Introduction
Breast cancer is one of the most common types of
malignant tumors in females and results in high mortality rates
(1). Although numerous diagnostic
techniques and therapeutic strategies have been identified,
metastatic breast cancer remains an incurable disease with little
response to current therapeutic approaches (2). Thus, the development of more
effective therapeutic strategies is required.
Oncolytic adenoviruses have been proposed as a
potential gene therapy vehicle for cancer cells as it enables
tumor-selective replication, viral self-spreading and guaranteed
efficient expression of transgene in cancer cells without
cross-resistance to current treatments (3,4).
Recently, numerous modified oncolytic adenoviruses are becoming
agents for cancer treatment. This includes tumor-specific promoters
which are used to drive the early viral gene, chimeric fibers which
are constructed to improve infection efficacy, and viral genes
which are deleted to prevent oncogene activation. The human
telomerase reverse transcriptase promoter (TERTp) (5,6),
hypoxia response elements (HRE)-containing promoter (7) and survivin promoter (8) are present in various cancer cells.
Moreover, a human α-fetoprotein (AFP) promoter has also been used
in AFP-producing cells (9,10) and a cyclooxygenase-2 (Cox2)
promoter (11,12) used in cells overexpressing Cox2.
For high infection activity of adenovirus serotype 5 (Ad5), which
requires the coxsackie and adenovirus receptor (CAR) localized on
the surface of cancer cells, the Ad5/35 chimeric fiber was created
(13,14). This chimeric fiber infected cells
in a CD46-dependent mechanism. In addition, an RGD-modified fiber
has also been used in cancer gene therapy (15).
The initial and most intensively engineered gene
deletion, adenoviral ONYX-015 (dl1520), was based on the
deletion of E1B-55 kDa, which prevents p53 binding and degradation
in normal cells (16,17). An adenovirus deletion mutant
dl922-947 (a 24-bp deletion) defective in pRb-binding was
engineered in order to improve potency. It has been shown that
dl922-947 activity was associated with deregulation of
multiple cell cycle checkpoints (18). ZD55 constructed by Professor Liu
(Chinese Academy of Sciences, Shanghai, China), harbored the E1B-55
kDa deletion and a BglII restriction site for carrying the
therapeutic gene within the genome. ZD55 has been demonstrated to
exhibit efficient activities in tumor therapy (19). One of our previous studies
introduced a replication-selective adenovirus with improved
potency, ZD55-IL-24. It was constructed by harboring the E1B-55 kDa
deletion and arming ZD55 with interleukin-24 (IL-24). IL-24 also
termed melanoma differentiation associated gene-7, exhibited
multiple antitumor activities, including suppressing growth and
inducing the apoptosis of tumor cells without harming normal cells
(20,21).
However, efficient treatments usually combine a
number of different therapies, including surgical removal,
chemotherapy and radiotherapy (22,23).
Safety and tumor selectivity of ONYX-015 was demonstrated; however,
efficacy was achieved only in combination with chemotherapeutic
agents, such as cisplatin or 5-FU in advanced cancer (24). A modest response of
dl922-947 was also reported in combination with gemcitabine
in pancreatic cancer cells compared with trials using the virus
alone (25,26). The microtubule-stabilizing drug
paclitaxel (PTX) exhibits activity in relapsed cancer and regimes
containing a weekly low-dose PTX are highly effective (27).
The present study aimed to investigate the
activities of ZD55-IL-24 in combination with PTX in breast cancer
cells. The results suggested that when ZD55-IL-24 was combined with
PTX, cancer cell cytotoxicity was markedly increased. PTX increased
viral uptake but did not affect the replication of ZD55-IL-24 in
breast cancer cells. Notably, it was demonstrated that ZD55-IL-24
conjugated with PTX promoted a markedly stronger cell apoptosis by
regulating the intrinsic apoptotic pathway. These results showed
that ZD55-IL-24 in combination with PTX exhibited a marked increase
in the induced cytotoxic and apoptotic effect in breast cancer
cells. This chemo-gene-viro therapeutic strategy is superior to
conventional chemotherapy or gene-viro therapy alone.
Materials and methods
Cell culture and adenoviral
construction
MDA-MB-231 and Bcap-37 human breast cancer cell
lines were obtained from the Institute of Biochemistry and Cell
Biology, Shanghai Institute for Biological Sciences, Chinese
Academy of Sciences (Shanghai, China). The (HEK)-293 human
embryonic kidney cell lines were obtained from Microbix Biosystems
Inc. (Toronto, ON, Canada). MDA-MB-231 was cultured in Leibovitz’s
L-15 medium (Life Technologies, Carlsbad, CA, USA) with fetal
bovine serum (FBS) to a final concentration of 10%. Bcap-37 was
cultured in RPMI-1640 medium (Life Technologies) with FBS to a
final concentration of 10%. HEK-293 was cultured in Dulbecco’s
minimal essential medium (Life Technologies) with FBS to a final
concentration of 10%. All media were supplemented with 4 mM
glutamine, 100 U/ml penicillin, and 100 mg/ml streptomycin. All
cells in the experiment were cultured under a 5% CO2
humidified atmosphere at 37°C.
