Introduction
The majority of females in their late 40s or early
50s may experience a variety of menopausal symptoms that include
vaginal atrophy and changes in uterine size and lining. Although
western medicine treats menopausal symptoms with various forms of
hormone replacement therapy (HRT), a number of females may elect to
forgo these therapies due to a number of side effects (1). However, Traditional Chinese Medicine
(TCM) has been used to relieve menopausal symptoms for centuries
with little adverse effects (2–5).
You Gui Wan (YGW) is a classical herbal formula in
TCM which has been used clinically in menopausal females with Yang
deficiency. In a previous study, YGW was observed to reverse the
atrophic effect of ovariectomy (OVX) in rat vaginal plica and blood
vessels in the lamina propria with little adverse effect on
endometrial hyperplasia (6). The
results suggested that YGW may be used as an alternative to HRT in
the management of menopausal vaginal atrophy. However, the
molecular mechanisms underlying the effect of YGW remain
unknown.
In a previous study, the major effect of YGW was
shown to recover the number of vaginal blood vessels which were
reduced by OVX (6), indicating
that YGW may alter angiogenesis regulators in the vaginal tissue
in vivo. It is well established that angiogenesis, the
formation of new blood vessels from existing blood vessels,
involves a number of processes, including the degradation of
existing vascular basement membrane, the proliferation and
migration of endothelial cells (ECs) into tubular structures and
the formation of a new matrix around neovessels (7). Angiogenesis also requires a number of
angiogenic proteins, including several growth factors such as basic
fibroblast growth factor (bFGF), vascular endothelial growth factor
(VEGF) and angiopoietin (Ang)1 and 2.
Estrogen is key in maintaining uterine and vaginal
health. Estrogen produces its effects by binding to estrogen
receptors (ER) in the reproductive organs. It has been reported
that estradiol increases the extracellular levels of VEGF in breast
cancer in vivo, which suggest that VEGF expression in the
breast is, at least in part, modulated by ER for which the VEGF
gene is a target (7). In cultured
human endometrial fibroblasts (8)
and a number of endometrial cancer cell lines (9), estrogens increase bFGF mRNA or
proteins. Furthermore, the regulation of Ang1 and 2 is associated
with VEGF expression within the microenvironment of angiogenic
blood vessels to ensure precise and stage-appropriate angiogenic
signals to ECs (10,11).
In addition, YGW has been shown to be capable of
recovering the expression of ER, despite having no significant
effect on estradiol production (6). Therefore, we hypothesized that there
may be specific components in the YGW decoction that produce an
estrogenic-like activity to reverse the atrophic effect caused by
OVX (6). In the current study, YGW
was hypothesized to upregulate angiogenic factors to reverse the
atrophic effect of OVX through the recovery of ER expression and
the cooperative interactions of specific angiogenic factors.
To investigate this hypothesis, the effects of YGW
on the expression of ER, VEGF, vascular endothelial growth factor
receptor-1 (VEGFR-1), bFGF and Ang1 and 2 were investigated in the
vagina following long-term (11 weeks) oral administration of YGW
decoctions in OVX-rats and the results were compared with OVX-rats
treated with estrogen replacement or saline.
Materials and methods
Materials
YGW was obtained from the Beijing Tongrentang Co.,
Ltd. (Beijing, China) with the herbal medicine license no.
Z23020593. YGW is composed of nine herbal ingredients (12), including Radix rehmanniae
preparata, Fructus corni officinalis, Fructus
lycii, Colla cornus cervi, Cuscuta chinensis LAM,
Eucommia ulmoides, Radix angelicae sinensis,
Cinnamomum and Radix aconiti lateralis preparata.
Decoctions of the formula were prepared using standard methods
(13), diluted with saline and
stored at 4°C prior to use. Premarin was purchased from Wyeth
Pharmaceuticals (Rouses Point, NY, USA).
Animals
The Animal Care and Use Committee at the Chengdu
University of Traditional Chinese Medicine approved the study and
all associated procedures as protocol no. 30472225.
In total, 73 Sprague Dawley female rats, aged 12–14
weeks, with an average weight of 230±30 g, were used as the test
subjects. The rats were supplied by the Laboratory Animal Service
Center at the Chengdu University of Traditional Chinese Medicine
and maintained in an air-conditioned room with a temperature of
22–25°C, a humidity level of 45–65% and a 12-h light/dark cycle.
The animals were randomly divided into five groups: i)
ovariectomized-treated with YGW decoctions (n=13); ii)
ovariectomized-treated with saline (n=12); iii)
ovariectomized-treated with Premarin (n=12); iv) a sham-surgery in
diestrus (n=17); and v) a normal control in diestrus (n=19). The
reproductive status of the animals was determined by a daily
vaginal lavage performed ~2 h following lights on for all rat
groups using traditional nomenclature for the estrous and diestrus
stages (14).
