Introduction
Atrial fibrillation (AF) is the most common cardiac
arrhythmia. It affects millions of individuals worldwide and the
incidence increases with age (1,2). The
increased number of cases of AF in the elderly may be due to
several factors, including disease, fibrosis and/or age-related
changes in cellular electrophysiological properties, which are
likely to lead to a disturbance in atrial activation and
repolarization (3–6). Calcium regulation is commonly
implicated in numerous forms of injury that may occur during
cellular aging. Altered calcium homeostasis has been correlated
with age-related phenomena (5,7,8).
Calpains are calcium-dependent enzymes that are readily activated
by elevated cellular calcium levels. In addition, calcium overload
is involved in the pathogenesis of AF (9–11)
and is also responsible for the initiation of other short-term
adaptation mechanisms through the inactivation of the functional
L-type Ca2+ current (ICa-L) (12,13).
Theoretically, calpain activation may be a process
that results in important cellular changes with aging and/or in AF.
Calpains have been demonstrated to degrade cytoskeletal,
contractile and L-type Ca2+ channel proteins involved in
atrial remodeling in AF (12,13).
The reduction in the expression of the L-type calcium channel a1c
subunit (LVDCCa1c) is suggested to be important in
electrical remodeling in AF (9,14,15).
This study aimed to investigate the correlation between the change
in the expression of atrial calpains (calpain 1, calpain 2 and
calpastatin) and cellular alterations in canines. In particular,
the characteristic reduction in LVDCCa1c in left atrial
cells and the accelerated fibrosis and apoptosis that occur with
aging and/or in AF (induced by chronic rapid atrial pacing) were
investigated.
Materials and methods
Animals
Fourteen adult (age, 1–3 years) and 14 aged (age,
>8 years) mongrels, weighing 18–26 kg each, were obtained from
the Animal Center of Xinjiang Medical University (Xinjiang, China).
The ages of the canines were estimated by a veterinarian based on
standard measures for age, including dentition, coat, eyes and
musculoskeletal and conformational descriptors. The canines were
kept in a temperature-controlled environment under a 12-h
light/dark cycle and fed a standard laboratory diet and water ad
libitum. The Animal Care and Use Committee of the Xinjiang
Medical University (Xinjiang, China) approved all experiments in
accordance with the Declaration of the National Institutes of
Health Guide for Care and Use of Laboratory Animals (16).
Six-lead electrocardiogram (ECG) measurements were
performed on resting, conscious canines to confirm sinus rhythm
(SR). In addition, echocardiograms were performed to exclude
canines with structural heart disease. Canines from both age groups
were randomly divided into 4 groups (n=7); the adult SR, the aged
SR, the adult AF and the aged AF groups. AF was induced by chronic
rapid atrial pacing and was defined as persistence of AF for at
least 5 days.
Induction of AF
In total, seven adult and seven aged dogs underwent
the procecure. Animals were anesthetized with pentobarbital sodium
(30 mg/kg, i.v.; Jinyao Company, Tianjin, China) and ventilated
with isoflurane (1.5–2%; Yapei Company, Shanghai, China), and
O2 (2 l/min). Morphine sulfate (0.15 mg/kg; The First
Pharmaceutical Company, Shenyang, China) was injected into the
epidural space to maintain postoperative analgesia. Using sterile
techniques, a right intercostal thoracotomy was performed, the
pericardium was opened and the heart was suspended in a pericardial
cradle. A lead was attached to the epicardium of the left atrial
appendage, tunneled subcutaneously and connected to a Pulse
Generator (Department of Electronic Engineering, Fudan University,
Shanghai, China). Pulse generators were implanted in subcutaneous
pockets on the left posterior chest wall. When the incisions had
been closed and the canines had recovered from anesthesia, they
were monitored for 2–3 days in the recovery room prior to being
moved for routine care. The canines were prophylactically treated
with cefazolin (25 mg/kg, i.v.) twice daily for 2 days following
surgery. They were allowed to stabilize for 1 week and were paced
at 600 bpm from the left atrial appendage to induce persistent AF.
Canines were used for the in vitro study when they exhibited
persistent AF for ≥5 days.
Atrial myocyte preparation
Subsequent to the experiments, the canines were
anesthetized with pentobarbital sodium (30 mg/kg, i.v.) and
sternotomies were performed. The hearts were quickly removed and
sections of the left atrial wall samples were rapidly frozen in
liquid nitrogen and stored separately at −80°C for further
analysis. One aliquot of each tissue sample was used to investigate
mRNA expression of target genes and another was used to determine
the protein levels. In addition, the hearts were rinsed in
oxygenated Ca2+-free Tyrode’s solution [137 mmol/l NaCl,
5.4 mmol/l KCl, 1.0 mmol/l MgCl2, 0.33 mmol/l
NaH2PO4, 10 mmol/l HEPES and 10 mmol/l
glucose (pH 7.4, NaOH)]. The aortae were cannulated and the hearts
were retrogradely perfused on a Langendorff apparatus (HEKA
Instruments, Inc., Lambrecht, Germany) at 37°C. A perfusion of
Ca2+-free Tyrode’s solution for 5 min was followed by
Ca2+-free Tyrode’s solution containing 0.03% collagenase
II (Worthington Biochemical, Lakewood, NJ, USA) and 1% bovine serum
albumin (BSA) for 35 min. The left atria were dissected, minced and
gently triturated with a pipette in a Ca2+ Tyrode’s
solution containing 1% BSA at 37°C for 10 min. The cells were
filtered through a 200-μm nylon mesh (Chenjie Company, Shanghai,
China) and resuspended in the Tyrode’s solution, in which the
Ca2+ concentration was gradually increased to 1.0
mmol/l. Only cells with a rod-shaped morphology and clear
cross-striation were used for the experiments.
Cellular electrophysiological
studies
Left atrium cells were maintained in a 1-ml bath,
continuously superfused with normal Tyrode’s solution (2–3 ml/min)
containing 137 mmol/l NaCl, 5.4 m mol/l KCl, 1.0 mmol/l,
MgCl2 1.8 mmol/l CaCl2, 0.33 mmol/l
NaH2PO4, 10 mmol/l HEPES and 10 mmol/l
glucose (the pH was adjusted with NaOH to 7.4) and bubbled with
100% O2. Membrane currents and action potentials (APs)
were recorded using whole-cell patch clamp techniques with an EPC
10/n Double amplifier (HEKA Instruments, Inc.) and Patchmaster
software (HEKA). Patch pipette resistances ranged from 2.0 to 3.0
MΩ when filled with an internal solution. The AP was recorded in
current-clamp mode. The solution for AP recording contained 137
mmol/l NaCl, 5.4 mmol/l KCl, 1.0 mmol/l MgCl2, 1.8
mmol/l CaCl2, 10 mmol/l HEPES and 20 mmol/l glucose (the
pH was adjusted with KOH to 7.4). The internal solution for the
electrode for the AP recording contained 140 mmol/l KCl, 2.0 mmol/l
MgCl2, 2.0 mmol/l egtazic acid, 5.0 mmol/l HEPES, 5
mmol/l EGTA and 4.0 mmol/l ATP-Na2 (the pH was adjusted
with KOH to 7.4). The calcium currents were recorded in the
voltage-clamp mode. The external solution for the ICa-L
recording contained 137 mmol choline-Cl, 2.0 mmol CaCl2,
1.0 mmol MgCl2, 5 mmol HEPES, 10 mmol glucose, 4.6 mmol
CsCl, 10 mmol TEA-Cl and 5 mmol 4-AP (pH 7.30, CsOH). The internal
solution for ICa-L recording contained 120 mmol CsCl,
1.0 mmol MgCl2, 5.0 mmol MgATP, 10 mmol BAPTA, 10 mmol
HEPES and 10 mmol TEA-Cl (pH 7.30, CsOH). In the present study,
data acquisition began 10 min following membrane rupture.