The replication-selective adenovirus, ZD55-IL-24,
had been described previously and was constructed by harboring the
E1B-55 kDa deletion and arming with IL-24 (21). ZD55-EGFP was also constructed as a
control virus, which carried enhanced green fluorescent protein
(EGFP) as a reporter gene. Large-scale preparations of these
adenoviruses were produced by ultracentrifugation (Optima L-100XP,
Beckman, USA) on cesium chloride. Paclitaxel (PTX) was purchased
from Nanjing Pharmaceutical Factory Co., Ltd. (Nanjing, Jiangsu,
China). A final concentration of 0.25 μg/ml was used as the
treatment dose based on preliminary experiments.
Reverse transcription-PCR
Bcap-37 cells were treated with 0.25 μg/ml PTX, 10
multiplicity of infection (MOI) ZD55-EGFP; 10 MOI ZD55-IL-24; 10
MOI ZD55-EGFP and 0.25 μg/ml PTX; or 10 MOI ZD55-IL-24 and 0.25
μg/ml PTX. After 48 h, total RNA was extracted using TRIzol reagent
(Invitrogen Life Technologies, Carlsbad, CA, USA). cDNA was
obtained from the total RNA using the One Step RT-PCR kit (Promega,
Madison, WI, USA) according to the manufacturer’s instructions.
Primers used for the quantification of E1A, IL-24 and
glyceraldehyde 3-phosphate dehydrogenase (GAPDH) mRNA were as
follows: Sense: 5′-ATTATCTGCCACGGAGGTGTT-3′ and antisense:
5′-CAAAGGTTGCCCAGACTC-3′ for E1A; sense:
5′-CCCGGTCGACATGAATTTTCAACAGAGG-3′ and antisense:
5′-GGGTGGATCCTCAGAGCTTGTAGAATT TCTGC-3′; for IL-24; and sense:
5′-TCCATGA CAACTTTGGTATC-3′ and antisense: 5′-TTCAGCTCAGG
GATGACCTT-3′ for GAPDH.
Western blot analysis
Following treatment, Bcap-37 cells were scraped into
200 μl lysis buffer [containing 150 mmol/l NaCl, 50 mmol/l Tris (pH
7.5), 0.05% sodium dodecyl sulphate (SDS) and 1% Triton X-100] and
sonicated on ice. Protein (50 μg) was electrophoresed on
SDS-polyacrylamide gels and transferred onto a nitrocellulose
filter by semi-dry blotting. The following antibodies were used:
Mouse anti-E1A (Santa Cruz Biotechnology, Inc., Santa Cruz, CA,
USA), rabbit anti-IL-24, rabbit anti-(cleaved) caspase-3, -7 and
-9, rabbit anti-(cleaved) poly ADP ribose polymerase (PARP), rabbit
anti-B-cell lymphoma-extra large (BCL-XL), rabbit anti-myeloid
leukemia cell differentiation protein (MCL-1) rabbit
anti-Bcl-2-associated X protein (BAX; Cell Signaling Technology,
Inc., Danvers, MA, USA), and mouse anti-β-actin (Santa Cruz
Biotechnology, Inc.). Detection was performed by AP-conjugated
antibodies and NBT/BCIP substrate (Promega, Madison, WI, USA).
Cell viability assay
For the cell viability assay, MDA-MB-231 and Bcap-37
breast cancer cells were plated on 96-well plates at a density of
1×104 cells/well 1 day prior to virus infection. Cells
were then infected with phosphate-buffered saline; 0.25 μg/ml PTX;
ZD55-EGFP; ZD55-IL-24; ZD55-EGFP and 0.25 μg/ml PTX; or ZD55-IL-24
and 0.25 μg/ml PTX, different adenoviruses were used at an MOI of
10. On days 1, 2, 3 and 4 following treatment, cell viability was
determined by an MTT assay according to the manufacturer’s
instructions at the indicated time. Cells were also infected with
ZD55-EGFP or ZD55-IL-24 at doses of different MOIs with different
concentrations of PTX (0.1 MOI + 0.01 μg/ml, 1 MOI + 0.05 μg/ml, 10
MOI + 0.25 μg/ml or 50 MOI + 0.5 μg/ml). Cell viability was
detected 4 days following infection. In each treatment group, eight
wells were measured for cell viability. All cell viability assays
were conducted in triplicate. Representative results are shown
unless otherwise stated.
Flow cytometric analysis
MDA-MB-231 and Bcap-37 cells were incubated with
0.25 μg/ml PTX for 24 h, followed by infection with 10 MOI
ZD55-EGFP for 2 h. On day 0, 1 or 2 following infection, EGFP
expression was observed under a fluorescence microscope. The cells
were then harvested 48 h after infection and the proportion of
EGFP-positive cells was determined by flow cytometry (FACS-Canto II
instrument, BD, UK), acquiring 20,000 events per sample from
duplicate wells using propidium iodide (PI) to exclude dead cells.
The analysis was conducted using Cell Quest software (BD
Biosciences, Franklin Lakes, NJ, USA).
Viral replication assay
For viral replication assays, cells were placed in
6-well plates at a density of 5×105 cells/well. The
cells were treated with 0.25 μg/ml PTX and 24 h subsequent to this,
were infected with ZD55-IL-24 or ZD55-EGFP at an MOI of 10
pfu/cell. Cells infected with ZD55-IL-24 or ZD55-EGFP but not added
to PTX served as controls. At 0 h (the beginning of infection) and
48 h following infection, the cells were harvested, lysed by three
cycles of freeze/thaw at −80°C and 37°C and serial dilutions of the
lysates were subsequently tittered on HEK293 cells with tissue
culture infectious dose 50 (TCID50) methods, as
described previously (28).