OVX/sham-surgery and treatment
Procedures for OVX and sham-surgery were described
previously (6). Briefly, rats in
diestrus were anesthetized intraperitoneally with a mixture of
ketamine hydrochloride (73 mg/kg) and xylazine (8.8 mg/kg). Surgery
was performed using aseptic precautions on a heating pad to
maintain a body temperature of 37°C. For OVX, the ovaries were
removed by slicing through the tissue on the rostral side of each
hemostat, while for sham-surgery, four similarly sized sections of
fat were sliced from this segment. Following determination that
there was no bleeding in the abdominal cavity, the wound was
closed. The rats were closely observed during the post-surgical
period for potential complications of which none occurred. OVX was
confirmed by daily vaginal lavages that consisted of leukocytes
indicative of constant diestrus. Constant diestrus continued until
saline, YGW treatment or estrogen replacement which were performed
~2 weeks later.
For groups i)–iii), the rats were orally
administered with saline-diluted YGW decoctions (preparation:
saline, 1:1 v/v and 1 ml/100 g body weight); saline (1 ml/100 g
body weight); and Premarin (62.5 mg/100 g body weight) for 11
weeks, respectively. For groups iv) and v), the rats were orally
administered with saline only. On the final day of oral
administration, the animals were sacrificed and their vaginas were
saved for histological examination, as well as immunohistochemical
and quantitative polymerase chain reaction (qPCR) analysis.
Vaginal histological examination
Procedures for vaginal histological examination were
described previously (6). Briefly,
following fixation of tissue samples in 4% paraformaldehyde in 0.1
M phosphate buffer (pH 7.4) for 24 h at 4°C, the samples were
dehydrated by a routine procedure through graded dilutions of
ethanol (70–100%). Samples were embedded in paraffin and 4 μm
sections were mounted on poly-lysine coated slides. Hematoxylin and
eosin (H&E) staining of the tissue sections was used for
histological examination and the results were summarized as a mean
of three regions observed.
Immunohistochemistry
Immunostaining of ER-α and -β, VEGF and VEGFR-1 in
vaginal tissue was performed using immunohistochemical kits from
Abcam (Cambridge, UK) according to the manufacturer’s instructions.
Briefly, deparaffinization of tissue sections was performed similar
to the aforementioned H&E staining. Following
deparaffinization, an antigen retrieval treatment was performed at
120°C (autoclave) for 5 min in a 10 nmol/l sodium citrate buffer
(pH 6.0). Endogenous peroxidase activity was blocked using a 0.03%
hydrogen peroxide solution containing sodium azide at room
temperature for 30 min. Then, anti-ER-α and -β, VEGF and VEGFR-1
antibodies were incubated at 4°C overnight. Primary antibody
sources and concentrations are listed in Table I. Subsequently, thorough washing in
a 0.01 M phosphate-buffered saline solution was performed. Binding
sites of the primary antibody were visualized using a secondary
antibody conjugated with horseradish peroxidase and
3,3′-diaminobenzidine substrate. Sections were then faintly
counterstained with hematoxylin and mounted with glycerol gelatin.
Negative controls were achieved by the omission of the primary
antibodies.
 | Table ISource and dilution of primary
antibodies used in the current study. |
Table I
Source and dilution of primary
antibodies used in the current study.
| Antibody | Clone | Manufacturer | Dilution |
|---|
| ER-α | 1D5 | ZSGB Bio | 1:100 |
| ER-β | B68 | ZSGB Bio | 1:200 |
| VEGF | VG1 | Maixin Bio | 1:400 |
| VEGFR-1 | Polyclone | Sigma-Aldrich | 1:100 |
Sections were analyzed using an Automated Cellular
Imaging System (ACIS; DAKO, Carpinteria, CA, USA). ACIS calculated
the average intensity within 12 representative regions per slide as
a measure of the integrated optical density (IOD). Scores for the
highest and lowest regions for each slide were excluded and the
average region score was calculated from the remaining 10 regions.
For comparison purposes, the IOD value was normalized to the entire
measured area by calculating IOD/0.25 mm (2,15).
qPCR
To assess the mRNA expression of ER-α, VEGF,
VEGFR-1, Ang1 and 2 and bFGF, qPCR was used as previously described
(16). Briefly, total RNA was
extracted using TRIzol reagents (Invitrogen Life Technologies,
Carlsbad, CA, USA) and complementary DNA was prepared from the
total RNA using oligo primers and Moloney murine leukemia virus
reverse transcriptase (Applied Biosystems, Inc., Foster City, CA,
USA). qPCR was performed with the SYBR-Green mix (Applied
Biosystems, Inc.) on an ABI Prism 7900 sequence detector (Life
Technologies, Carlsbad, CA, USA). Specific primers for ER-α, VEGF,
VEGFR-1, Ang1 and 2, bFGF and glyceraldehyde 3-phosphate
dehydrogenase (GAPDH) are listed in Table II. For each PCR product, the
melting curve was determined using the comparative threshold cycle
number (2−ΔΔCt) method, with the results presented as
fold change in the expression of ER-α, VEGF, VEGFR-1, Ang1, and 2
and bFGF relative to OVX-rats treated with saline (17).
