Introduction
Tuberculosis (TB) continues to be an increasing
cause of morbidity globally and is a leading cause of human
mortality in the developing world. According to the most recent
World Health Organization statistics, there were an estimated 8.7
million new cases of TB and 1.4 million deaths from the disease in
2011. Geographically, the burden of TB is highest in Asia and
Africa. India and China together account for ~40% of the global
cases of TB (1). Over the past two
decades, drug-resistant TB and TB/human immunodeficiency virus
(HIV) co-infection have further threatened to undermine the control
of TB (2). Although TB diagnostics
have improved (3), they are not
suitable to be used in regions with low and middle incomes.
Moreover, there are no new efficient drugs to treat drug-resistant
TB. Thus, the development of effective immunodiagnostics and
vaccines against TB remains a global priority.
Recently, interferon-γ (IFN-γ) release assays
(IGRAs) have been introduced into clinical practice for the
diagnosis of Mycobacterium tuberculosis (M.
tuberculosis) infection, and two licensed IGRAs are
commercially available: QuantiFERON®-TB Gold In-Tube
(Cellestis, Carnegie, Victoria, Australia) and
T-SPOT®.TB (Oxford Immunotec, Abingdon, UK).
Furthermore, novel antigens incorporating these assays may improve
detection sensitivity (4). The
only TB vaccine presently used in humans, Bacillus Calmette-Guérin
(BCG), is widely used and has been available since 1921; however,
it provides partial and inconsistent protection (5). A number of hypotheses have been
suggested to explain the inconsistent efficacy of the BCG vaccine,
including differences among BCG vaccine strains, modulation of the
immune responses by previous exposure to environmental mycobacteria
and host genetics (6–8). As a result, the antigenic components
that are absent in the BCG vaccine, which elicit critical
protective immune responses to TB, has been an area of intense
investigation (9,10).
Comparative genomic studies have identified several
M. tuberculosis-specific genomic regions of difference (RDs)
that are absent in the avirulent Mycobacterium bovis BCG
strains (11), which may be useful
for TB diagnosis and vaccine design. For example, early secreted
antigenic target 6 kDa (ESAT-6) and culture filtrate protein 10 kDa
(CFP-10) from RD1 (12), Rv0222
from RD4 (13) and Rv3425 from
RD11 (14,15) have been identified as useful
biomarkers for the immunodiagnosis of TB. Moreover, ESAT-6 and
Rv3425 have also been used in TB vaccine studies (9,16,17),
respectively. In a previous study, we identified a novel B-cell
antigen, Rv3117, which was encoded by DNA segment RD5 of M.
tuberculosis(18). Therefore,
in this study, we evaluated the humoral and cellular immune
responses to this antigen in human subjects, as well as in
immunized C57BL/6 mice, and compared them using two RD1-encoded
antigens, CFP-10 and ESAT-6.
Materials and methods
Generation of recombinant antigen
Rv3117
The procedures used for the cloning, expression and
purification of M. tuberculosis RD5-encoded antigen Rv3117
were as described previously (18). The endotoxin was removed using
Polymyxin affinity chromatography, in accordance with the
manufacturer’s instructions (Bio-Rad Laboratories Ltd., Shanghai,
China), prior to the recombinant protein being concentrated using
an Amicon Ultra 10K device (Millipore, Billerica, MA, USA). The
protein purity and content were then assessed using Coomassie blue
staining, western blotting and Bradford’s assay, using bovine serum
albumin (BSA) as the protein standard, respectively. The level of
endotoxin was measured using the commercially available
Quantitative Chromogenic Endpoint Tachypleus Amebocyte Lysate
reactivity endotoxin kit (Chinese Horseshoe Crab Reagent
Manufactory Co., Xiamen, China).
Animals and immunization
C57BL/6 mice were purchased from the Shanghai
SIPPR/BK Experimental Animal Co., Ltd. (Shanghai, China). The
immunization procedure was performed as previously described
(19) and the experiments
performed adhered strictly to the 1986 Scientific Procedures Act.
Three groups of 25-g, 6-week-old, female C57BL/6 mice were used for
the experiments. The mice received free access to food and water
throughout the study. The six mice of each group were injected
subcutaneously with 100 μg purified recombinant protein Rv3117,
CFP-10 or phosphate-buffered saline (PBS) mixed with Incomplete
Freund’s adjuvant (IFA; Sigma, St. Louis, MO, USA), respectively,
with two boosters, two weeks apart. Sera were collected from the
caudal vein of the immunized mice every two weeks for antibody
analysis. Two weeks subsequent to the final immunization, the
animals were sacrificed to harvest splenocytes for cytokine
analysis.
Human sera samples
A total of 65 serum samples (n=65) from patients
with active pulmonary TB (PTB; age range, 15–66 years) and 59 serum
samples (n=59) from healthy control subjects (age range, 8–30
years) were collected from Wuxi No. 5 People’s Hospital (Wuxi,
China). The patients with active PTB were diagnosed by the
isolation and identification of M. tuberculosis, as well as
by clinical and radiological findings. Mycobacterial isolates were
obtained from Lowenstein-Jensen cultures, identified to the species
level by biochemical procedures (20) and then confirmed using genotyping
based on the 16S–23S rRNA gene internal transcribed spacer sequence
(21). None of the patients had
received anti-TB chemotherapy when the serum samples were
collected. The healthy controls had not previously suffered from TB
and had negative chest X-rays and sputum culture results for M.
tuberculosis. The sera collected were stored at −20°C. The
study was approved by the Research Ethics Committees of Shanghai
Jiao Tong University School of Medicine (Shanghai, China) and
written informed consent was obtained from all the participants
after a full explanation of the study.
