Introduction
MicroRNAs (miRNAs) are small non-coding RNAs that
are essential for normal cellular processes and are commonly
dysregulated in cell proliferation, differentiation, survival and
motility (1). miRNAs, initially
transcribed as long primary transcripts (pri-miRNAs), are processed
in the nucleus by the RNase III enzyme Drosha to generate 60- to
120-nt-long precursors containing a stem-loop structure, termed
pre-miRNAs (2). These precursors,
which are exported into the cytoplasm by the nuclear export factor
Exportin-5 and the Ran-GTP cofactor, are cleaved by the RNase
enzyme Dicer to release the mature miRNAs (3). miRNAs predominantly bind to the
3′UTRs of their target mRNAs (1).
This process, requiring only partial matching, leads to
translational repression; while target mRNAs with more stringent
pairing requirements may be cleaved (4,5).
Numerous seminal studies have demonstrated that disease-associated
miRNAs may therefore represent a novel class of therapeutic
targets. It has been shown that inhibition of miRNA-122 (miR-122)
reduced viral load in non-human primates (6,7) and
hepatitis C patients, and thus, miRNA modulators may be candidates
for therapeutics. However, the predominant limitations of miRNA
applications, similar to the majority of antisense- or
nucleic-acid-based strategies, are the charge density, molecular
weight and instability in the presence of nucleases. Furthermore,
intracellular accumulation and endosomal escape remain to be
significant barriers in the delivery of these macromolecules.
Numerous delivery vectors, viral and non-viral, have
been developed to facilitate cellular uptake. One group of
non-viral vectors that is increasingly utilized for the delivery of
various cargoes is the cell-penetrating peptides (CPPs). Over the
past 20 years, CPPs have been successfully applied in vitro
and in vivo to trigger the movement of a large panel of
cargoes, including plasmid DNA, oligonucleotides, siRNA, RNA,
proteins, peptides, liposomes and nanoparticles, across the
cellular membrane into the cytoplasm of cells (8). CPPs are subdivided into two
predominant classes, the first requiring chemical linkage with the
cargo (9–12) and the second involving the
formation of stable, non-covalent complexes (13–16).
MPG is a promising non-covalent strategy that appears to be more
appropriate for siRNA delivery and yields a significant biological
response (17–20).
MPG is a 27-residue peptide vector which contains a
hydrophobic domain derived from the fusion sequence of HIV-1 gp41
and a hydrophilic domain derived from the nuclear localization
sequence of SV40 T-antigen. MPG is a bipartite amphipathic peptide
derived from the fusion peptide domain of HIV-1 glycoprotein 41
(gp41) protein and the nuclear localization signal (NLS) of SV40
large T antigen, which forms stable non-covalent complexes with
siRNAs, increases the stability, promotes the cellular uptake
without the requirement for prior chemical covalent coupling and
enables robust downregulation of target mRNAs (17,21).
The hydrophobic moiety of MPG (GALFLGFLGAAGSTMGA) derived from the
HIV-1 gp41, is required for efficient targeting to the cell
membrane and cellular uptake. The hydrophilic lysine-rich domain
(PKKKRKV) derived from the NLS of the SV40 large T antigen is
involved in the predominant interactions with nucleic acids and is
required to improve intracellular trafficking of the cargo. The
spacer domain (WSQ), improves the flexibility and the integrity of
the hydrophobic and hydrophilic domains (22). A variant of MPG with a single
mutation in the NLS (MPGΔNLS) was designed to favor the
rapid release of the siRNA into the cytoplasm, thereby allowing for
a greater biological response (17). MPG carriers have been used for the
delivery of siRNAs into a large range of cell lines, including
adherent cell lines, cells in suspension, cancer cell lines and
primary cells, which are not transfected using other non-viral
approaches (21,23,24).
In addition, the final subcellular localization of the siRNA is
dependent on the MPG carrier used.