 | Table IIList of the primers used in the qPCR
analysis. |
Table II
List of the primers used in the qPCR
analysis.
| Biomarkers | Primers | Amplified size |
|---|
| ER-α |
5′-GCTCTTGGACAGGAACCAGG-3′ | |
|
5′-AAGATCTCCACCATGCCCTCT-3′ | 187 |
| VEGF |
5′-AGGCCAGCACATAGGAGAGA-3′ | |
|
5′-TTTCTTGCGCTTTCGTTTTT-3′ | 232 |
| VEGFR-1 |
5′-TGTGTGGCTGCGACACTCTT-3′ | |
|
5′-ATAGGGCAGCCGTTCACACT-3′ | 150 |
| Ang1 |
5′-GACAAGACCCCCACGTGTCT-3′ | |
|
5′-CCAGATCAGGTGGTGGCATT-3′ | 150 |
| Ang2 |
5′-GGTCAAGGCCTACTGTGACATG-3′ | |
|
5′-TCGTTGCCCAGCCAATACTC-3′ | 150 |
| bFGF |
5′-GACCCTCACATCAAGCTACAACT-3′ | |
|
5′-AAAGAAACACTCATCCGTAACACA-3′ | 141 |
| GAPDH |
5′-GAAGGTGAAGGTCGGAGT-3′ | |
|
5′-GAAGATGGTGATGGGATTTC-3′ | 226 |
Data analysis
Statistical analysis was performed using the SPSS
software package (SPSS Inc., Chicago, IL, USA). Since the data did
not violate assumptions of homogeneity of variance and normal
distribution, differences in all morphological and histological
parameters of the vagina, as well as the IOD scores and
2−ΔΔCt method among the experimental groups, were
compared using the one-way analysis of variance. If significance
was found in the analysis, the data underwent post-hoc comparisons.
P<0.05 was considered to indicate a statistically significant
difference.
Results
Effect of YGW on vaginal atrophy in
OVX-rats
All parameters of vaginal histomorphology in
OVX-rats treated with saline were significantly lower than those in
normal and sham-surgery animals (all P<0.05). In the group with
Premarin replacement, all the parameters of the vagina in OVX-rats