ICa-L magnitudes were normalized by each cellular
membrane capacitance (pF) and expressed as current density (pA/pF).
Recordings were filtered at low pass (2 Hz) and high pass (30 Hz).
Activation voltage dependence was assessed by
depolarization-induced currents, with the driving force corrected
by dividing membrane potential (MP)-Erev, where Erev is the voltage
axis intercept of the ascending limb of the current-voltage
relation. Inactivation was assessed with 1-sec prepulses at −60,
−50, −40, −35, −30, −25, −20, −15, −10, 0, 10, 20, 30 and 40 mV,
followed by 240-msec test pulses to +10 mV. The Boltzmann equation
was used to fit data. ICa-L recovery was studied with
paired 240-msec pulses to 10 mV (0.1 Hz) delivered at progressively
increasing interpulse intervals (P-P) of 3, 5, 8, 10, 20, 40, 60,
80, 160, 300, 500 and 1000 msec.
Morphological evaluation
Tissues from the left atrial wall were immediately
fixed with 4% paraformaldehyde at 4°C and embedded in paraffin.
Light microscopy was performed using 2-μm sections stained with
hematoxylin and eosin, and Masson’s trichrome stain (Yisha
Biological Technology Co., Ltd., Shanghai, China). To quantify the
extent of myolysis in the cardiomyocytes, at least two sections per
atrial site and ≥200 cells/section were observed. The extent of
cell change was only analyzed in cells in which the nucleus was
present in the plane of the section. The myolytic area of the
cardiomyocytes was measured using a digital imaging system (Motic
Images Advanced, Richmond, BC, Canada). Cells were scored as either
mildly myolytic when myolysis involved 10–25% of the cytosol or
severely myolytic when >25% of the sarcomeres were absent.
For electron microscopy, ultrathin sections (50–100
nm) were cut from each sample, counterstained with uranium acetate
and lead citrate and observed under a transmission electron
microscope (Philips 201, Philips Scientific, Mahwah, NJ, USA) at
high magnification (x10,000).
TUNEL assay
Sections were transferred to xylene and rehydrated
in decreasing concentrations of alcohol. Slides were then incubated
for 10 min at room temperature with 10 μg proteinase K
(Sigma-Aldrich, St. Louis, MO, USA) per 1 ml of phosphate-buffered
saline. Encanineenous peroxydase was inactivated by ImmunoPure
peroxydase suppressor for 30 min (Pierce Biotechnology Inc.,
Rockford, IL, USA). Tissue sections were permeabilized with 1%
Triton X-100 at 4°C for 2 min and stained using an in situ
cell detection system (POD, Boehringer Mannheim, Mannheim,
Germany). DNA strand breaks were identified by labeling free 3′-OH
termini with deoxyuridine triphosphate-fluorescein isothiocyanate
using terminal deoxynucleotidyl transferase (Tdt) for terminal
deoxynucleotidyl-transferase-mediated dUTP nick end labeling
(TUNEL) analysis. The incorporated fluorescein was detected by
anti-fluorescein antibody Fab fragments from sheep conjugated with
horseradish peroxydase (POD). Following the reaction with the
metal-enhanced DAB substrate (Boehringer Mannheim), the sections
were counterstained with hematoxylin. For positive controls, fixed
and permeabilized sections were incubated with DNase I (1 μg/ml)
for 10 min at room temperature. For negative controls, sections
were incubated in labeling solution without Tdt. The percentage of
TUNEL-positive nuclei was calculated by examining 50 randomly
selected fields/section, containing ~700 cells, at high
magnification (x400).
Gene expression
Total RNA was extracted from samples of the left
artium appendage using TRIzol (Invitrogen Life Technologies,
Carslbad, CA, USA). The expression levels of the target genes were
measured via quantitative (q)PCR using SYBR-Green qPCR Master mix
(Bio-Rad, Hercules, CA, USA) in a 20-μl reaction volume containing
50 ng cDNA. All reactions were performed in triplicate and included
a negative control. PCR reactions were conducted using the ABI
Prism 7500 Sequence Detection System (Invitrogen Life
Technologies). Cycling conditions were 2 min at 50°C, 10 min at
95°C, 40 cycles for 15 sec at 95°C and 1 min at 60°C. Relative
quantification of mRNA levels was conducted using the 7500 system
software (Invitrogen Life Technologies), which uses the comparative
method. Fluorescence signals were normalized to the housekeeping
gene β-actin. The comparative threshold (Ct) cycle method was used
(ΔCt) for relative quantification. For every sample, each gene was
quantified in duplicate in three separate experiments. The values
were averaged and then used for the 2−ΔΔCt calculation,
where 2−ΔΔCt corresponds to expression relative to that
of β-actin. The expected size of the amplicons were confirmed by
gel electrophoresis. The sequences of the genes studied were
obtained from GenBank, and the primers were designed using the
Primer 5.0 software (Invitrogen Life Technologies). The following
primer sequences, amplicon size and annealing temperatures were
used: Forward, 5′-AAGGACCTGTATGCCAACACA-3′ and reverse,
5′-ATCCACACAGAATACTTGCGTT-3′ (152 bp, 57°C) for β-actin; forward,
5′-GACGCTATGGGCTATGAGTTAC-3′ and reverse,
5′-AGTCCAGGTAGCCCTTTAGGT-3′ (199 bp, 58°C) for LVDCCa1c;
forward, 5′-TCACCCTCAATGACACGCTT-3′ and reverse,
5′-GCAGCAGGTCATCCACGA-3′ (135 bp, 58°C)for calpain 1; forward,
5′-TCACCCTCATGAGTTAC3′ and reverse, 5′-ATCCACACTGCCAACACA-3′ (125
bp, 57.5°C) for calpain 2; and forward, 5′-GCTGGTTCCTCAAACACTTCA-3′
and reverse, 5′-CATCGTCCAGTTCTTTGTTGTC-3′ (154 bp, 57.2°C) for
calpastatin.