Cell apoptosis analysis
MDA-MB-231 and Bcap-37 cells were infected with 0.25
μg/ml PTX; 10 MOI ZD55-EGFP; 10 MOI ZD55-IL-24; 10 MOI ZD55-IL-24
and 0.25 μg/ml PTX; or ZD55-IL-24 and 0.25 μg/ml PTX. The apoptotic
cells were assayed with Annexin V-fluorescein isothiocyanate (FITC)
and propidium iodide (Annexin V-FITC Apoptosis Detection Kit,
KeyGen Biotech, Nanjing, China) under a fluorescence microscope
after 48 h. The fluorescence signal in cells probed by Annexin
V-FITC was green and that probed by PI was red. The number of
apoptotic cells was counted in five fields of vision chosen at
random. The average value was calculated as the rational data.
Hoechst 33258 staining
Cells were treated with drugs or adenovirus as
described above for the fluorescence microscopy analysis. Following
2 days, cells were fixed with 4% paraformaldehyde and stained with
Hoechst 33258 (Apoptotic Cell Hoechst 33258 Detection kit, KeyGen
Biotech) for 10 min at room temperature. Fluorescence was
visualized with a Nikon standard fluorescence microscope (Ti-U,
Nikon, Japan). The number of cells with nuclei condensation and
fragmentation were counted.
Statistical analysis
Data are expressed as the mean ± SD unless otherwise
stated. Results were compared for statistical significance by a
t-test. P<0.05 and P<0.01 were considered to indicate a
statistically significant difference.
Results
PTX results in an increase in E1A and
IL-24 mRNA but not protein expression levels in breast cancer
cells
To investigate the effect of PTX on adenovirus gene
expression, the Bcap-37 human breast cancer cell line was treated
with 0.25 μg/ml PTX, infected with 10 MOI ZD55-EGFP or ZD55-IL-24,
or ZD55-EGFP or ZD55-IL-24 in combination with PTX. RT-PCR analysis
showed an increase in E1A mRNA level 48 h following infection with
ZD55-EGFP or ZD55-IL-24 in combination with PTX; and an IL-24 mRNA
level in ZD55-IL-24 plus PTX group (Fig. 1A and B). The quantity of E1A mRNA
in the ZD55-EGFP + PTX group was 1.28-fold greater than that in the
ZD55-EGFP group, 2.23-fold greater than that in the ZD55-IL-24 +
PTX group than that for the ZD55-IL-24 group in the Bcap-37 cells.
The quantity of IL-24 mRNA for the ZD55-IL-24 + PTX group was
1.49-fold greater than that in the ZD55-IL-24 group in Bcap-37
cancer cells. There was no E1A and IL-24 mRNA expression in the PTX
group. These results indicated that PTX enhanced adenoviral gene
mRNA expression.
The expression levels of associated proteins were
also investigated. Western blot analysis was performed when Bcap-37
cells had been treated as previously described. Cell lysates were
harvested after 48 h. As shown in Fig.
1C, no increase in protein levels of E1A or IL-24 were observed
in any oncolytic adenovirus plus PTX treatment group. The control
PBS and PTX groups exhibited no E1A and IL-24 expression as
expected. These results indicated that PTX increased the transgenic
mRNA but not protein expression mediated by oncolytic adenoviruses
defective in p53-binding (E1B-55 kDa deletion).
Administration of oncolytic adenovirus in
combination with PTX induces increased cytotoxicity in breast
cancer cells compared with that in the groups with virus alone
To investigate whether breast cancer cells were able
to be sensitized to PTX by oncolytic adenoviruses, 0.25 μg/ml PTX
was added with 10 MOI ZD55-EGFP or ZD55-IL-24 in MDA-MB-231 and
Bcap-37 cells. Cell cytotoxicity was markedly increased in all
tested cell lines treated with ZD55-EGFP or ZD55-IL-24 in
combination with PTX compared with that of the control groups
(Fig. 2A and B). In addition, we
also wanted to confirm whether the strong synergistic cell
cytotoxic effect was viral dose-dependent. MDA-MB-231 and Bcap-37
cells were infected with ZD55-EGFP or ZD55-IL-24 at MOIs of 0.1, 1,
10, 50 combined with or without varying concentrations of PTX. Cell
viability was also detected following 4 days of infection. The most
potent synergistic responses were observed in cells with 0.25 μg/ml
PTX with 10 MOI adenovirus ZD55-IL-24 infection (Fig. 2C and D). Moreover, ZD55-IL-24 plus
PTX exhibited higher cytotoxicity than ZD55-EGFP plus PTX in these
cells. In Bcap-37 cells, the difference between 10 MOI and 50 MOI
ZD55-IL-24 combined with PTX and the PTX control group was
significant (*P<0.05). In MDA-MB-231 cells, the
difference between the 10 MOI ZD55-IL-24 combined with PTX and the
PTX group was significant (**P<0.01). In conclusion,
oncolytic adenoviruses and PTX increased cell cytotoxicity and to a
certain extent, oncolytic adenoviruses enhanced the sensitivity of
breast cancer cells to PTX.