As the miRNA mimic and inhibitors are chemically
similar to antisense oligonucleotides and therapeutic siRNAs,
positively charged MPG strategies developed for siRNA delivery may
also be applicable to the delivery of miRNAs. The development of
clinically relevant miRNA formulations frequently involves a
thorough evaluation of existing technologies to identify those that
may be utilized for miRNA and its chemistry. It was observed that
miR-122, a liver-specific miRNA, is involved in diverse aspects of
hepatic function and in the progression of liver diseases. miR-122
was also the first miRNA identified to fine-tune lipid metabolism
(25). Notably, inhibition of
miR-122 in vivo has pronounced effects on cholesterol and
fatty acid metabolism. Using antisense strategies, several studies
demonstrated that the antagonism of miR-122 in the liver resulted
in sustained decreases in plasma cholesterol levels in mice and
non-human primates (25–28). Mice treated with antisense
oligonucleotides (ASO) to miR-122 showed 25–35% reductions in total
cholesterol, which reflected decreases in the low-density
lipoprotein and high-density lipoprotein fractions (25). In African green monkeys, efficient
silencing of miR-122 in the liver was achieved with only three
doses of the miR-122 inhibitor, and led to sustained decreases in
the total plasma cholesterol without any apparent liver toxicity or
histopathological change (7).
Furthermore, the prolonged antagonism of miR-122 in chimpanzees,
achieved by weekly injections of the miR-122 inhibitor for 12
weeks, also reduced plasma cholesterol by 30%. Notably, the
reduction in cholesterol levels persisted for several weeks
following treatment, suggesting that miR-122 ASO treatment
prolonged the effects on hepatic gene expression and cholesterol
metabolism. There are respective advantages for the aforementioned
approaches; however, all of these modifications have impeded the
large-scale production due to the low encapsulation efficiency and
low endosomal escape. In the present study, a rapid and inexpensive
non-covalent strategy using MPG family members was used to deliver
an miR-122 mimic and inhibitor into the NCTC 1469 mouse liver cell
line, mouse primary hepatocytes and C. elegans.
High-performance liquid chromatography (HPLC) analysis demonstrated
that MPGΔNLS mediated the delivery of the miR-122 mimic
and inhibitor and was able to fine-tune cholesterol levels in NCTC
1469 cells.
Materials and methods
Peptides and the miR-122 mimic and
inhibitor
Peptides were synthesized by solid-phase peptide
synthesis using standard 9-fluorenylmethyl oxycarbonyl (Fmoc)
chemistry (29). HPLC analysis
indicated that the synthetic peptide had a purity of ≥95%. The
mouse miR-122 sequence was obtained from the miRBase Sequence
Database (http://mi-crorna.sanger.ac.uk, Release 18.0).
Synthetic miR-122 duplexes were chemically synthesized and supplied
by RiboBio Biotechnology (Guangzhou, China). The sequences used
were as follows: Sense: 5′-UGGAGUGUGACAAUGGUGUUUG-3′ and antisense:
5′-AACACCAUUGUCACACUCCAUU-3′ for Mmu-miR-122-5p; sense:
5′-UUCUCCGAACGUGUCACGUTT-3′ and antisense:
5′-ACGUGACACGUUCGGAGAATT-3′ for the negative control;
5′-CAAACACCAUUGUCACACUCCA-3′ for the miR-122 inhibitor and
5′-CAGUACUUUUGUGUAGUACAA-3′ for the 2′Ome, miR-122 inhibitor
negative control. The 5′-end of the mmu-miR-122-5p sense strand was
modified with Fam and Cy3 dye. Synthetic miR-122 duplexes and
fluorescently labeled mmu-miR-122-5p sense strand (5′-Fam and
5′-Cy3) were chemically synthesized and supplied by RiboBio
Biotechnology (Guangzhou, China).
Cell culture and MPG-mediated
transfection
NCTC 1469 and A549 cell lines were derived from
mouse liver cells (American Type culture Collection, Manassas, VA,
USA). A549 cells were maintained in Dulbecco’s modified Eagle’s
medium (DMEM) supplemented with 2 mM glutamine, 1% antibiotics
(10,000 mg/ml streptomycin and 10,000 IU/ml penicillin) and 10%
(w/v) fetal bovine serum (FBS, Invitrogen Life Technologies,
Carlsbad, CA, USA). NCTC 1469 cells were grown in low glucose DMEM
(5 mmol/l glucose; Gibco-BRL, Carlsbad, CA, USA) supplemented with
10% (v/v) horse serum (Hyclone, Rockford, IL, USA), 100 U/ml
penicillin (Gibco-BRL) and 0.1 mg/ml streptomycin (Gibco-BRL). Cell
lines were cultured at 37°C in a humidified atmosphere of 95%
O2 and 5% CO2. Fluorescent labeling of the
miR-122 mimic was performed using Fam or Cy3. For peptide (MPG and
MPGΔNLS)-mediated delivery, the miR-122 mimic and
inhibitor were incubated with carrier peptide at a molecular ratio
of 1:20 in phosphate-buffered saline for 30 min at 37°C, and then
diluted to the required concentration in 500 μl DMEM. Cells, grown
to 60% confluence, were then rinsed twice and overlaid with
preformed complexes. Following incubation for 30 min at 37°C, 1 ml
fresh DMEM supplemented with 10% fetal calf serum was added
directly to the cells (without removing the overlay of
peptide/miR-122 complexes) and cells were returned to the incubator
for 48 h. For cellular localization experiments, cells were grown
on acid-treated glass coverslips to 60% confluence and then
overlaid with preformed peptide/miR-122 complexes. After 1 h, cells
were rinsed twice and the cellular localization of Fam-labeled
miR-122 mimic was monitored by fluorescence or confocal
microscopy.