Enzyme-linked immunosorbent assay
(ELISA)
In order to assess the antibody responses, ELISA was
performed as in our previous studies (15,18).
Briefly, 96-well polystyrene flat-bottomed microtiter plates
(Costar, Cambridge, MA, USA) were coated with 1–5 μg/ml of Rv3117,
CFP-10 or ESAT-6 (Linc-Bio Science Co., Ltd., Shanghai, China) and
incubated overnight at 4°C. Subsequent to being washed four times
with PBST [0.05% (v/v) Tween-20 in PBS], the plates were blocked
with 200 μl blocking buffer (3% BSA in PBST) for 2 h at 37°C. The
plates were then washed a further four times with PBST. Following
this, 200-fold diluted human serum samples or serial dilutions of
mouse serum samples (100 μl) in blocking buffer were added to the
wells and incubated for 30 min at 37°C. The pooled sera from the
patients with active PTB and healthy donors were used as positive
and negative controls, respectively. All the samples were tested in
duplicate. The plates were thoroughly washed with PBST and then
incubated with horseradish peroxidase (HRP)-conjugated goat
anti-human immunoglobulin G (IgG), IgM and IgA antibodies or
HRP-conjugated goat anti-mouse IgG, IgG1 and IgG2a antibodies
(Southern Biotech, Birmingham, AL, USA), at certain dilutions
recommended by the manufacturer, respectively. Following this, the
plates were incubated at 37°C for 30 min and then thoroughly washed
a further four times, prior to 100 μl
3,3′,5,5′-tetramethylbenzidine (TMB) substrate solution (0.04% TMB
and 0.04% urea peroxide in 0.1 M sodium acetate-citric acid buffer,
pH 4.0) being added. Following 10 min incubation in the dark at
room temperature, the reaction was stopped by the addition of 50 μl
of 2 M H2SO4 to each well. The optical
densities were subsequently measured at 450 nm
(OD450).
Enzyme-linked immunospot (ELISPOT)
assay
The mouse spleens were removed aseptically and
gently ground through a 70-μm cell strainer. Following this,
single-cell suspensions were prepared using mouse lympholyte
EZ-Sep™ density gradient centrifugation (Dakewe Biotech Co., Ltd.,
Beijing, China). IFN-γ ELISPOT kits (eBioscience, San Diego, CA,
USA) were used in accordance with the instruction manual. In brief,
96-well polyvinylidene difluoride (PVDF; Millipore, Beverly, MA,
USA) plates were coated with 100 μl anti-IFN-γ monoclonal antibody
overnight at 4°C. The plates were then washed twice with 200 μl
ELISPOT coating buffer and blocked with 200 μl complete RPMI-1640,
supplemented with penicillin, streptomycin and 10% fetal calf
serum, at room temperature for 1 h. The blocking solution was
subsequently discarded from the plates, and freshly isolated
splenocytes were plated in duplicate at a density of
5×104 cell/well in 200 μl and stimulated with Rv3117 or
CFP-10 (0.1, 1 or 10 μg/ml) for 24 h at 37°C in 5% CO2,
using phytohemagglutinin (PHA) and medium as positive and negative
controls, respectively. Following this, the cells and medium were
decanted from the plates and the plates were washed three times
with PBST. A total of 100 μl diluted biotinylated detection
antibodies were then added to each well and the plates were
incubated for 2 h at room temperature. Subsequently, the antibody
solution was decanted and washed four times with PBST, with the
wells being left to soak for 1 min per wash, in order to reduce the
background staining. Following this, 100 μl diluted
streptavidin-HRP-conjugated solution was added to each well and the
plates were incubated for 45 min at room temperature. The two sides
of the membrane were then washed five times with PBST and the
plates were washed twice with PBS, without Tween-20. Thawed
3-amino-9-ethylcarbazole (AEC) substrate solution (100 μl/well) was
subsequently added. Following this, the plates were incubated for a
further 30 min at room temperature in the dark. Once spots were
able to be observed in the wells under an inverted microscope, the
reaction was stopped by thoroughly rinsing the two sides of the
membrane with distilled water. The plates were then air dried, and
the spots were counted using an immunospot image analyzer.
Statistical analysis
The differences were compared using a Student’s
t-test and a non-parametric test within the SPSS 13.0 data analysis
program (SPSS, Chicago, IL, USA). P<0.05 was considered to
indicate a statistically significant difference.
Results
Rv3117 was expressed as a His-tagged
fusion protein in Escherichia coli (E. coli)
The gene Rv3117 was expressed in E.
coli BL21 (DE3) PlysS cells and purified as a 6xHis-tag fusion
protein in the soluble fraction under non-denaturing conditions.