PTX increases viral uptake in breast
cancer cells and viral replication is not altered in response to
PTX
To determine whether the observed synergy was due to
increased permissiveness to viral infection in the presence of PTX,
MDA-MB-231 and Bcap-37 cells were infected with an EGFP-expressing
virus (ZD55-EGFP) to monitor viral uptake. On day 0, 1 or 2
following infection, EGFP expression was observed by fluorescence
microscopy (Fig. 3A). The EGFP
expression level was low 24 h following infection, but was
significantly increased after 48 h. Moreover, EGFP expression in
these cells was increased in ZD55-EGFP combined with PTX compared
with ZD55-EGFP alone. Furthermore, the expression of EGFP tagged
virus in these cells was determined by flow cytometry analysis. The
cells were harvested 48 h following infection and the proportion of
EGFP-positive cells was determined by acquiring 20,000 events per
sample from duplicate wells using PI to exclude dead cells. As
shown in Fig. 3B, viral uptake
increased with the administration of PTX in the two breast cancer
cell lines. The difference was significant between combinations of
virus and PTX and mock (virus without PTX). The number of
EGFP-positive cells in ZD55-EGFP combined with PTX was 5-fold that
in ZD55-EGFP infected MDA-MB-231 cells, and 3-fold that in Bcap-37
cells.
The study also aimed to detect the potential
drug-induced effects of a combination treatment on factors of the
viral life cycle, such as replication. As shown in Fig. 3C, viral replication was high after
48 h at a MOI of 10 for ZD55-EGFP and ZD55-IL-24 infected
MDA-MB-231 and Bcap-37 cells. When cells were pretreated with PTX
at 0.25 μg/ml, viral replication was marginally attenuated in the
two cell lines. The expression of E1A was also detected in the
treatment group and there was no significant change in the presence
of PTX (data not shown). The replication of the virus was closely
associated with the expression of E1A. The results also
demonstrated that the involvement of E1A in replication and was
consistent with the fact that PTX did not increase protein
expression mediated by oncolytic adenovirus defective in
p53-binding (E1B-55 kDa deletion) (Fig. 1C).
Oncolytic adenovirus in combination with
PTX induced increased apoptosis in breast cancer cells
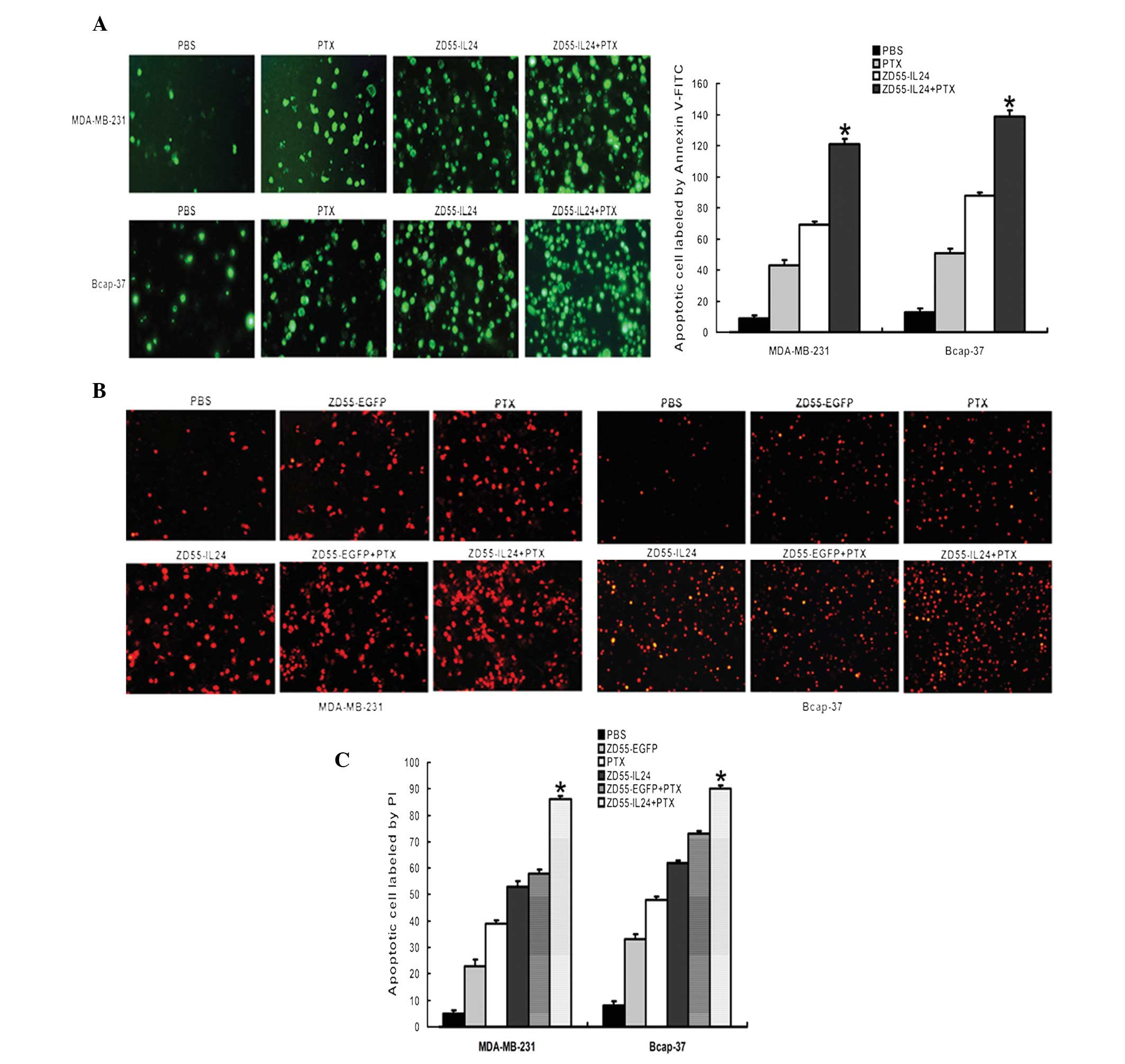
Apoptosis in MDA-MB-231 and Bcap-37 cells were
infected with ZD55-EGFP or ZD55-IL-24, PTX, or a combination of
both. For the early apoptosis assay, cells were incubated with
Annexin V-FITC following treatment for 48 h. As shown in Fig. 4A, there was a marked cytopathic
effect and cells became rounded. The number of green fluorescent
cells was greatest in the ZD55-IL-24+PTX group compared with the
other groups. As ZD55-EGFP itself expressed green fluorescence and
due to the difficulty in distinguishing EGFP with FITC, apoptosis
in the ZD55-EGFP or ZD55-EGFP+PTX groups was not analyzed in this
assay. The results from the analysis of apoptosis using PI were
similar to that when analyzed with FITC (Fig. 4B and C). A combination of
ZD55-IL-24 and PTX resulted in large scale cell apoptosis. The
quantization of positive cells was analyzed by Image-J software.