were reversed to the level of normal and sham-surgery controls.
Treatment with YGW decoctions on OVX-rats reversed the effect of
OVX on the number of vaginal fold and blood vessels in the lamina
propria. These results were in agreement with a previous report
(6).
Effects of YGW on protein expression of
ER, VEGF and VEGFR-1 in OVX-rats
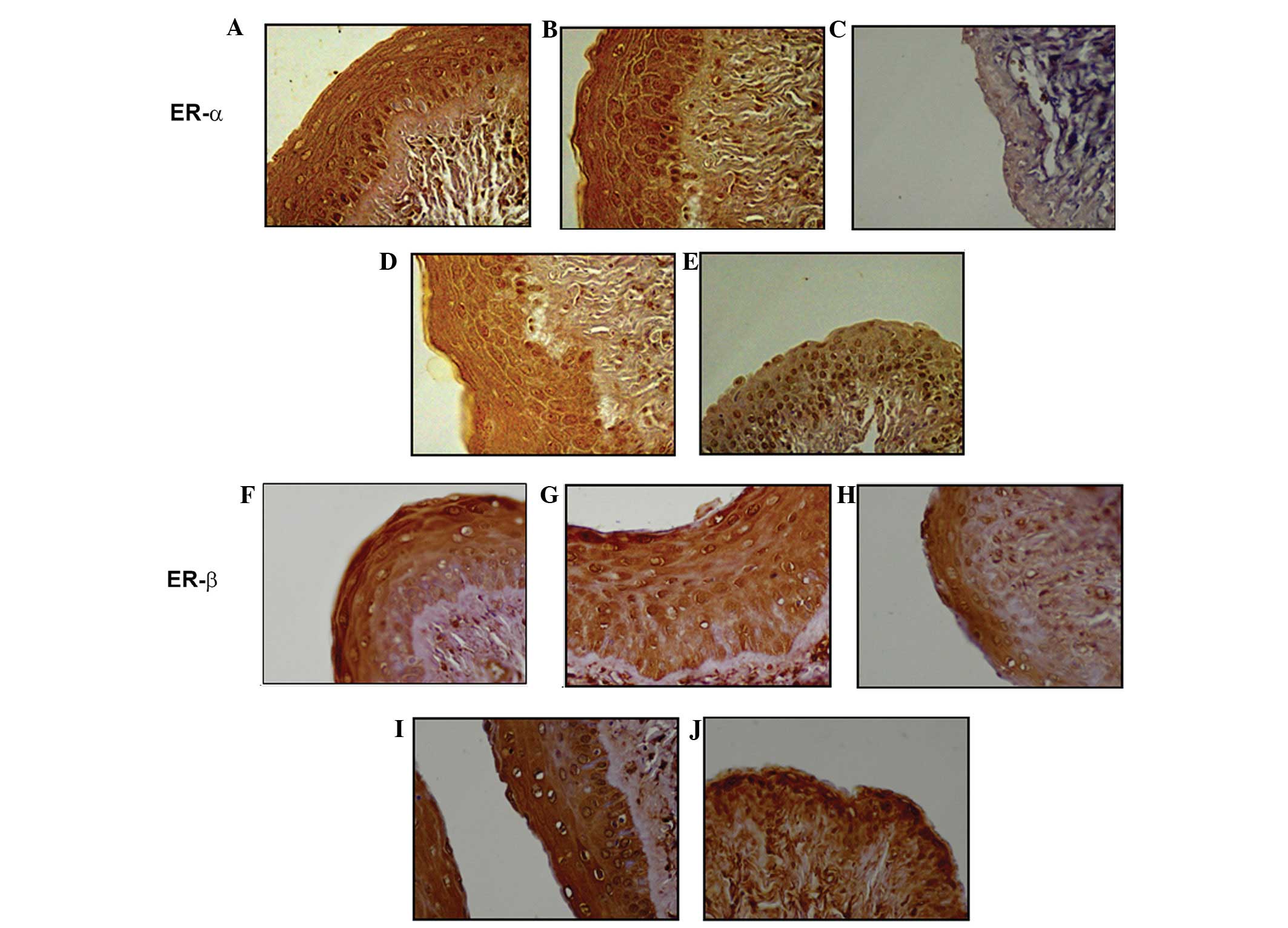
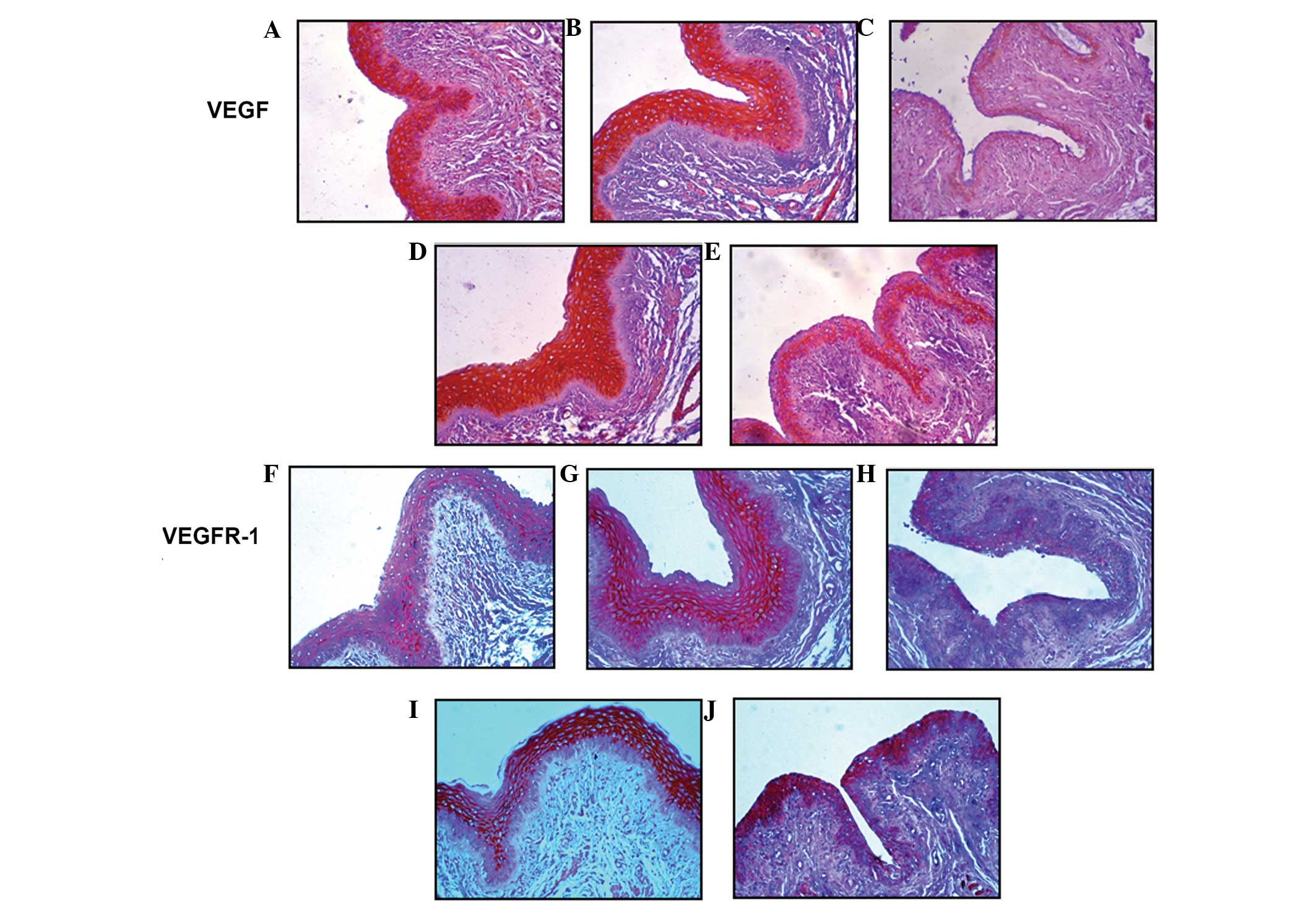
ER-α or -β immunostaining was primarily restricted
to the nucleus of the cells, while VEGF and VEGFR-1 were observed
in membrane and cytoplasmic staining. The effects of OVX, estrogen
replacement and the administration of YGW on the protein expression
of ER-α or -β, VEGF and VEGFR-1 in the rat vagina are summarized in
Table III. In OVX-rats, OVX
decreased the expression of ER-α or -β, VEGF and VEGFR-1 when
compared with normal and sham-surgery animals (P<0.05). Estrogen
replacement and YGW treatment recovered OVX-induced suppression
when compared with normal and sham-surgery controls. Fig. 1 shows representative samples of the
effects of OVX, estrogen replacement and the administration of YGW
on the expression of ER-α or -β. Fig.
2 shows representative samples of the effects of OVX, estrogen
replacement and the administration of YGW on the expression of VEGF
and VEGFR-1.
 | Table IIIEffects of Premarin and YGW on the
expression of ER, VEGF and VEGFR-1. |
Table III
Effects of Premarin and YGW on the
expression of ER, VEGF and VEGFR-1.
| Groups | n | ER-α IOD value,
mean ± SE | ER-β IOD value,
mean ± SE | VEGF IOD value,