The purified recombinant protein Rv3117 was fractionated using
electrophoresis on a 12% polyacrylamide gel. A single band
corresponding to the 48.1 kDa (including the mass of the N-terminal
fusion domain of pET32a) protein was observed (Fig. 1) and confirmed using anti-His
monoclonal antibody (data not shown). A total of <5.0 EU/mg
endotoxins was typically observed and subsequently used for animal
immunization.
Rv3117 elicited strong, specific antibody
responses, consistent with CFP-10 and ESAT-6
To evaluate the immunological nature of the B-cell
antigen Rv3117, two RD1-encoded antigens of M. tuberculosis,
CFP-10 and ESAT-6, were selected and the antibody responses to
these antigens in individual sera were measured using ELISA
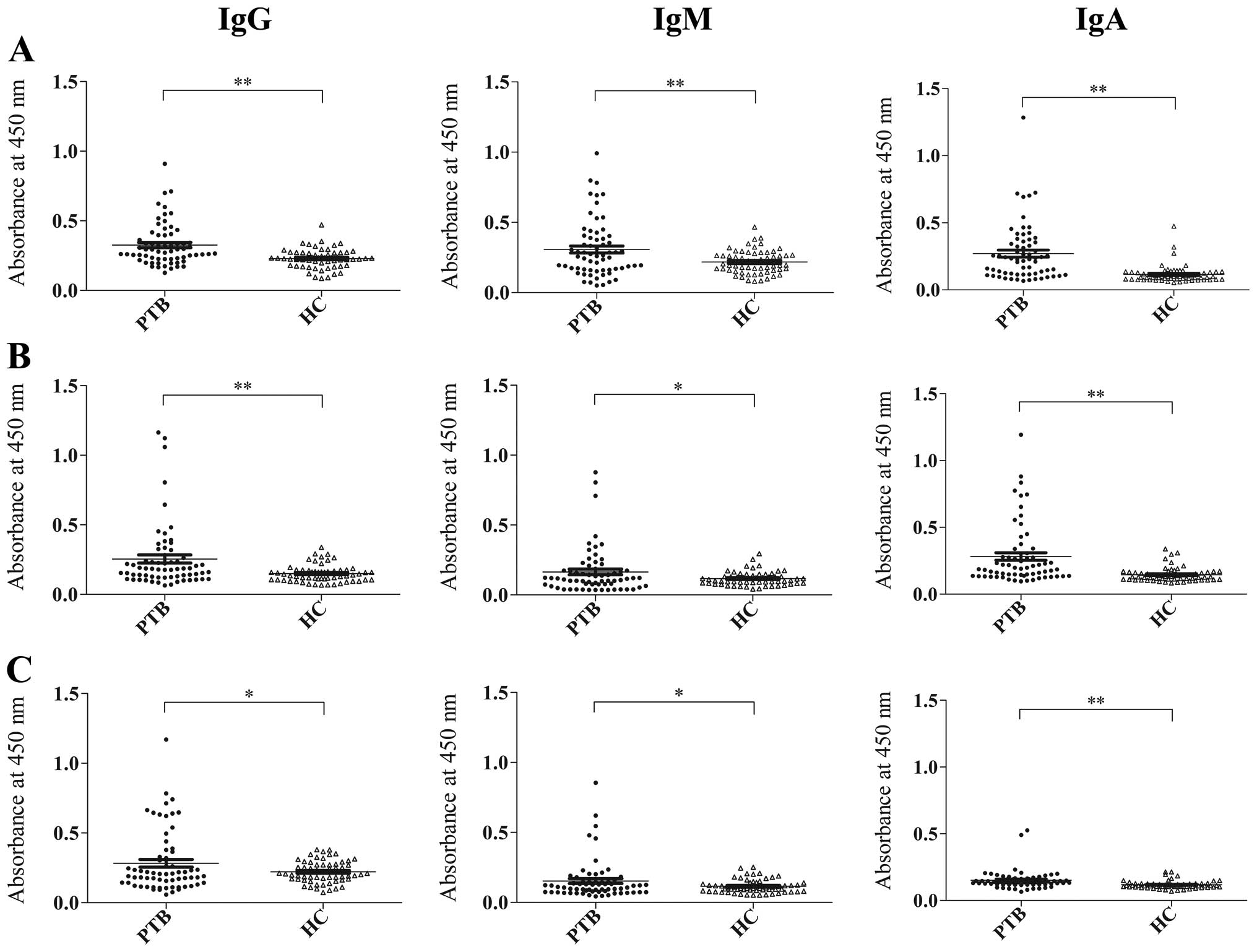
(Fig. 2). Consistent with the
results from the RD1-encoded antigens, CFP-10 and ESAT-6, the IgG,
IgM and IgA antibody responses to Rv3117 were able to statistically
distinguish between the 65 patients with active PTB and the 59
healthy controls (P<0.01, respectively). When the ELISA results
were determined by the cut-off values, equal to the mean OD value
for the healthy control serum samples plus two standard deviations,
the sensitivity of the IgG antibody responses to Rv3117 (26.2%)
were no lower than those to CFP-10 (24.6%) and ESAT-6 (24.6%) in
the 65 patients with active PTB. Moreover, in these patients, the
sensitivities of the IgM and IgA responses to Rv3117 (26.2 and
43.1%) were higher than those to CFP-10 (23.1 and 33.8%) and ESAT-6
(13.8 and 20.0%), respectively. Of note, the specificities of the
antibody responses to any one of the three antigens remained ≥93.0%
(Table I).