The fluorescence signal in cells probed by Annexin V-FITC (green)
and PI (red) was obtained by fluorescence microscopy. The number of
apoptotic cells was counted in five fields of vision chosen at
random. The average value was calculated as the rational data.
Oncolytic adenovirus in combination with
PTX induces apoptosis in breast cancer cells via a
mitochondria-mediated pathway
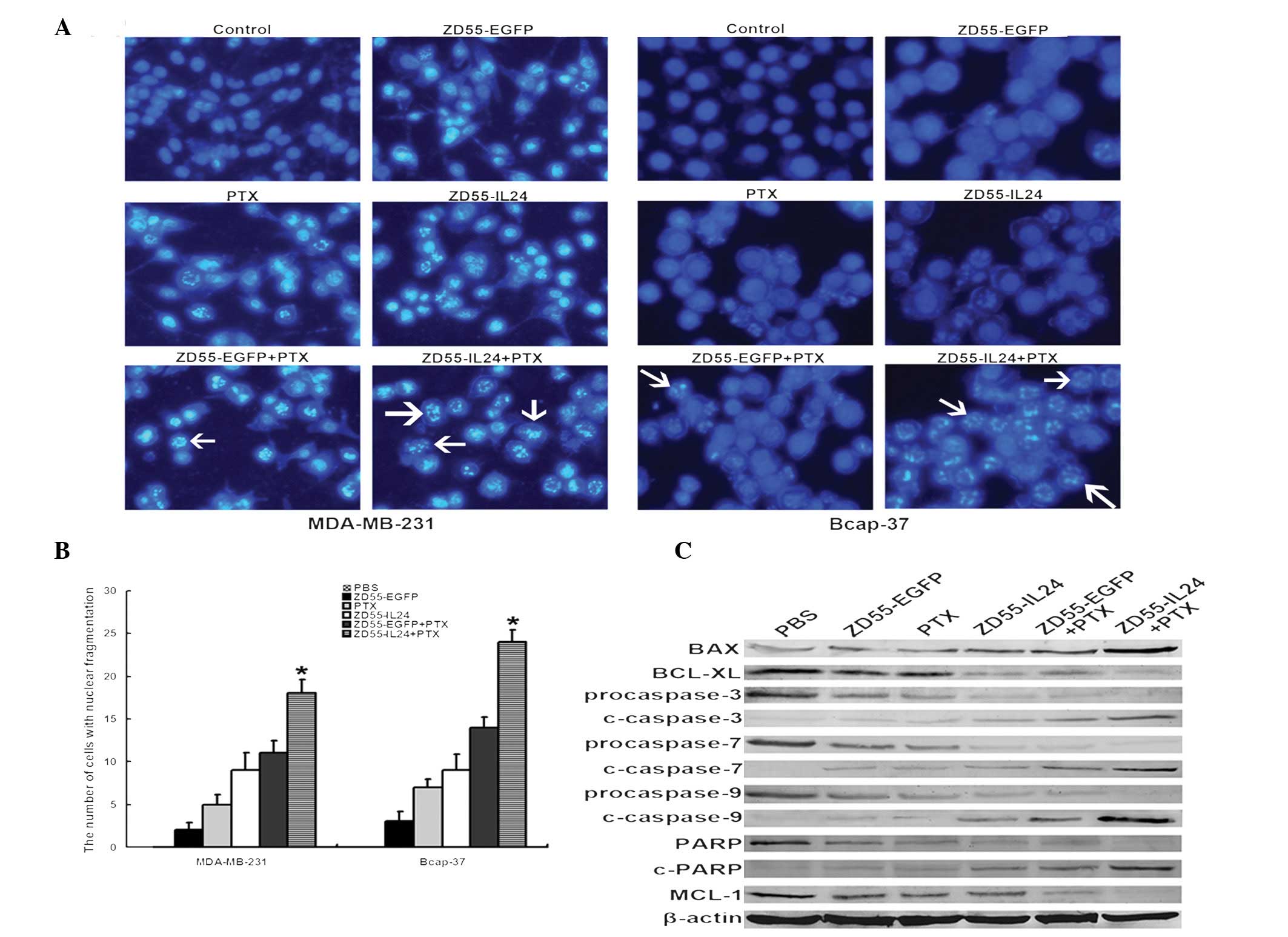
The apoptosis of cells was also analyzed by Hoechst
33258 staining following treatment (Fig. 5A). Nuclear fragmentation and
chromatin clumping were observed in cells in the ZD55-IL-24 plus
PTX group. The number of cells with nuclear fragmentation was
greatest in the ZD55-IL-24 plus PTX group (Fig. 5B). The components of the apoptosis
signaling cascade were investigated by immunoblotting in Bcap-37
cells. As shown in Fig. 5C, BAX,
cleaved caspase-3, -7 and -9, and PARP were upregulated and the
anti-apoptotic proteins BCL-XL and MCL-1 were decreased following
treatment with oncolytic adenovirus, PTX or a combination of the
two. It was observed that the ZD55-IL-24 plus PTX group exhibited a
higher expression level of pro-apoptotic protein (BAX), lower
anti-apoptotic protein (BCL-XL and MCL-1) and higher downstream
active caspase family members. These results showed that ZD55-IL-24
in combination with PTX induced cell apoptosis efficiently and
exhibited enhanced anti-tumor activities in breast cancer
cells.