Assessment of protein expression
Membrane proteins were extracted from tissue samples
of the left atrium (LA) with 5 mmol/l Tris-HCl (pH 7.4), 2 mmol/l
EDTA, 5 μg/ml leupeptin, 10 μg/ml benzamidine and 5 μg/ml soybean
trypsin inhibitor. Subsequent to this, tissue homogenization was
conducted. All procedures were performed at 4°C. Equal quantities
(100 μg/sample) of LA membrane proteins were separated on 8% sodium
dodecyl sulfate-polyacrylamide gel electrophoresis gels and
transferred on polyvinylidene difluoride membranes. The membranes
were blocked in 5% non-fat dry milk in TBST (tris-buffered saline
with Tween 20; 50 mmol/l Tris-HCl, 500 mmol/l NaCl; pH 7.5, 0.05%
Tween-20) for 2 h and incubated with primary antibody (dilution,
1:500) in 5% non-fat dry milk in TBST for 4 h at room temperature.
The membranes were then incubated with the following antibodies:
rabbit polyclonal anti-LVDCCa1c (Santa Cruz
Biotechnology, Santa Cruz, CA, USA) and rabbit polyclonal
anti-calpain 1, -calpain 2 and -calpastatin (Santa Cruz
Biotechnology). The membranes were washed three times in TBST,
reblocked in 5% non-fat dry milk and TBST for 15 min and incubated
with horseradish peroxidase (HRP)-conjugated goat anti-rabbit
antibodies (dilution, 1:5,000) in 5% non-fat dry milk in TBST (40
min). Immunoreactive bands were detected by Immun-Star HRP
Substrate (Bio-Rad) and quantified through densitometry analysis
using an ImageQuant 350 imager and ImageQuant TL-1 software (GE
Healthcare, Little Chalfront, UK). Anti-β-actin antibody (Santa
Cruz Biotechnology) was used to control equal protein loading and
normalize ion channel protein band intensity. All western blot
analysis target bands were expressed quantitatively by
normalization to the control band in the same lane. Western blot
analysis band intensities were expressed in optical density units,
according to the densitometric analysis of band intensity following
background subtraction, divided by the β-actin signal intensity for
the same sample.
Statistical analysis
The AP properties measured include maximum diastolic
potential (MDP), amplitude of phase 0 (APA), maximum upstroke
velocity, potential at the peak of the plateau and AP duration to
90% repolarization (APD90). Quantitative data were
presented as the mean ± standard deviation. Comparisons between the
quantitative and qualitative data were performed using a t-test and
the χ2 method, respectively. SPSS 10.0 software (SPSS
Inc., Chicago, IL, USA) was used for statistical analysis.
P<0.05 was considered to indicate a statistically significant
difference.
Results
ECG data
The ECGs of the aged canines manifested a longer
P-wave duration and dispersion than adult canines (66.1±6.4 versus
75.9±5.3 msec and 19.1±4.1 versus 26.7±3.1 msec, respectively; n=7;
P<0.05). Other variables did not differ. There was no
significant difference identified between the two groups at the
time of persistent AF onset; the adult canines developed persistent
AF following 40±5 days and the aged canines following 52±7 days of
atrial pacing (P>0.05).
Changes in AP characteristics
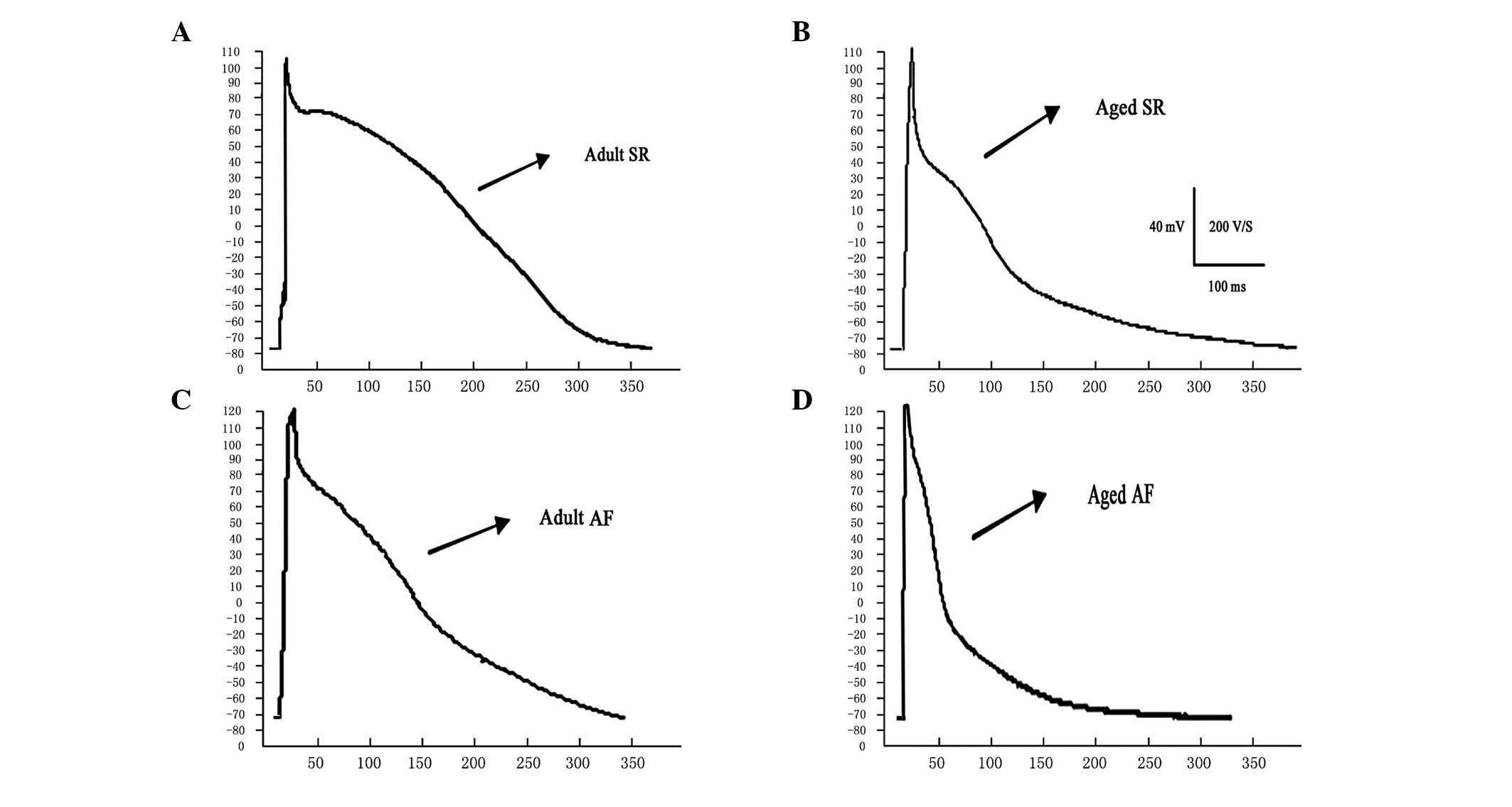
Representative recordings and summary data for the
predominant AP parameters of adult and aged canines in SR and those
with AF are shown in Table I and
Fig. 1. Cardiomyocytes from aged
atria exhibited longer APD90, a significantly lower AP
plateau potential compared with adult canines and no significant
differences in MDP and APA. APD90 was shortened with AF
in the adult and aged groups with increased shortening in the aged
group, which resulted in no difference between APD90 in
the two AF groups. AF led to a higher MDP, a significant increase
of APA and a lower AP plateau potential in the two age groups. AF
was associated with significant depolarization of the cellular
membrane in adult and aged LA. The extent of depolarization was the
same in the two AF groups, resulting in increased depolarization in
the adult cardiomyocytes compared with that in aged
cardiomyocytes.