mean ± SE | VEGFR-1 IOD value,
mean ± SE |
|---|
| Normal | 17 | 483.72±65.66 | 557.06±53.40 | 483.72±65.66 | 557.06±53.40 |
| Sham-surgery | 19 | 507.10±53.71 | 549.70±44.39 | 507.10±53.71 | 549.70±44.39 |
| OVX-rats with
saline | 12 |
340.25±35.03a |
352.08±69.68a |
340.25±35.03a |
352.08±69.68a |
| OVX-rats with
Premarin | 12 | 506.67±43.32 | 550.75±46.78 | 506.67±43.32 | 550.75±46.78 |
| OVX-rats with
YGW | 13 | 470.08±64.72 | 411.00±98.54 | 470.08±64.72 | 411.00±98.54 |
Effects of YGW on the transcription of
ER-α, VEGF, VEGFR-1, Ang1 and 2 and bFGF
Comparisons of gene transcription of ER-α, VEGF,
VEGFR-1, Ang1 and 2 and bFGF in vaginal tissue among the groups are
shown in Fig. 3. The levels of
ER-α mRNA expression in normal, sham-surgery, OVX- with Premarin
and OVX-rats with YGW were 1.83, 1.54, 1.53 and 1.36 times higher
compared with OVX-rats with saline, respectively (P<0.05;
Fig. 3A). Moreover, mRNA
expression of VEGF, bFGF and Ang1 in normal, sham-surgery, OVX-
with Premarin and OVX- with YGW rats were also significantly higher
compared with OVX-rats with saline (VEGF mRNA were 4.21, 3.54, 3.20
and 5.92 times higher; bFGF mRNA were 1.97, 1.74, 2.02 and 1.92
times higher and Ang1 mRNA were 2.73, 2.50, 5.50 and 4.46 times
higher, respectively; all P<0.05; Fig. 3B, D and E). Notably, the VEGF mRNA
expression in the vaginal tissue of OVX-rats treated with YGW was
significantly higher compared with the estrogen replacement group
(3.20 for the estrogen replacement group vs. 5.92 for the OVX-rats
treated with YGW; P<0.05; Fig.