Discussion
The incidence of breast carcinoma has increased
annually and the prognosis for this cancer is remains poor
regardless of the improved treatment options during the past
decades (29). Traditional
treatment, such as surgery, chemotherapy or radiotherapy are not
breakthrough therapies and are associated with significant side
effects, thus the development of novel potent curative treatments
is required. Oncolytic adenoviruses provide numerous advantages in
cancer therapy (30) due to
selective and efficient replication in tumor cells, which reduced
the therapeutic dose. The viruses replicate within and lyse tumor
cells, which leads to cell death. Novel progeny viruses released
from host cells infect neighboring cells continually and ultimately
eliminate tumor cells. The combination of viral and gene therapy
developed into the novel strategy of gene-viro therapy, in which
therapeutic genes amplified abundantly with adenovirus replication
and functioned as antitumor agents (31,32).
Recently, it has been demonstrated that the combination of
replication-selective oncolytic adenoviruses with chemotherapeutic
agents is a promising cancer therapy (33).
PTX was approved as a novel drug for the treatment
of ovarian cancer by the FDA in 1992 (34). Numerous studies have confirmed that
a variety of proteins are related to paclitaxel-induced apoptosis
such as Bcl-2 protein family of Raf-1, caspase-3, PARP, P34/cdc2,
p53, and p27 (35–38). Like most chemotherapeutic drugs,
paclitaxel exhibits side effects, and the treatment efficacy is
attenuated when the dosage is reduced. Therefore, utilization of
lower doses of PTX combined with other treatments may achieve
similar or improved therapeutic effects without increasing the side
effects.
The results of the present study suggested that
oncolytic IL-24 armed adenovirus with the E1B-55 kDa deletion
(ZD55-IL-24) exhibited significant activity when combined with low
doses (0.25 μg/ml) of the microtubule-stabilizing drug, PTX,
through the mitochondria-mediated pathway in breast cancer cells.
The mRNA and protein expression were investigated when cells had
been infected with ZD55-EGFP or ZD55-IL-24 in combination with PTX.
The mRNA level of E1A and transgenes (EGFP and IL-24) in oncolytic
adenoviruses increased; however, the protein expression did not
increase accordingly (Fig. 1C).
These results demonstrated the different roles of PTX in the
expression of oncolytic adenovirus transgenic mRNA and protein. A
possible mechanism explaining this result is that PTX is a
microtubule-stabilizing drug and adenoviruses require microtubules
for nuclear transport. Further studies are required to determine
the contribution of PTX in adenovirus mediated cancer therapy.
It was also demonstrated that the cell cytotoxic
efficacy of ZD55-IL-24 combined with PTX was greater than that with
virus or drug treatment alone. The synergistic cell cytotoxic
effect of the virus plus PTX groups was virus dose-dependent.
ZD55-IL-24 and PTX may have a synergistic effect depending on
different mechanisms. ZD55-IL-24 promoted the sensitivity of cells
to cytotoxic drugs. The results also demonstrated that although PTX
increased viral uptake, viral replication was not affected in
response to PTX in breast cancer cells. As adenoviral infection and
replication were different biological behaviors, cell surface
receptors (e.g. CAR) were required for infection and key viral
genes (e.g. E1A, the first viral gene expressed) were important for
viral replication. Annexin V-FITC/PI staining and the Hoechst 33258
assay indicated that ZD55-IL-24 induced greater cell apoptosis when
combined with PTX. Furthermore, the possible mechanism was
investigated. ZD55-IL-24 expressed IL-24 with high efficiency,
which was concomitant with increased levels of pro-apoptotic
proteins, activated caspase-3, -7 and -9 and downregulated
anti-apoptotic proteins. These results showed that ZD55-IL-24
conjugated with PTX exhibited a markedly increased cytotoxic and
apoptotic effect in breast cancer cells.