 | Table IAP characteristics recorded in adult
and aged atria in SR and AF at a cycle length of 2,000 msec. |
Table I
AP characteristics recorded in adult
and aged atria in SR and AF at a cycle length of 2,000 msec.
| Group | n | MDP (mV) | APA (mV) | Plateau (mV) | APD90
(msec) |
|---|
| SR adult | 24 | −78.8±0.8 | 109.8±1.4 | −4.0±0.7 | 320.0±7.9.. |
| SR aged | 30 | −79.2±1.4 | 110.5±4.9 | −7.5±1.7a | 340.5±10.1a |
| AF adult | 28 | −71.8±0.9b | 121.8±1.1b | −6.4±1.1b | 297.0±5.6b |
| AF aged | 26 | −72.2±1.2b | 122.5±2.9b | −9.8±1.1b | 300.5±7.1b |
Changes in ICa-L
characteristics
Typical recordings of ICa-L and
comparative predominant ICa-L parameters in adult and
aged canines in SR and those with AF are shown in Table II and Fig. 2. Aged LA cardiomyocytes
demonstrated lower peak ICa-L densities than adult LA
cells. In addition, this decrease was observed in the adult and
aged AF groups; however, it was lowest in the aged groups.
Activation voltage dependence exhibited no significant difference
in the half-activation voltage and slope factor of each group.
Furthermore, no significant difference in the half-inactivation
voltage and slope factor of each group was observed. However, this
current reduction during aging and in AF was not accompanied by a
significant change in its recovery time from inactivation.
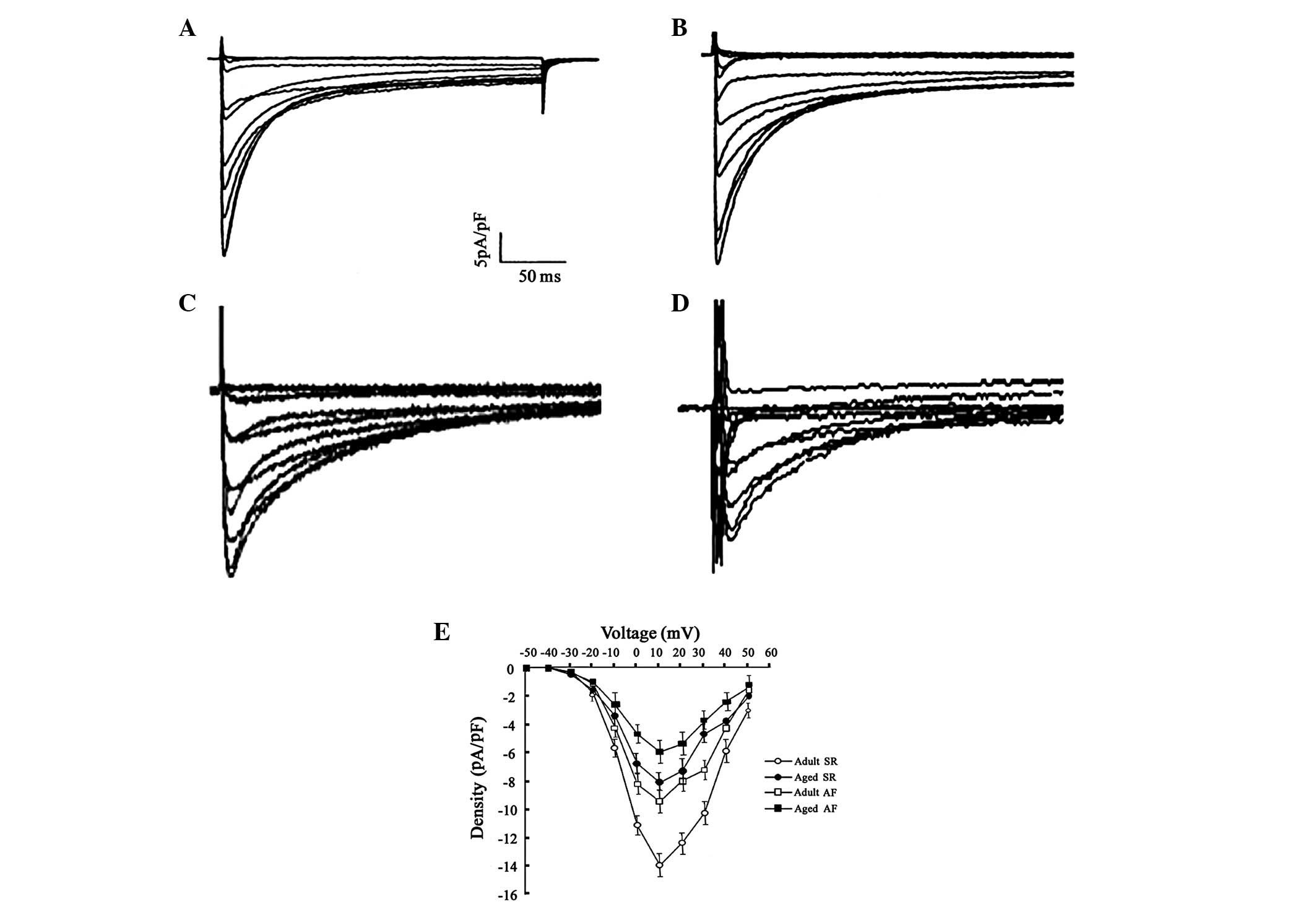 | Figure 2L-type Ca2+ current
(ICa-L) tracings between adults and aged left atrium
(LA) in sinus rhythm (SR) and atrial fibrillation (AF), with a
holding voltage of −70 mV to various test voltages. (A) Adult sinus
rhythm (SR), (B) aged SR, (C) adult atrial fibrillation (AF) and
(D) aged AF groups. (E) Average peak ICa-L density in
adult and aged cells. All data were collected simultaneously
following establishment of whole cell configuration (adults, 17±0.8
min and aged, 18±1.1 min). (SR adults, 14 cells; SR aged, 16 cells;
AF adults, 15 cells; and AF aged, 19 cells from the 7 canines in
each group, respectively). |
 | Table IIElectrophysiological characteristics
of ICa-L between adult and aged LA in SR and AF. |
Table II
Electrophysiological characteristics
of ICa-L between adult and aged LA in SR and AF.
