimental study. Toxicol Lett.
219:178–193. 2013.PubMed/NCBI
|
|
18
|
Alzamora R, O’Mahony F, Ko WH, Yip TW,
Carter D, Irnaten M and Harvey BJ: Berberine reduces cAMP-induced
chloride secretion in T84 human colonic carcinoma cells through
inhibition of basolateral KCNQ1 channels. Front Physiol. 2:332011.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Kim HS, Kim MJ, Kim EJ, Yang Y, Lee MS and
Lim JS: Berberine-induced AMPK activation inhibits the metastatic
potential of melanoma cells via reduction of ERK activity and COX-2
protein expression. Biochem Pharmacol. 83:385–394. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Kim MK, Min DJ, Rabin M and Licht JD:
Functional characterization of Wilms tumor-suppressor WTX and
tumor-associated mutants. Oncogene. 30:832–842. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Hardie DG: AMP-activated protein kinase:
an energy sensor that regulates all aspects of cell function. Genes
Dev. 25:1895–1908. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Egan DF, Shackelford DB, Mihaylova MM, et
al: Phosphorylation of ULK1 (hATG1) by AMP-activated protein kinase
connects energy sensing to mitophagy. Science. 331:456–461. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Gwinn DM, Shackelford DB, Egan DF, et al:
AMPK phosphorylation of raptor mediates a metabolic checkpoint. Mol
Cell. 30:214–226. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Liu L, Ulbrich J, Müller J, et al:
Deregulated MYC expression induces dependence upon AMPK-related
kinase 5. Nature. 483:608–612. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Jones RG, Plas DR, Kubek S, et al:
AMP-activated protein kinase induces a p53-dependent metabolic
checkpoint. Mol Cell. 18:283–293. 2005. View Article : Google Scholar : PubMed/NCBI
|