In conclusion, replication-selective oncolytic
adenoviruses carrying the IL-24 gene, ZD55-IL-24, significantly
enhanced the effects of PTX on the suppression of MDA-MB-231 and
Bcap-37 cell proliferation and induced efficient cell apoptosis.
PTX did not affect the protein expression of the therapeutic genes
and the replication of ZD55-IL-24 in breast cancer cells. Thus,
this study suggested that gene-viro therapy combined with
chemotherapy represent a potent approach for breast cancer
therapy.
Acknowledgements
This study was supported by grants from the National
Natural Science Foundation of China (grant nos. 81101702, 81071854
and 30972976); the Science and Technology Department of Jiangsu
province (grant nos. BK2011207, BK2009091 and BK2009089); the
College Science and Technology Foundation of Jiangsu province
(grant nos. 12KJA320001 and 11KJA320002); the Science Foundation of
Xuzhou Medical College (grant no. 2010KJZ04); and the Science and
Technology Department of Xuzhou (no. XF11C061).
References
|
1
|
Krychman ML and Katz A: Breast cancer and
sexuality: multi-modal treatment options. J Sex Med. 9:5–13. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Haas JS, Liang SY, Hassett MJ, Shiboski S,
Elkin EB and Phillips KA: Gene expression profile testing for
breast cancer and the use of chemotherapy, serious adverse effects,
and costs of care. Breast Cancer Res Treat. 130:619–626. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Choi JW, Lee JS, Kim SW and Yun CO:
Evolution of oncolytic adenovirus for cancer treatment. Adv Drug
Deliv Rev. 64:720–729. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Huang JH, Zhang SN, Choi KJ, et al:
Therapeutic and tumor-specific immunity induced by combination of
dendritic cells and oncolytic adenovirus expressing IL-12 and
4-1BBL. Mol Ther. 18:264–274. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Hashimoto Y, Tazawa H, Teraishi F, et al:
The hTERT promoter enhances the antitumor activity of an oncolytic
adenovirus under a hypoxic microenvironment. PLoS One.
7:e392922012. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Fang L, Pu YY, Hu XC, et al:
Antiangiogenesis gene armed tumor-targeting adenovirus yields
multiple antitumor activities in human HCC xenografts in nude mice.
Hepatol Res. 40:216–228. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Cho WK, Seong YR, Lee YH, et al: Oncolytic
effects of adenovirus mutant capable of replicating in hypoxic and
normoxic regions of solid tumor. Mol Ther. 10:938–949. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Ulasov IV, Zhu ZB, Tyler MA, et al:
Survivin-driven and fiber-modified oncolytic adenovirus exhibits
potent antitumor activity in established intracranial glioma. Hum
Gene Ther. 18:589–602. 2007. View Article : Google Scholar
|
|
9
|
Kim KI, Park JH, Lee YJ, et al: In vivo
bioluminescent imaging of α-fetoprotein producing hepatocellular
carcinoma in diethylnitrosamine-treated mouse using recombinant
adenoviral vector. J Gene Med. 14:513–520. 2012.
|
|
10
|
Kwon OJ, Kim PH, Huyn S, Wu L, Kim M and
Yun CO: A hypoxia- and α-fetoprotein-dependent oncolytic adenovirus
exhibits specific killing of hepatocellular carcinomas. Clin Cancer
Res. 16:6071–6082. 2010.
|
|
11
|
Nakagawa T, Tanaka H, Shirakawa T, et al:
Cyclooxygenase 2 promoter-based replication-selective adenoviral
vector for hypopharyngeal cancer. Arch Otolaryngol Head Neck Surg.
135:282–286. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Bauerschmitz GJ, Guse K, Kanerva A, et al:
Triple-targeted oncolytic adenoviruses featuring the cox2 promoter,
E1A transcomplementation, and serotype chimerism for enhanced
selectivity for ovarian cancer cells. Mol Ther. 14:164–174. 2006.
View Article : Google Scholar
|
|
13
|
Ganesh S, Gonzalez-Edick M, Gibbons D,
Waugh J, Van Roey M and Jooss K: Evaluation of biodistribution of a
fiber-chimeric, conditionally replication-competent (oncolytic)
adenovirus in CD46 receptor transgenic mice. Hum Gene Ther.
20:1201–1213. 2009. View Article : Google Scholar
|
|
14
|
Hoffmann D, Meyer B and Wildner O:
Improved glioblastoma treatment with Ad5/35 fiber chimeric
conditionally replicating adenoviruses. J Gene Med. 9:764–778.
2007. View
Article : Google Scholar : PubMed/NCBI
|
|
15
|
Wakayama M, Abei M, Kawashima R, et al:
E1A, E1B double-restricted adenovirus with RGD-fiber modification
exhibits enhanced oncolysis for CA-deficient biliary cancers. Clin
Cancer Res. 13:3043–3050. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Ries SJ, Brandts CH, Chung AS, et al: Loss
of p14ARF in tumor cells facilitates replication of the adenovirus
mutant dl1520 (ONYX-015). Nat Med. 6:1128–1133. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Harada JN and Berk AJ: p53-Independent and
-dependent requirements for E1B-55K in adenovirus type 5
replication. J Virol. 73:5333–5344. 1999.PubMed/NCBI
|
|
18
|
Heise C, Hermiston T, Johnson L, et al: An
adenovirus E1A mutant that demonstrates potent and selective
systemic anti-tumoral efficacy. Nat Med. 6:1134–1139. 2000.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Zhang ZL, Zou WG, Luo CX, et al: An armed
oncolytic adenovirus system, ZD55-gene, demonstrating potent
antitumoral efficacy. Cell Res. 13:481–489. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Tian H, Wang J, Zhang B, et al:
MDA-7/IL-24 induces Bcl-2 denitrosylation and ubiquitin-degradation
involved in cancer cell apoptosis. PLoS One. 7:e372002012.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Jiang G, Liu YQ, Wei ZP, Pei DS, Mao LJ
and Zheng JN: Enhanced anti-tumor activity by the combination of a
conditionally replicating adenovirus mediated interleukin-24 and
dacarbazine against melanoma cells via induction of apoptosis.