| Group | n | ICa-L
density (pA/pF) | Steady-state
activation | Steady-state
inactivation | Monoexponential
recovery time constant (msec) |
|---|
|
|
|---|
| V0.5
(mV) | k (mV) | V0.5
(mV) | k (mV) |
|---|
| SR adult | 14 | −14.1±0.8 | −7.1±1.5 | 5.7±0.4 | −23.1±2.1 | 6.2±0.3 | 51.9±3.3 |
| SR aged | 16 | −8.1±0.5a | −6.7±2.8 | 5.5±0.5 | −22.9±3.3 | 6.4±0.5 | 53.1±3.1 |
| AF adult | 15 | −9.4±0.7b | −6.9±1.2 | 5.1±0.3 | −22.1±1.9 | 6.2±0.3 | 51.2±2.3 |
| AF aged | 19 | −5.9±0.3b | −6.8±2.1 | 5.9±0.3 | −21.9±2.3 | 6.8±0.6 | 52.1±5.1 |
Fibrosis change
As shown in Fig. 3,
compared with those in the adult group, the myocardial fibers of
tissue from the aged group appeared to be more compact and were
closer together. Muscle bundles were separated by large strands of
connective tissue and a significantly higher deposition of
connective tissue was observed (4.1±0.9 versus 8.4±1.0%; n=15;
P<0.05). Moreover, the degree of myocardial fiber disarray was
greater in the adult (4.1±0.9 versus 14.7±2.1%; n=15; P<0.01)
and aged (8.4±1.0 versus 18.2±2.4%; n=15; P<0.01) groups with AF
than that in the corresponding groups in SR. Moreover, the degree
of myocardial fiber disarray among muscle bundles was greater in
the aged group with AF than in the adult group with AF (14.7±2.1
versus 18.2±2.4%; n=15; P<0.05).
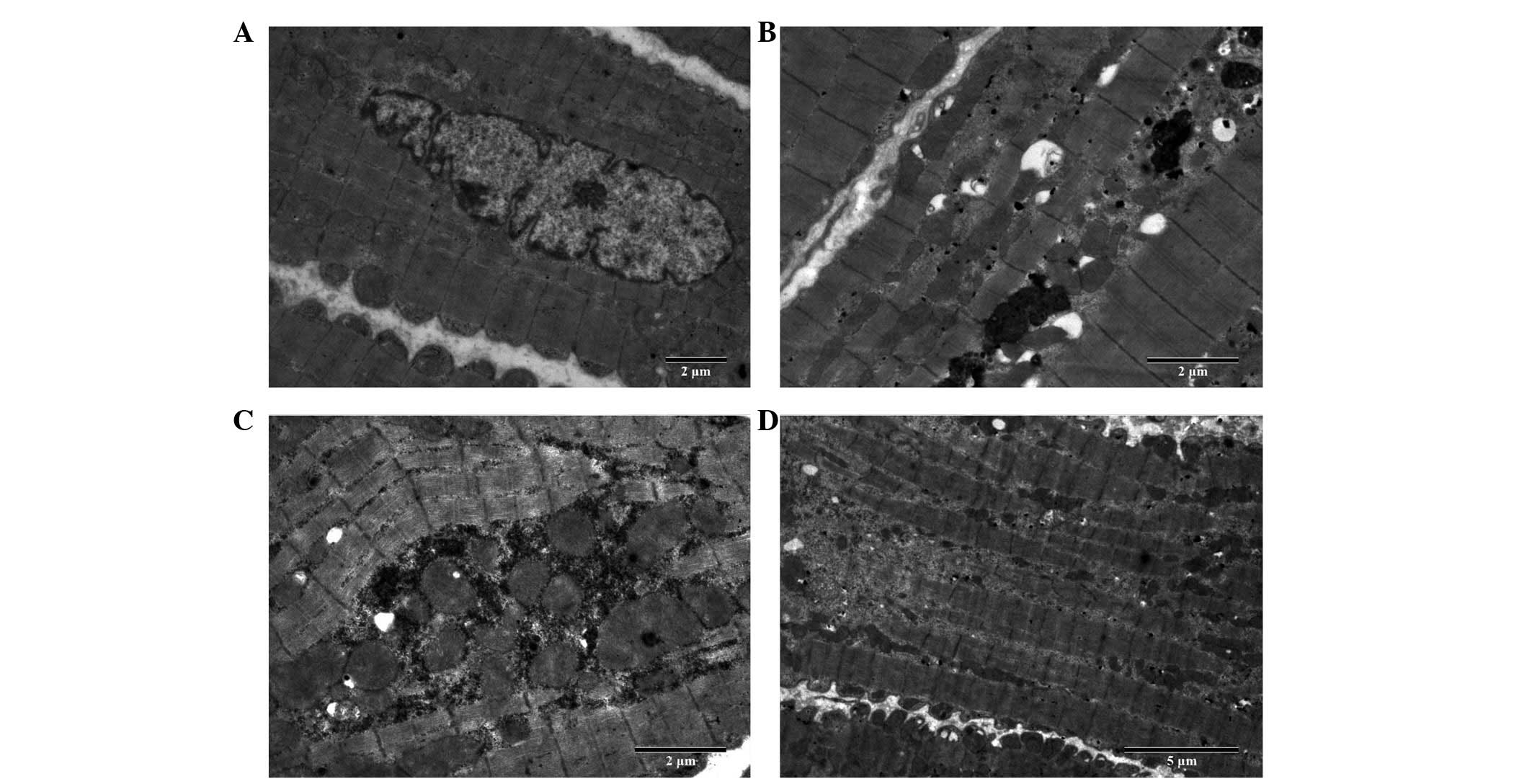
Cell ultrastructural changes
The ultrastructure of the atrial myocardium was
observed by electron microscopy and representative transmission
electron micrographs are shown in Fig.
4. Atrial myocytes of the LA wall from the adult canines
exhibited a regular sarcomere organisation, uniformly sized
mitochondria and nuclei showing normal clumping of chromatin at the
nuclear membrane (n=3, samples from randomly selected adult
canines) (Fig. 4A). Atrial
myocytes of the LA wall from the aged canines exhibited an abnormal
ultrastructure with mild and severe sarcomere degeneration,
swelling of several mitochondria with a reduction in the density
and organisation of the cristae, karyopyknosis with chromatin
margination to the nuclear membrane, expanded endoplasmic
reticulum, increased glucogen and mild compact and close myocardial
fibers (n=3, samples from randomly selected aged canines) (Fig. 4B). Atrial myocytes of the LA wall
from the adult canines with AF appeared to show a more abnormal
ultrastructure, including severe sarcomere degeneration, increased
mitochondrial swelling, karyopyknosis indicating cell apoptosis,
several secondary lysosomes, expanded endoplasmic reticulum,
decreased glucogen and irregular and disorderly myocardial fibers
(n=3, samples from randomly selected adult canines with AF)
(Fig. 4C). Atrial myocytes of the
LA wall from the aged canines with AF also exhibited an abnormal
ultrastructure, including more severe sarcomere degeneration,
mitochondria with the presence of vacuoles, increased karyopyknosis
indicating cell apoptosis, expanded endoplasmic reticulum and
secondary lysosomes and disintegration of certain myofilaments
(n=3, samples from randomly selected aged canines with AF)
(Fig. 4D).