3B). VEGFR-1 mRNA expression in normal, sham-surgery and
OVX-rats with Premarin were 1.39, 1.47 and 1.34 times higher
compared with OVX-rats with saline, respectively (P<0.05), but
no significant difference was found between OVX-rats with YGW and
OVX-rats with saline (Fig. 3C).
Ang-2 mRNA expression in normal rats was similar to that of the
OVX-rats with saline (0.94). However, for sham-surgery and OVX-rats
with Premarin or YGW, Ang2 mRNA expression was 1.25, 2.44 or 2.32
times higher compared with the OVX- rats with saline (P<0.05;
Fig. 3E). The ratios of Ang1 and 2
mRNA expression in normal, sham-surgery and OVX-rats with Premarin
or YGW were 2.18, 2.66, 2.25 and 2.22 times higher compared with
the OVX-rats with saline (P<0.05; Fig. 3F).
 | Figure 3Effects of YGW on the mRNA levels of
(A) ER-α; (B) VEGF; (C) VEGFR-1; (D) bFGF; and (E) Ang1 and 2 in
rat vaginal tissues by real-time polymerase chain reaction. Fold
changes (2−ΔΔCt) in the expression of these angiogenic
factors were calculated from the ΔCt values for the angiogenic
factors, obtained following the subtraction of Ct values for GAPDH
(internal control), relative to those for OVX-rat with saline. (F)
Ratios of Ang1 mRNA to Ang2 mRNA in normal, sham-surgery, OVX- with
saline, OVX- with Premarin and OVX-rats with YGW are shown.
Vertical error bars indicate ± standard error.
*P<0.05 vs. OVX-rats with saline and
#P<0.05 for Premarin- vs. YGW-treated OVX-rats. YGW,
You Gui Wan; ER, estrogen receptor; VEGF, vascular endothelial
growth factor; VEGFR-1, vascular endothelial growth factor
receptor-1; bFGF, basic fibroblast growth factor; Ang,
angiopoietin; GAPDH, glyceraldehyde 3-phosphate dehydrogenase; OVX,
ovariectomy. |
Discussion
In the current study, the effects of the
administration of YGW decoctions in reversing rat vaginal atrophy
caused by OVX were confirmed and were in agreement with a previous
study (6). The major observations
of the present study may be summarized as follows: i) OVX reduces
the expression of vaginal ER and specific angiogenic factors in
vaginal tissue; ii) estrogen replacement may recover OVX effects
and iii) YGW decoction may also recover expression of ER and
specific angiogenic factors. The upregulation effects of YGW were
not implemented by endogenous estrogen production as YGW had no
significant effect on the circulating estrogen levels reduced by
OVX (6).
The promotion of angiogenesis by estrogen
replacement may be via ER signaling pathways since reduced estrogen
production following OVX decreased the number of ER in the blood
vessel walls, as well as changes in the post-ER signaling
mechanisms (18). ER has been
observed to mediate angiogenesis through classical genomic and
rapid non-genomic mechanisms (19–21).
How YGW implements its effects on the various
angiogenic factors remains to be determined as the chemical profile
of YGW is not yet known. However, there may be one or more main
active components in each of the herb in YGW according to published
data. The primary active component of Radix rehmanniae
reparata has been reported to be oligosaccharides (22). In Fructus corni officinalis,
the primary active components are morroniside, loganin and gallic
acid (23). In Fructus
lycii, it is Lycium Barbarum polysaccharide (24). For Cuscuta chinensis LAM,
the component is hypothesized to be flavones glycoside (25) and for Eucommia ulmoides, the
components consist of acidic polysaccharide metal salt and
glucosidic metal salt (26).
Radix angelicae sinensis is reported to contain phthalides,
organic acids and their esters (27) while Cinnamomum has
phenylpropanoids cinnamaldehyde and eugenol (28). Radix aconiti lateralis
preparata is reported to contain benzoylmesaconine (29).
A number of these active components have been
hypothesized to possess estrogenic activity. For example, flavones
glycoside, the component of Cuscuta chinensis LAM, may be a
phytoestrogen (30). A number of
phytoestrogens have been defined as selective estrogen receptor
modulators. These substances may also provide cardiovascular
benefits, including regulation of ECs proliferation,
differentiation, adhesion, migration and kinase activation through
interaction with ER (31,32). Other components may directly exert
angiogenic activity. For instance, morroniside, one of the active
components of Fructus corni officinalis, may exert a
beneficial effect on preventing diabetic angiopathies (33). However, accumulated clinical
observations have concluded that the herbal formula of YGW is more
effective than any single herb since the formula may possess much
broader actions. Therefore, the effects of the YGW herbs may be a
synthetic action of all main constituents rather than a single
component.