Cancer Lett. 294:220–228. 2010. View Article : Google Scholar
|
|
22
|
Halldén G: Optimisation of
replication-selective oncolytic adenoviral mutants in combination
with chemotherapeutics. J BUON. 14(Suppl 1): S61–S67.
2009.PubMed/NCBI
|
|
23
|
Saito K, Shirasawa H, Isegawa N, Shiiba M,
Uzawa K and Tanzawa H: Oncolytic virotherapy for oral squamous cell
carcinoma using replication-competent viruses. Oral Oncol.
45:1021–1027. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Khuri FR, Nemunaitis J, Ganly I, et al: A
controlled trial of intratumoral ONYX-015, a
selectively-replicating adenovirus, in combination with cisplatin
and 5-fluorouracil in patients with recurrent head and neck cancer.
Nat Med. 6:879–885. 2000. View
Article : Google Scholar : PubMed/NCBI
|
|
25
|
Cherubini G, Kallin C, Mozetic A, et al:
The oncolytic adenovirus AdΔΔ enhances selective cancer cell
killing in combination with DNA-damaging drugs in pancreatic cancer
models. Gene Ther. 18:1157–1165. 2011.
|
|
26
|
Bhattacharyya M, Francis J, Eddouadi A,
Lemoine NR and Halldén G: An oncolytic adenovirus defective in
pRb-binding (dl922-947) can efficiently eliminate pancreatic cancer
cells and tumors in vivo in combination with 5-FU or gemcitabine.
Cancer Gene Ther. 18:734–743. 2011. View Article : Google Scholar
|
|
27
|
Ingemarsdotter CK, Baird SK, Connell CM,
Öberg D, Halldén G and McNeish IA: Low-dose paclitaxel synergizes
with oncolytic adenoviruses via mitotic slippage and apoptosis in
ovarian cancer. Oncogene. 29:6051–6063. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Gao Y, Nankya I, Abraha A, et al:
Calculating HIV-1 infectious titre using a virtual TCID(50) method.
Methods Mol Biol. 485:27–35. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Walker RA, Hanby A, Pinder SE, Thomas J
and Ellis IO; National Coordinating Committee for Breast Pathology
Research Subgroup. Current issues in diagnostic breast pathology. J
Clin Pathol. 65:771–785. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Wong HH, Lemoine NR and Wang Y: Oncolytic
viruses for cancer therapy: overcoming the obstacles. Viruses.
2:78–106. 2010. View
Article : Google Scholar : PubMed/NCBI
|
|
31
|
Cun B, Song X, Jia R, et al: Combination
of oncolytic adenovirus and dacarbazine attenuates antitumor
ability against uveal melanoma cells via cell cycle block. Cancer
Biol Ther. 13:77–84. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Abate-Daga D, Andreu N, Camacho-Sánchez J,
et al: Oncolytic adenoviruses armed with thymidine kinase can be
traced by PET imaging and show potent antitumoural effects by
ganciclovir dosing. PLoS One. 6:e261422011. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Chu RL, Post DE, Khuri FR and Van Meir EG:
Use of replicating oncolytic adenoviruses in combination therapy
for cancer. Clin Cancer Res. 10:5299–5312. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Menzin AW, King SA, Aikins JK, Mikuta JJ
and Rubin SC: Taxol (paclitaxel) was approved by FDA for the
treatment of patients with recurrent ovarian cancer. Gynecol Oncol.
54:1031994.PubMed/NCBI
|
|
35
|
Flores ML, Castilla C, Ávila R,
Ruiz-Borrego M, Sáez C and Japón MA: Paclitaxel sensitivity of
breast cancer cells requires efficient mitotic arrest and
disruption of Bcl-xL/Bak interaction. Breast Cancer Res Treat.
133:917–928. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Lu PH, Yu CC, Chiang PC, et al: Paclitaxel
induces apoptosis through activation of nuclear protein kinase C-δ
and subsequent activation of Golgi associated Cdk1 in human hormone
refractory prostate cancer. J Urol. 186:2434–2441. 2011.
|
|
37
|
Le XF, Mao W, He G, et al: The role of
p27(Kip1) in dasatinib-enhanced paclitaxel cytotoxicity in human
ovarian cancer cells. J Natl Cancer Inst. 103:1403–1422. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Heikaus S, Matuszek KS, Suschek CV, et al:
Paclitaxel (Taxol)-induced apoptosis in human epithelioid sarcoma
cell lines is enhanced by upregulation of CD95 ligand
(FasL/Apo-1L). J Cancer Res Clin Oncol. 134:689–695. 2008.
View Article : Google Scholar : PubMed/NCBI
|