 | Table ISensitivities and specificities for
antibody responses to the region of difference 5 (RD5)-encoded
protein Rv3117. |
Table I
Sensitivities and specificities for
antibody responses to the region of difference 5 (RD5)-encoded
protein Rv3117.
| Rv3117 | CFP-10 | ESAT-6 |
|---|
|
|
|
|
|---|
| Items | IgG | IgM | IgA | IgG | IgM | IgA | IgG | IgM | IgA |
|---|
| Cut-offa | 0.371 | 0.386 | 0.250 | 0.273 | 0.213 | 0.253 | 0.378 | 0.210 | 0.177 |
| Sensitivityb (n=65) (%) | 26.2 (17) | 26.2 (17) | 43.1 (28) | 24.6 (16) | 23.1 (15) | 33.8 (22) | 24.6 (16) | 13.8 (9) | 20.0 (13) |
| Specificityc (n=59) (%) | 98.3 (1) | 96.6 (2) | 94.9 (3) | 94.9 (3) | 96.6 (2) | 93.2 (4) | 96.6 (3) | 96.6 (3) | 93.2 (4) |
Rv3117 evoked high levels of
antigen-specific IFN-γ in C57BL/6 mice
Twenty-four hours subsequent to stimulation,
single-splenocyte suspensions from immunized mice were obtained and
assayed for IFN-γ. An ELISPOT assay was used to determine the
relative number of IFN-γ-expressing mouse cells in the
single-splenocyte suspensions. The number of such cells were shown
by spot-forming units. Levels of antigen-specific IFN-γ were
evaluated in the supernatants of the in vitro cultures of
immune splenocytes restimulated with the proteins. As shown in
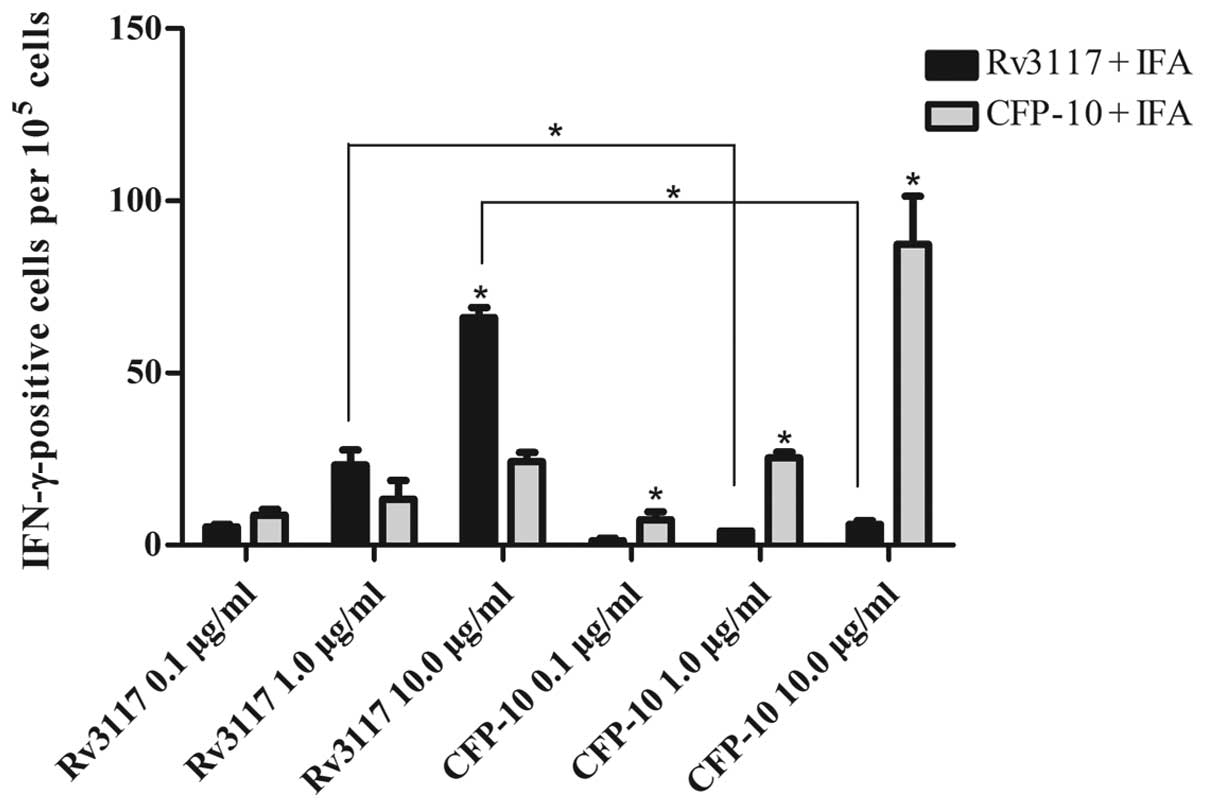
Fig. 3, the RD1-encoded antigen
CFP-10 evoked high levels of antigen-specific IFN-γ in C57BL/6
mice. This was consistent with the results from the RD5-encoded
antigen Rv3117, in which it was observed that the IFN-γ production
in response to Rv3117 (10.0 μg/ml) was statistically significant
(P<0.05) in the cultures of splenocytes from Rv3117-immunized
C57BL/6 mice when compared with control CFP-10-immunized C57BL/6
mice (Fig. 3). Moreover,
splenocytes from the Rv3117-immunized mice produced high levels of
IFN-γ when stimulated in vitro with Rv3117, whereas low
levels of IFN-γ were produced when the splenocytes were stimulated
in vitro with the control protein CFP-10 (Fig. 3).