Although YGW and Premarin may reverse the expression
of ER and specific angiogenic factors, varying potency was observed
in the current study. For example, VEGF mRNA expression in the
vaginal tissue of OVX-rats treated with YGW was higher compared
with that of the estrogen replacement group, while YGW had no
significant effect on the repression of VEGFR-1 mRNA. It is
possible that YGW may contain a number of components that may
directly affect VEGF expression other than via ER signaling
pathways. These observations also suggest that YGW may have
stronger effects on angiogenesis since VEGF is a critical and
specific factor stimulating physiological and pathological
angiogenesis (34). However, to
verify this, further studies are required in the future.
In conclusion, the current observations indicate
that YGW, similar to estrogen replacement, may recover the
expression of ER and various angiogenic factors in the vaginal
tissue of OVX-rats. The induction of the expression of various
angiogenic factors by the herbal formula was, at least in part,
mediated through the activation of the ER pathways. The current
study provides an explanation for the underlying mechanisms of YGW
to reverse vaginal atrophy induced by OVX, although future research
is required to elucidate the herb-induced signaling pathways. These
data may aid in the development of improved approaches to stimulate
angiogenesis, as well as to provide an improved understanding of
the potential health benefits of the herbal agents of YGW in
treating vaginal atrophy induced by OVX or menopause.
Acknowledgements
This study was supported by grants from the National
Basic Research Program in China (973 Plan, no. 2010CB530403 and
2010CB530400).
References
|
1
|
Anderson GL, Limacher M, Assaf AR, et al:
Effects of conjugated equine estrogen in postmenopausal women with
hysterectomy: the Women’s Health Initiative randomized controlled
trial. JAMA. 291:1701–1712. 2004.
|
|
2
|
Haines CJ, Lam PA, Chung TK, Cheng KF and
Leung PC: A randomized, double-blind, placebo-controlled study of
the effect of a Chinese herbal medicine preparation (Dang Gui Buxue
Tang) on menopausal symptoms in Hong Kong Chinese women.
Climacteric. 11:244–251. 2008. View Article : Google Scholar
|
|
3
|
Chan CC, Lau WN, Chiu SP, Chen LC, Choi WK
and Tang GW: A pilot study on the effects of a Chinese herbal
preparation on menopausal symptoms. Gynecol Endocrinol. 22:70–73.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Kwee SH, Tan HH, Marsman A and Wauters C:
The effect of Chinese herbal medicines (CHM) on menopausal symptoms
compared to hormone replacement therapy (HRT) and placebo.
Maturitas. 58:83–90. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Zell B, Hirata J, Marcus A, Ettinger B,
Pressman A and Ettinger KM: Diagnosis of symptomatic postmenopausal
women by traditional Chinese medicine practitioners. Menopause.
7:129–134. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Hu X, Wang J, Yin QZ, Lu H and Yie SM: You
Gui Wan can reverse atrophic effect of ovariectomy on rat vaginal
fold and blood vessels in the lamina propria. Biol Pharm Bull.
34:1808–1814. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Applanat MP, Buteau-Lozano H, Herve MA and
Corpet A: Vascular endothelial growth factor is a target gene for
estrogen receptor and contributes to breast cancer progression. Adv
Exp Med Biol. 617:437–444. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Fujimoto J, Hori M, Ichigo S and Tamaya T:
Ovarian steroids regulate the expression of basic fibroblast growth
factor and its mRNA in fibroblasts derived from uterine
endometrium. Ann Clin Biochem. 34:91–96. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Presta M: Sex hormones modulate the
synthesis of basic fibroblast growth factor in human endometrial
adenocarcinoma cells: implications for the neovascularization of
normal and neoplastic endometrium. J Cell Physiol. 137:593–597.
1988. View Article : Google Scholar
|
|
10
|
Hangai M, Murata T, Miyawaki N, Spee C,
Lim JI, He S, Hinton DR and Ryan SJ: Angiopoietin-1 upregulation by
vascular endothelial growth factor in human retinal pigment
epithelial cells. Invest Ophthalmol Vis Sci. 42:1617–1625.
2001.PubMed/NCBI
|
|
11
|
Fiedler U and Augustin HG: Angiopoietins:
a link between angiogenesis and inflammation. Trends Immunol.