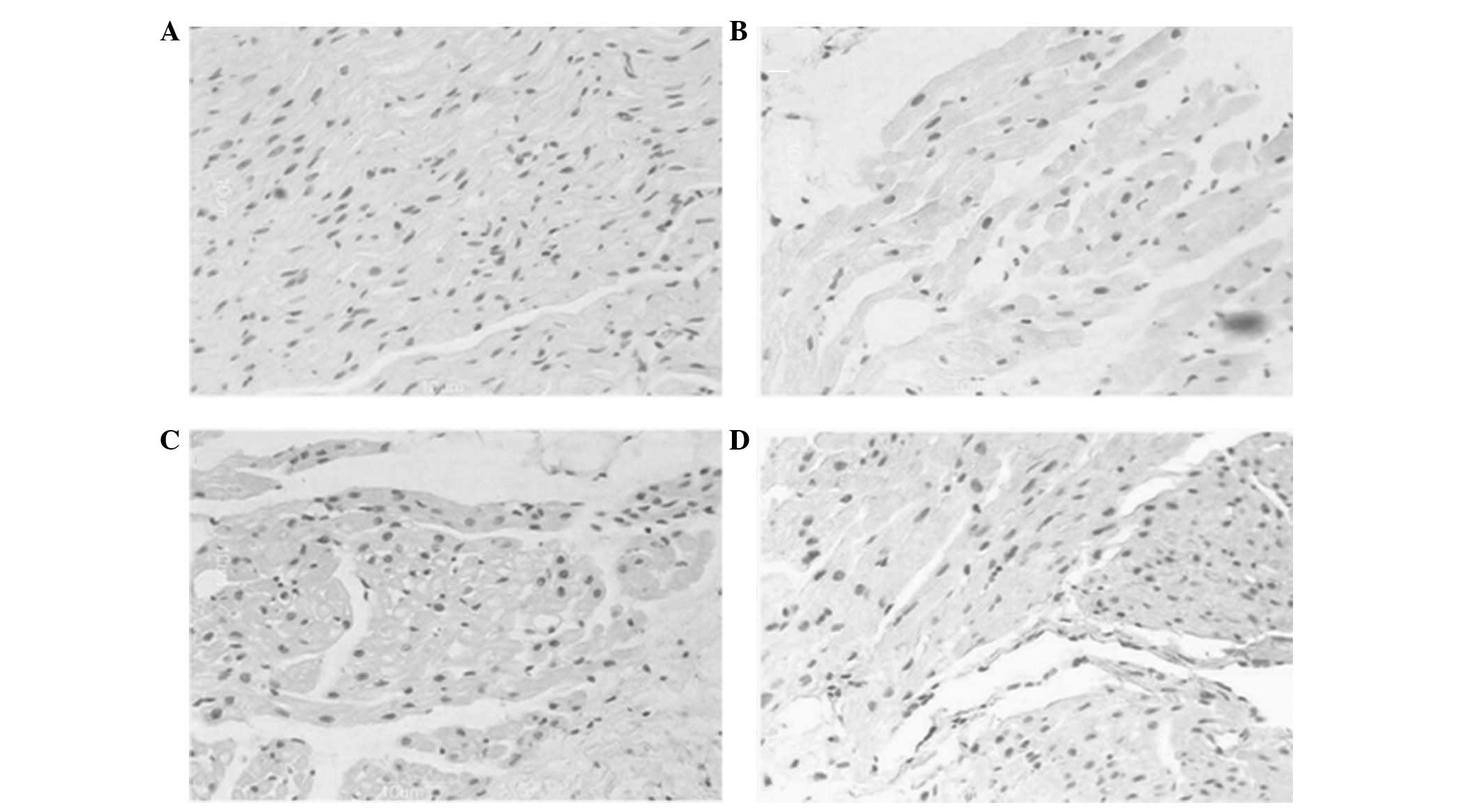
Apoptosis indices
As shown in Fig. 5,
a higher percentage of myocytes with myolysis in aged canines
exhibited TUNEL-positive nuclei compared with that of the adult
group (22.0±5.45 versus 32.9±3.4%; n=12; P<0.05). The majority
of the nuclei were large in size with a uniform distribution of
heterochromatin and exhibited weak TUNEL staining (Fig. 5A and B). Moreover, the frequency of
apoptosis significantly increased in the adult (22.0±5.45 versus
33.4±3.9%; n=12; P<0.01) and aged (32.9±3.4 versus 51.2±3.4%;
n=12; P<0.001) groups with AF, compared with their counterpart
groups in SR. The frequency in the aged groups was greater than
that in the adult groups. In addition, several nuclei decreased in
size and stained strongly with TUNEL, indicating extensive DNA
cleavage (Fig. 5C and D).
Left atrial mRNA and protein expression
of LVDCCa1c and calpains
As shown in Tables
III and IV, compared with the
adult group, the mRNA and protein expression levels of
LVDCCa1c in the aged groups were significantly lower
than those in the adult groups (P<0.05). The mRNA and protein
expression of calpain 1, calpain 2 and calpastatin showed
upregulation in the aged group; however, these were not
significantly different between the two groups (P>0.05).
 | Table IIIThe expression of mRNA in the left
atrial myocardium in adult and aged LA in SR and AF. |
Table III
The expression of mRNA in the left
atrial myocardium in adult and aged LA in SR and AF.
| | Expression |
|---|
| |
|
|---|
| Group | n |
LVDCCa1c | Calpain 1 | Calpain 2 | Calpastain |
|---|
| SR adult | 7 | 2.38±1.03 | 1.45±0.68 | 1.23±0.59 | 1.38±0.71 |
| SR aged | 7 | 1.17±0.75a | 1.79±0.75 | 1.43±0.89 | 1.47±0.84 |
| AF adult | 7 | 0.27±0.25b | 2.53±0.85b | 1.48±0.78 | 1.45±0.74 |
| AF aged | 7 | 0.10±0.07b | 2.72±0.66b | 1.53±1.01 | 1.71±0.62 |
 | Table IVThe expression of protein in the left
atrial myocardium in adult and aged LA in SR and AF. |
Table IV
The expression of protein in the left
atrial myocardium in adult and aged LA in SR and AF.
| | Expression |
|---|
| |
|
|---|
| Group | n |
LVDCCa1c | Calpain 1 | Calpain 2 | Calpastain |
|---|
| SR adult | 7 | 0.28±0.11 | 0.39±0.13 | 0.36±0.16 | 0.42±0.16 |
| SR aged | 7 | 0.13±0.10a | 0.47±0.21 | 0.39±0.09 | 0.44±0.23 |
| AF adult | 7 | 0.13±0.01b | 0.61±0.19b | 0.41±0.20 | 0.38±0.08 |
| AF aged | 7 | 0.07±0.05b | 0.78±0.31b | 0.44±0.17 | 0.45±0.62 |
Compared with the control groups, the mRNA and
protein expression of LVDCCa1c was significantly lower
and the mRNA and protein expression levels of calpain 1 were
significantly higher in the adult and aged groups with AF
(P<0.05). The aged group appeared to be the higher of the two AF
groups (P<0.05). The protein level of LVDCCa1c was
negatively correlated with that of calpain 1 (r=−0.583, P=0.019).