Rv3117 induced a specific humoral
response in C57BL/6 mice
Sera were collected from the caudal vein of mice 0–6
weeks subsequent to immunization in order to perform antibody
analyses. Specific IgG, IgG2a and total IgG antibody responses were
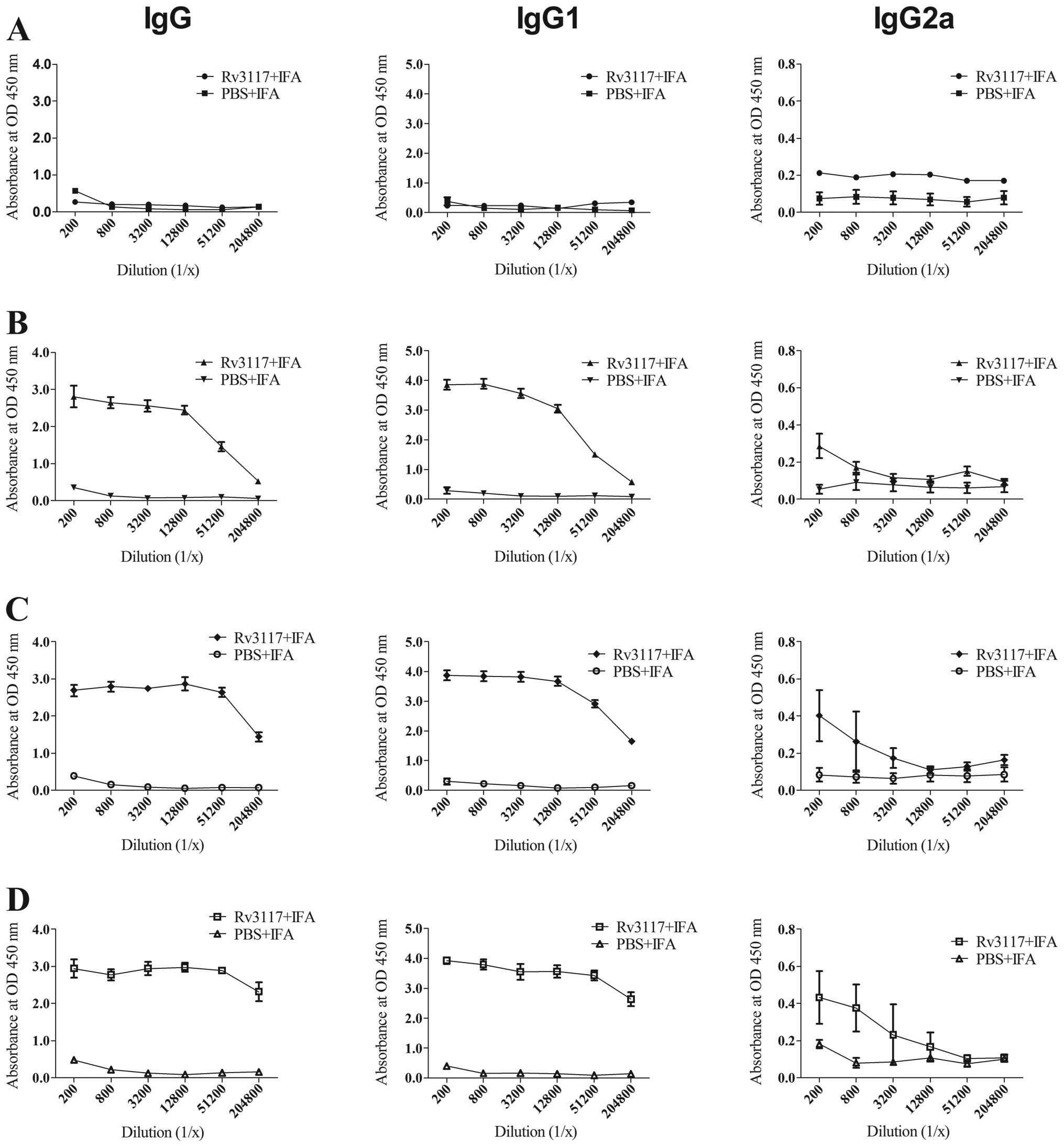
measured using ELISA. Fig. 4 shows
the level of antibody responses in the sera of mice immunized with
IFA-emulsified protein Rv3117 or emulsified PBS against the protein
in different weeks subsequent to the immunization. Compared with
the PBS control group, the mice immunized with Rv3117 produced
higher levels of antibodies against Rv3117 protein. High titers of
total IgG and IgG1 antibodies were detected in the sera of the mice
immunized with the Rv3117 antigen during the 2–6-week period and
the antibody levels of IgG2a isotype against the protein Rv3117
increased from the 2-week time-point following the immunization.