27:552–558. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Zhou J and Qu F: Treating gynaecological
disorders with traditional Chinese medicine: a review. Afr J Tradit
Complement Altern Med. 6:494–517. 2009.PubMed/NCBI
|
|
13
|
Sze SC, Tong Y, Zhang YB, Zhang ZJ, Lau
AS, Wong HK, Tsang KW and Ng TB: A novel mechanism: Erxian
Decoction, a Chinese medicine formula, for relieving menopausal
syndrome. J Ethnopharmacol. 123:27–33. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Becker JB, Arnold AP, Berkley KJ,
Blaustein JD, Eckel LA, Hampson E, Herman JP, Marts S, Sadee W,
Steiner M, et al: Strategies and methods for research on sex
differences in brain and behavior. Endocrinology. 146:1650–1673.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Oberholzer M, Ostreicher M, Christen H and
Brühlmann M: Methods in quantitative image analysis. Histochem Cell
Biol. 105:333–355. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Yie SM, Li LH, Li GM, Xiao R and Librach
CL: Progesterone enhances HLA-G gene expression in JEG-3
choriocarcinoma cells and human cytotrophoblasts in vitro. Hum
Reprod. 21:46–51. 2006.PubMed/NCBI
|
|
17
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(−Delta DeltaC(T)) Method. Methods. 25:402–408. 2001.
|
|
18
|
Masood DE, Roach EC, Beauregard KG and
Khalil RA: Impact of sex hormone metabolism on the vascular effects
of menopausal hormone therapy in cardiovascular disease. Curr Drug
Metab. 11:693–714. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Losordo DW and Isner JM: Estrogen and
angiogenesis: A review. Arterioscler Thromb Vasc Biol. 21:6–12.
2001. View Article : Google Scholar
|
|
20
|
Kim KH, Moriarty K and Bender JR: Vascular
cell signaling by membrane estrogen receptors. Steroids.
73:864–869. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Kim-Schulze S, McGowan KA, Hubchak SC, Cid
MC, Martin MB, Kleinman HK, Greene GL and Schnaper HW: Expression
of an estrogen receptor by human coronary artery and umbilical vein
endothelial cells. Circulation. 94:1402–1407. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Liu WX, Lu YW, Du HT and Wu ZZ:
Pharmacological actions of Radix Rehmanniae and its active
components: research advances. J Int Pharm Res. 36:277–280.
2009.
|
|
23
|
Wang SF, Chen XG, Hu ZD and Ju Y: Analysis
of three effective components in Fructus corni and its
preparations by micellar electrokinetic capillary chromatography.
Biomed Chromatogr. 17:306–311. 2003.PubMed/NCBI
|
|
24
|
Li SY, Yang D, Yeung CM, Yu WY, Chang RC,
So KF, Wong D and Lo AC: Lycium barbarum polysaccharides
reduce neuronal damage, blood-retinal barrier disruption and
oxidative stress in retinal ischemia/reperfusion injury. PLoS One.
6:e163802011. View Article : Google Scholar
|
|
25
|
Jin X, Li J and Yan M: Flavonoids in the
seed of Cuscuta chinensis Lam. Zhongguo Zhong Yao Za Zhi.
17:292–294. 1992.(In Chinese).
|
|
26
|
Deyama T, Nishibe S and Nakazawa Y:
Constituents and pharmacological effects of Eucommia and
Siberian ginseng. Acta Pharmacol Sin. 22:1057–1070. 2001.PubMed/NCBI
|
|
27
|
Yi L, Liang Y, Wu H and Yuan D: The
analysis of Radix Angelicae Sinensis (Danggui). J Chromatogr
A. 1216:1991–2001. 2009.
|
|
28
|
Lockwood GB: Phenylpropanoids from a
Nigerian sample of Cinnamomum cassia [proceedings]. J Pharm
Pharmacol. 31:8P1979. View Article : Google Scholar
|
|
29
|
Xie Y, Zhou H, Wong YF, Liu Z, Xu H, Jiang
Z and Liu L: An optimized high-performance liquid chromatography
(HPLC) method for benzoylmesaconine determination in Radix
Aconiti Lateralis Preparata (Fuzi, aconite roots) and
its products. Chin Med. 3:62008. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Matsuda H, Shimoda H, Morikawa T and
Yoshikawa M: Phytoestrogens from the roots of Polygonum
cuspidatum (Polygonaceae): structure-requirement of
hydroxyanthraquinones for estrogenic activity. Bioorg Med Chem
Lett. 11:1839–1842. 2001.
|
|
31
|
Valachovicova T, Slivova V and Sliva D:
Cellular and physiological effects of soy flavonoids. Mini Rev Med
Chem. 4:881–887. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Kostelac D, Rechkemmer G and Briviba K:
Phytoestrogens modulate binding response of estrogen receptors
alpha and beta to the estrogen response element. J Agric Food Chem.
51:7632–7635. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Xu HQ, Hao HP, Zhang X and Pan Y:
Morroniside protects cultured human umbilical vein endothelial
cells from damage by high ambient glucose. Acta Pharmacol Sin.
25:412–415. 2004.PubMed/NCBI
|
|
34
|
Zhang K, Lu J, Mori T, Smith-Powell L,
Synold TW, Chen S and Wen W: Baicalin increases VEGF expression and
angiogenesis by activating the ERR alpha/PGC-1alpha pathway.
Cardiovasc Res. 89:426–435. 2011. View Article : Google Scholar : PubMed/NCBI
|