No significant changes in the mRNA and protein levels of calpain 2
and calpastatin were observed between the SR and AF groups
(P>0.05). In the adult and aged groups with AF, the protein
expression of calpain 1 was positively correlated with the degree
of myocardial fiber disarray (r=0.421, P=0.041 and r=0.475,
P=0.036, respectively) and directly correlated with the frequency
of apoptosis (r=0.503, P=0.043 and r=0.528, P=0.036,
respectively).
Discussion
In this study, it was demonstrated that the most
notable alteration with aging was a significant decrease in the AP
plateau potential and APD90 was prolonged in aged LA
cardiomyocytes. Moreover, P-wave duration and dispersion were
significantly longer in aged canines. The results may reflect an
aging-associated degree of slower conduction of the atria. Previous
studies have demonstrated that ICa-L is reduced in aged
canine right atria cardiomyocytes compared with that of adult
canines (3); however, no published
data are available concerning the effects of age on LA
cardiomyocyte ICa-L. The present study demonstrated that
there was a significant reduction in peak ICa-L in aged
canine LA cardiomyocytes, while decreased LVDCCa1c
protein levels may be a predominant reason for the reduced
ICa-L. However, the current reduction in aged atrial
cardiomyocytes was not accompanied by a significant change in
calcium channel availability or recovery from inactivation. The
currents that determine the plateau level of AP in the atria are
ultrarapid delayed rectifier potassium current (IKur),
outward potassium current (Ito) and
ICa-L(15,17). Therefore, a decrease in
depolarizing current ICa-L or an increase in
repolarizing currents (IKur and/or Ito) may
lead to a lower plateau of AP. Thus, the results suggested that the
decrease in ICa-L may be a mechanism for the low plateau
potential in aged canine LA cardiomyocytes. The longer
APD90 in aged atria suggested certain aging-induced
changes of delayed rectifier potassium currents (IK) or
may be a consequence of the low plateau potential in aged canines.
Previous studies demonstrated that negative plateau potentials
exhibited a lower driving force for the conduction of early
premature beats (18,19). Therefore, the results of the
present study suggested that the change in the AP in aged atria may
lead to a decreased conduction of premature beats. Slow conduction
of early premature impulses may further facilitate the onset of
AF.
Numerous studies have demonstrated that AF remodels
atrial electrophysiology, which facilitates AF recurrence (20,21).
The predominant electrophysiological characteristics of electrical
remodeling are a reduction in the atrial refractory period and a
loss of the APD adaptation to rate (22,23).
To date, studies have been performed in normal adult animals
(17,20,21).
AF-induced electrophysiological remodeling in adults results from
rapid atrial activation, and rapid atrial pacing produces similar
AP changes. However, the mechanism of AP changes observed with this
remodeling may differ, as shown in the present study. It was
observed that there was a reduced ICa-L in persistent AF
cardiomyocytes versus controls. The change was interpreted as a
result of the reduced LVDCCa1c protein levels. It
appears reasonable to suggest that calcium ion channel remodeling
is the basis of the atrial electrical remodeling of AF. Based on
the present results, it was demonstrated that AF led to a notable
shortening of APD90, a higher MDP, a significant
increase of APA and a significant decrease of AP plateau potential
in the two age groups. AF-induced decreases in APD90 may
be explained by the reduction in ICa-L, as AF was
associated with a significant depolarization of the cellular
membrane in adult and aged LA. This membrane depolarization in AF
may be a consequence of the decreased basal ICa-L.
Structurally, the most notable change in the aged
atria was the increased quantity of fibrous tissue interspersed
between myocytes. These alterations were observed in fibrillating
and aged atria. Cardiac fibrosis is characterized by the excessive
accumulation of fibrillar collagen in the extracellular space. In
addition, interstitial fibrosis is known to reduce electrical
coupling in the heart (24) and it
significantly increases the complexity of the myocardial
architecture by electrically insulating cardiac cells and/or muscle
bundles (25,26). As a direct consequence, the typical
uniform anisotropic conduction in the atrial myocardium is replaced
by nonuniform anisotropic conduction (27,28).
Fibrosis preferentially affects lateral (or transverse)
connections, rather than longitudinal cell-cell connections
(29,30), resulting in a slower, zigzag
transverse propagation (31,32).
Thus, a premature response occurring in the aged atrial myocardium
has a higher probability of undergoing unidirectional block from an
imbalance between source/sink currents and initiating re-entry due
to the underlying arrhythmogenic substrate, which is produced as a
result of electrical and/or structural remodeling (5,14,33).
Notable cytoarchitectural and cellular alterations
were observed in the aging LA myocardium in SR; however, these
alterations were also present in fibrillating and aging atria. As a
result of the persistence of pathogenic factors, such as increased
pressure load or high frequency of beating, certain myocytes with
structural alterations may activate a programmed cell death (PCD)
pathway. The results of the present study indicated that aging
and/or fibrillating atria contained several myocytes undergoing
apoptosis. Myocytes with myolysis may exhibit certain features of
dedifferentiation. However, whether the cells undergoing lysis are
in a long-term viable state that may be reversed or whether they
will die by activating PCD, remains to be determined. In
particular, in aging atria, minor additional noxious stimuli, such
as high frequency of beating, may be sufficient to trigger the
death of occasional vulnerable myocytes in a random manner.
Although calcium signaling is mediated through an
array of calcium-dependent enzymes, calpian exhibits the greatest
effect on cell function (10). By
carrying out selective, limited proteolytic cleavages, calpains
modulate the activity of enzymes and induce specific cytoskeletal
rearrangements in various aging phenomena and late-life diseases
(13). The results of the present
study demonstrated that the majority of parameters correlated with
one another. The overexpression of the calpain 1 protein was
consistent with general cellular alterations, including
characteristic reductions in LVDCCa1c that resulted in
electrical remodeling in the atrial cells and accelerated fibrosis
and apoptosis. The data suggested that calpain 1 activation, due to
overexpression of calpain 1 in fibrillating and aging atria, may
have resulted in gross cellular damage (the structural and
molecular changes associated with remodeling). Thus, its activation
is likely to contribute to the progress of atrial tissue
deterioration. Therefore, intervention in the calpain pathway may
be important in determining the sequence of cellular and molecular
events in aging and/or AF, and may aid in the identification of a
novel strategy for pharmacological intervention.
In conclusion, the results of the present study are
limited as the plateau potential of AP is determined by
IKur, Ito and ICa-L; however,
IKur and Ito were not included in this study.