The mice immunized with PBS showed lower detectable titers of IgG,
IgG1 and IgG2a antibodies.
Discussion
The causative agent of TB, M. tuberculosis,
is an intracellular pathogen, and Type 1 T helper (Th1)
cell-mediated immune responses are indispensable for protective
immunity against TB (22,23). The only vaccine that is used at
present, the attenuated M. bovis strain BCG, induces Th1
cell responses; however, it has been inconsistent in providing
protection from the disease, with low or unmeasurable efficacy in
many of those regions with the highest incidence (24). Therefore, additional immune
responses are required for a vaccine demonstrating optimal
efficacy. Previous studies have suggested a number of hypotheses to
explain the inconsistent efficacy, including differences among BCG
vaccine strains, modulation of the immune responses by previous
exposure to environmental mycobacterium and host genetics (6–8).
However, there is still uncertainty and certain hypotheses remain
to be tested. Although it has been suggested that the explanation
regarding differences in strains may be discounted, as parallel
studies in the UK and Malawi using the same strain of vaccine still
showed differences in efficacy (8), Keyser et al(25) revealed that BCG substrains had the
capacity to drive Th2 immunity and induced variable protection
against M. tuberculosis infection (25). Moreover, further study is required
with regard to host genetics, as human Toll-like receptor 1 (TLR1)
and TLR6-deficiency altered immune responses to BCG vaccination in
South African infants (26). In
adults and adolescents, pre-exposure to environmental mycobacteria
is likely to influence immune responses (27). However, studies in neonates and
infants have demonstrated that the same vaccine may have a
different immunogenicity in different populations; therefore,
pre-exposure to environmental mycobacteria is probably not a factor
in efficacy (28,29).
The low efficacy of BCG may be interrelated with the
fact that it lacks important antigens (30). The Rv3117 selected for the present
study, located in RD5, is absent in the BCG strains (11). This antigen was revealed to encode
a probable rhodanese-like thiosulfate sulfurtransferase and to be
involved in intermediary metabolism and respiration (31,32).
To the best of our knowledge, most of the important antigens of TB
were initially screened and identified in the IgG antibody response
studies, as with Rv3117. In our previous study, all five M.
tuberculosis RD5-specific proteins were cloned and then used to
screen patients with active TB, and Rv3117 was identified as a
novel B-cell antigen (18). In
this study, the results of IgG, as well as IgM and IgA, antibody
responses to Rv3117, when compared with two RD1-encoded antigens,
CFP-10 and ESAT-6, further indicated that Rv3117 was a potential
candidate for the development of TB immunodiagnostics or vaccine
study. However, the reason for IgA antibody responses to Rv3117,
CFP-10 or ESAT-6 being able to distinguish patients with active PTB
and healthy controls (P<0.01, respectively) has yet to be
elucidated, as shown in Fig. 2.
The low sensitivity of antibody responses to Rv3117 suggested that
Rv3117 was not suitable for the serodiagnosis of TB compared with
other B-cell antigens (15,33,34).
However, the high specificity of the results suggested that it may
be useful in further IGRA studies for TB immunodiagnostics.
The development of a Th1 immune response mediated by
IFN-γ is a prerequisite for mounting efficient protection against
the M. tuberculosis challenge (22). Therefore, we investigated whether
Rv3117 was able to evoke antigen-specific INF-γ in C57BL/6 mice. In
the study, the cytokine secretion pattern from Rv3117-immunized
mice showed statistically higher IFN-γ compared with that from the
control group mice (Fig. 3),
confirming a Th1-type response to the chosen protein and a
potential protective effect against M. tuberculosis.
Similarly, Mustafa and Al-Attiyah demonstrated that synthetic
peptides from RD5-encoded proteins were also able to evoke high
levels of IFN-γ in patients with PTB (35). In the present study, we
demonstrated that IFA-emulsified antigen Rv3117 was able to
increase the production of IFN-γ (Fig.
3) and induce increasing concentrations of antigen-specific
IgG2a antibodies subsequent to immunization (Fig. 4). These results were consistent
with those of the RD11-encoded antigen Rv3245 (19), which is now in further and
preclinical studies in China (9,36–38).
These data indicated that IFA may be used as one of the most
optimal adjuvants for Rv3117-based vaccine studies in animal
models. Studies in mouse models of TB have shown that BCG efficacy
may be enhanced through supplementation with other RD antigens
(39). Further studies by the
authors are planned to investigate the cellular immune response
induced by an Rv3117-modified BCG in C57BL/6 mice and its
protective efficacy prior to or subsequent to M.
tuberculosis H37Rv challenge.
In conclusion, we evaluated the humoral and cellular
immune responses to a novel M. tuberculosis RD5-encoded
antigen, Rv3117, and compared the results with those from two
RD1-encoded antigens, ESAT-6 and CFP-10. The results of the
immunological characterization suggested that Rv3117 may be used as
a candidate for the study of TB immunodiagnostic development and
vaccine design.
Acknowledgements
This study was supported by the China Mega-Projects
of Science Research for the 12th Five Year Plan (grant no.
2013ZX10003002-005), the National Natural Science Foundation of
China (grant no. 81271794), the National High Technology Research
and Development Program of China (grant no. 2011AA02A119), the
Science and Technology Commission of Shanghai Municipality, China
(grant no. 12441903300) and the Doctor Innovation Fund of the
Shanghai Jiao Tong University School of Medicine, China (grant no.
BXJ201204).
References
|
1
|
WHO. Global tuberculosis report 2012.
Geneva: World Health Organization. URL: http://www.who.int/tb/publications/global_report/en/index.html.
2012
|
|
2
|
Lawn SD and Zumla AI: Tuberculosis.
Lancet. 378:57–72. 2011. View Article : Google Scholar
|
|
3
|
Boehme CC, Nabeta P, Hillemann D, et al:
Rapid molecular detection of tuberculosis and rifampin resistance.
N Engl J Med. 363:1005–1015. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Dosanjh DP, Hinks TS, Innes JA, et al:
Improved diagnostic evaluation of suspected tuberculosis. Ann
Intern Med. 148:325–336. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Tu HA, Vu HD, Rozenbaum MH, Woerdenbag HJ
and Postma MJ: A review of the literature on the economics of
vaccination against TB. Expert Rev Vaccines. 11:303–317. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Lagranderie MR, Balazuc AM, Deriaud E,
Leclerc CD and Gheorghiu M: Comparison of immune responses of mice
immunized with five different Mycobacterium bovis BCG
vaccine strains. Infect Immun. 64:1–9. 1996.PubMed/NCBI
|
|
7
|
Black GF, Dockrell HM, Crampin AC, et al:
Patterns and implications of naturally acquired immune responses to
environmental and tuberculous mycobacterial antigens in northern
Malawi. J Infect Dis. 184:322–329. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Black GF, Weir RE, Floyd S, et al:
BCG-induced increase in interferon-gamma response to mycobacterial
antigens and efficacy of BCG vaccination in Malawi and the UK: two
randomised controlled studies. Lancet. 359:1393–1401. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Wang J, Qie Y, Zhu B, et al: Evaluation of
a recombinant BCG expressing antigen Ag85B and PPE protein Rv3425
from DNA segment RD11 of Mycobacterium tuberculosis in
C57BL/6 mice. Med Microbiol Immunol. 198:5–11. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Sweeney KA, Dao DN, Goldberg MF, et al: A
recombinant Mycobacterium smegmatis induces potent
bactericidal immunity against Mycobacterium tuberculosis.
Nat Med. 17:1261–1268. 2011.
|
|
11
|
Behr MA, Wilson MA, Gill WP, et al:
Comparative genomics of BCG vaccines by whole-genome DNA
microarray. Science. 284:1520–1523. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Arend SM, Andersen P, van Meijgaarden KE,
et al: Detection of active tuberculosis infection by T-cell
response to early-secreted antigenic target 6-kDa protein and
culture filtrate protein 10. J Infect Dis. 181:1850–1854. 2000.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Rosenkrands I, Aagaard C, Weldingh K, et
al: Identification of Rv0222 from RD4 as a novel serodiagnostic
target for tuberculosis. Tuberculosis (Edinb). 88:335–343. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Zhang HM, Wang JL, Lei JQ, et al: PPE
protein (Rv3425) from DNA segment RD11 of Mycobacterium
tuberculosis: a potential B-cell antigen used for serological
diagnosis to distinguish vaccinated controls from tuberculosis
patients. Clin Microbiol Infect. 13:139–145. 2007.PubMed/NCBI
|
|
15
|
Zhang SL, Zhao JW, Sun ZQ, et al:
Development and evaluation of a novel multiple-antigen ELISA for
serodiagnosis of tuberculosis. Tuberculosis (Edinb). 89:278–284.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Samten B, Wang X and Barnes PF: Immune
regulatory activities of early secreted antigenic target of 6-kD
protein of Mycobacterium tuberculosis and implications for
tuberculosis vaccine design. Tuberculosis (Edinb). 91(Suppl 1):
S114–S118. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Lin PL, Dietrich J, Tan E, et al: The
multistage vaccine H56 boosts the effects of BCG to protect
cynomolgus macaques against active tuberculosis and reactivation of
latent Mycobacterium tuberculosis infection. J Clin Invest.
122:303–314. 2012. View
Article : Google Scholar : PubMed/NCBI
|
|
18
|
Zhang MM, Zhao JW, Sun ZQ, et al:
Identification of RD5-encoded Mycobacterium tuberculosis
proteins as B-cell antigens used for serodiagnosis of tuberculosis.
Clin Dev Immunol. 2012:7380432012.PubMed/NCBI
|
|
19
|
Wang J, Qie Y, Zhang H, et al: PPE protein
(Rv3425) from DNA segment RD11 of Mycobacterium
tuberculosis: a novel immunodominant antigen of
Mycobacterium tuberculosis induces humoral and cellular
immune responses in mice. Microbiol Immunol. 52:224–230.