Furthermore, APA is related to INa, which was not
observed in this study. Finally, the results are limited to studies
of cells from LA and may not be extended to cells of other regions
of the atria. It may have been beneficial to determine the
activation of calpain 1 during aging and/or in AF-related atrial
structural remodeling; however, calpain 1 requires low (micromolar)
intracellular calcium concentrations to be able to reach
half-maximum activity, which is expected during AF.
Acknowledgements
This study was supported by the Program of National
Natural Science Foundation of China (grant no. 308660299), the
Program of Natural Science Foundation of the Xinjiang Uygur
Autonomous Region (grant nos. 200821143 and 2011211A074) and the
Program of Doctoral Fund of Ministry of Education (grant no.
200807600004).
References
|
1
|
Nattel S: New ideas about atrial
fibrillation 50 years on. Nature. 415:219–226. 2002.PubMed/NCBI
|
|
2
|
Chen LY and Shen WK: Epidemiology of
atrial fibrillation: a current perspective. Heart Rhythm. 4(Suppl
3): S1–S6. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Dun W, Yagi T, Rosen MR and Boyden PA:
Calcium and potassium currents in cells from adult and aged canine
right atria. Cardiovasc Res. 58:526–534. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Anyukhovsky EP, Sosunov EA, Plotnikov A,
et al: Cellular electrophysiologic properties of old canine atria
provide a substrate for arrhythmogenesis. Cardiovasc Res.
54:462–469. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Anyukhovsky EP, Sosunov EA, Chandra P, et
al: Age-associated changes in electrophysiologic remodeling: a
potential contributor to initiation of atrial fibrillation.
Cardiovasc Res. 66:353–363. 2005. View Article : Google Scholar
|
|
6
|
Spach MS, Heidlage JF, Dolber PC and Barr
RC: Mechanism of origin of conduction disturbances in aging human
atrial bundles: experimental and model study. Heart Rhythm.
4:175–185. 2007. View Article : Google Scholar
|
|
7
|
Eisner DA, Diaz ME, Li Y, O’Neill SC and
Trafford AW: Stability and instability of regulation of
intracellular calcium. Exp Physiol. 90:3–12. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Katra RP and Laurita KR: Cellular
mechanism of calcium-mediated triggered activity in the heart. Circ
Res. 96:535–542. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
van Wagoner DR, Pond AL, Lamorgese M,
Rossie SS, McCarthy PM and Nerbonne JM: Atrial L-type
Ca2+ currents and human atrial fibrillation. Circ Res.
85:428–436. 1999.
|
|
10
|
Croall DE and DeMartino GN:
Calcium-activated neutral protease (calpain) system: structure,
function, and regulation. Physiol Rev. 71:813–847. 1991.PubMed/NCBI
|
|
11
|
Brundel BJ, Ausma J, van Gelder IC, et al:
Activation of proteolysis by calpains and structural changes in
human paroxysmal and persistent atrial fibrillation. Cardiovasc
Res. 54:380–389. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Daoud EG, Bogun F, Goyal R, et al: Effect
of atrial fibrillation on atrial refractoriness in humans.
Circulation. 94:1600–1606. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Ausma J, Dispersyn GD, Duimel H, et al:
Changes in ultrastructural calcium distribution in goat atria
during atrial fibrillation. J Mol Cell Cardiol. 32:355–364. 2000.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Allessie M, Ausma J and Schotten U:
Electrical, contractile and structural remodeling during atrial
fibrillation. Cardiovasc Res. 54:230–246. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Bers DM: Calcium cycling and signaling in
cardiac myocytes. Annu Rev Physiol. 70:23–49. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Schiefer HB: Guide to the Care and Use of
Experimental Animals, Volume 2. Can Vet J. 26:591985.
|
|
17
|
Yue L, Feng J, Gaspo R, Li GR, Wang Z and
Nattel S: Ionic remodeling underlying action potential changes in a
canine model of atrial fibrillation. Circ Res. 81:512–525. 1997.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Bosch RF and Nattel S: Cellular
electrophysiology of atrial fibrillation. Cardiovasc Res.
54:259–269. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Verheule S, Wilson E, Banthia S, et al:
Direction-dependent conduction abnormalities in a canine model of
atrial fibrillation due to chronic atrial dilatation. Am J Physiol
Heart Circ Physiol. 287:H634–H644. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Wijffels MC, Kirchhof CJ, Dorland R and
Allessie MA: Atrial fibrillation begets atrial fibrillation. A
study in awake chronically instrumented goats. Circulation.
92:1954–1968. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Willems R, Holemans P, Ector H, Sipido KR,
Van de Werf F and Heidbüchel H: Mind the model: effect of
instrumentation on inducibility of atrial fibrillation in a sheep
model. J Cardiovasc Electrophysiol. 13:62–67. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Nattel S, Khairy P and Schram G:
Arrhythmogenic ionic remodelling: adaptive responses with
maladaptive consequences. Trends Cardiovasc Med. 11:295–301. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Baba S, Dun W, Hirose M and Boyden PA:
Sodium current function in adult and aged canine atrial cells. Am J
Physiol Heart Circ Physiol. 291:H756–H761. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Tanaka K, Zlochiver S, Vikstrom KL, et al:
Spatial distribution of fibrosis governs fibrillation wave dynamics
in the posterior left atrium during heart failure. Circ Res.
101:839–847. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Wit AL and Boyden PA: Triggered activity
and atrial fibrillation. Heart Rhythm. 4(Suppl 3): S17–S23. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Weiss JN, Qu Z, Chen PS, et al: The
dynamics of cardiac fibrillation. Circulation. 112:1232–1240. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Everett TH IV and Olgin JE: Atrial
fibrosis and the mechanisms of atrial fibrillation. Heart Rhythm.
4(Suppl 3): S24–S27. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Qu Z, Garfinkel A, Chen PS and Weiss JN:
Mechanisms of discordant alternans and induction of reentry in
simulated cardiac tissue. Circulation. 102:1664–1670. 2000.
View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Miragoli M, Gaudesius G and Rohr S:
Electrotonic modulation of cardiac impulse conduction by
myofibroblasts. Circ Res. 98:801–810. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Koura T, Hara M, Takeuchi S, et al:
Anisotropic conduction properties in canine atria analyzed by
high-resolution optical mapping: preferential direction of
conduction block changes from longitudinal to transverse with
increasing age. Circulation. 105:2092–2098. 2002. View Article : Google Scholar
|
|
31
|
Thijssen VL, Ausma J, Liu GS, Allessie MA,
van Eys GJ and Borgers M: Structural changes of atrial myocardium
during chronic atrial fibrillation. Cardiovasc Pathol. 9:17–28.
2000. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Mandapati R, Skanes A, Chen J, Berenfeld O
and Jalife J: Stable microreentrant sources as a mechanism of
atrial fibrillation in the isolated sheep heart. Circulation.
101:194–199. 2000.PubMed/NCBI
|
|
33
|
Ausma J, Wijffels M, Thoné F, Wouters L,
Allessie M and Borgers M: Structural changes of atrial myocardium
due to sustained atrial fibrillation in the goat. Circulation.
96:3157–3163. 1997. View Article : Google Scholar : PubMed/NCBI
|