2008.PubMed/NCBI
|
|
20
|
Metchock B, Nolte FS and Wallace RJ:
Mycobacterium. Manual of Clinical Microbiology. Murray PR, Baron
EJ, Pfaller MA, Tenover FC and Yolken RH: 7th edition. ASM Press;
Washington, DC: pp. 399–437. 1999
|
|
21
|
Zhang SL, Shen JG, Shen GH, et al: Use of
a novel multiplex probe array for rapid identification of
Mycobacterium species from clinical isolates. World J
Microbiol Biotechnol. 23:1779–1788. 2007. View Article : Google Scholar
|
|
22
|
Cooper AM: Cell-mediated immune responses
in tuberculosis. Annu Rev Immunol. 27:393–422. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
O’Garra A, Redford PS, McNab FW, Bloom CI,
Wilkinson RJ and Berry MP: The immune response in tuberculosis.
Annu Rev Immunol. 31:475–527. 2013.PubMed/NCBI
|
|
24
|
Colditz GA, Brewer TF, Berkey CS, et al:
Efficacy of BCG vaccine in the prevention of tuberculosis.
Meta-analysis of the published literature. JAMA. 271:698–702. 1994.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Keyser A, Troudt JM, Taylor JL and Izzo
AA: BCG sub-strains induce variable protection against virulent
pulmonary Mycobacterium tuberculosis infection, with the
capacity to drive Th2 immunity. Vaccine. 29:9308–9315. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Randhawa AK, Shey MS, Keyser A, et al:
Association of human TLR1 and TLR6 deficiency with altered immune
responses to BCG vaccination in South African infants. PloS Pathog.
7:e10021742011. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Weir RE, Black GF, Nazareth B, et al: The
influence of previous exposure to environmental mycobacteria on the
interferon-gamma response to bacille Calmette-Guerin vaccination in
southern England and northern Malawi. Clin Exp Immunol.
146:390–399. 2006. View Article : Google Scholar
|
|
28
|
Lalor MK, Ben-Smith A, Gorak-Stolinska P,
et al: Population differences in immune responses to Bacille
Calmette-Guerin vaccination in infancy. J Infect Dis. 199:795–800.
2009. View
Article : Google Scholar : PubMed/NCBI
|
|
29
|
Lalor MK, Floyd S, Gorak-Stolinska P, et
al: BCG vaccination induces different cytokine profiles following
infant BCG vaccination in the UK and Malawi. J Infect Dis.
204:1075–1085. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Aagaard C, Hoang T, Dietrich J, et al: A
multistage tuberculosis vaccine that confers efficient protection
before and after exposure. Nat Med. 17:189–194. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Cole ST, Brosch R, Parkhill J, et al:
Deciphering the biology of Mycobacterium tuberculosis from
the complete genome sequence. Nature. 393:537–544. 1998.
|
|
32
|
Krawczyk J, Kohl TA, Goesmann A,
Kalinowski J and Baumbach J: From Corynebacterium glutamicum
to Mycobacterium tuberculosis - towards transfers of gene
regulatory networks and integrated data analyses with MycoRegNet.
Nucleic Acids Res. 37:e972009.
|
|
33
|
Steingart KR, Dendukuri N, Henry M, et al:
Performance of purified antigens for serodiagnosis of pulmonary
tuberculosis: a meta-analysis. Clin Vaccine Immunol. 16:260–276.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Mukherjee P, Dutta M, Datta P, et al: The
RD1-encoded antigen Rv3872 of Mycobacterium tuberculosis as
a potential candidate for serodiagnosis of tuberculosis. Clin
Microbiol Infect. 13:146–152. 2007.PubMed/NCBI
|
|
35
|
Mustafa AS and Al-Attiyah R:
Identification of Mycobacterium tuberculosis-specific
genomic regions encoding antigens inducing protective cellular
immune responses. Indian J Exp Biol. 47:498–504. 2009.
|
|
36
|
Wang S, Chen J, Zhang Y, et al:
Mycobacterium tuberculosis region of difference (RD) 2
antigen Rv1985c and RD11 antigen Rv3425 have the promising
potential to distinguish patients with active tuberculosis from
M. bovis BCG-vaccinated individuals. Clin Vaccine Immunol.
20:69–76. 2013. View Article : Google Scholar
|
|
37
|
Wang J, Qie Y, Liu W and Wang H:
Protective efficacy of a recombinant BCG secreting antigen
85B/Rv3425 fusion protein against Mycobacterium tuberculosis
infection in mice. Hum Vaccin Immunother. 8:2012. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Chen F, Zhai MX, Zhu YH, Qi YM, Zhai WJ
and Gao YF: In vitro and in vivo identification of a novel
cytotoxic T lymphocyte epitope from Rv3425 of Mycobacterium
tuberculosis. Microbiol Immunol. 56:548–553. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Kalra M, Grover A, Mehta N, et al:
Supplementation with RD antigens enhances the protective efficacy
of BCG in tuberculous mice. Clin Immunol. 125:173–183. 2007.
View Article : Google Scholar : PubMed/NCBI
|