MPG-mediated delivery of miR-122 into
mouse primary hepatocytes
Hepatocytes were isolated from male C57BL/6J mice
(age, 8–12 weeks; Peking University Health Science Center, Haidian,
China) with collagenase solution (Worthington Biochemicals,
Lakewood Township, NJ, USA), as described previously (30). Following filtration and
centrifugation, the isolated hepatocytes were dispersed in
pre-warmed William’s medium E (Sigma-Aldrich, St. Louis, MO, USA)
supplemented with 20 ng/ml dexamethasone (Sigma-Aldrich), ITS (5
mg/l insulin, 5 mg/l transferrin, 5 μg/l sodium selenate;
Sigma-Aldrich), 10 μg/ml gentamicin (Invitrogen Life Technologies)
and 10% (v/v) FBS, at a density of 300,000 cells/well in
collagen-coated 12-well plates or 5×106
cells/90-mm-diameter dish. The cultures were maintained for an
additional 24 h prior to transient transfection or nuclear extract
preparation. Subsequent procedures for fluorescence imaging were
the same as previously mentioned.
MPG-mediated Cy3-labeled miR-122 delivery
into C. elegans
The nematode Caenorhabditis elegans (C.
elegans) was provided by the Caenorhabditis Genetics Center
(St. Paul, MN, USA). Wild-type worms were incubated in 8 μl
MPGΔNLS/miR-122 mimic complexes for 10 min to 24 h and
were washed in M9 solution (43.6 mM Na2HPO4,
22.0 mM KH2PO4, 8.6 mM NaCl and 18.7 mM
NH4Cl). Subsequent to this, the worms were transferred
to a fresh nematode growth media plate and were allowed to crawl
for several minutes to remove excess fluorescent dye.
RNA extraction and miRNA
quantification
Total RNA was extracted from cultured cells using
TRIzol® Reagent (Invitrogen Life Technologies) according
to the manufacturer’s instructions. Stem-loop qPCR was used to
quantify mature miRNA. Initially, total RNA (1 μg) was
reverse-transcribed using specific primers for either miR-122 or U6
in a 10-μl reaction volume to synthesize cDNA. The synthesized cDNA
was diluted up to 150 μl, and 6 μl cDNA dilution was added to 10 μl
of the 2X SYBR-Green PCR master mix (Takara Bio, Inc., Shiga,
Japan), with 800 nM of each primer in a total volume of 20 μl. The
reactions were amplified for 15 sec at 95°C and 1 min at 60°C for
40 cycles. All reactions were run in triplicate and included no
template or reverse transcription controls. The cycle number at
which the reaction crossed an arbitrarily placed threshold (CT) was
determined, and the relative quantity of miR-122 to U6 RNA was
calculated using the 2−ΔΔCT method.
Confocal microscopy
Specimens were observed with a Zeiss confocal
microscope (LSM; Carl Zeiss Microimaging, Thornwood, NY, USA) or
with a Nikon fluorescent microscope (TE2000U; Nikon, Tokyo, Japan).
Image collection from the Nikon microscope was conducted with a
Hamamatsu Photonics (Hamamatsu, Japan) C4880 cooled CCD camera and
the images were processed with Image Pro-Plus (Media Cybernetics,
Bethesda, MD, USA) and Photoshop (Adobe, San Jose, CA, USA).
Cytotoxicity assay
The
3,(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide
tetrazole (MTT) reduction assay was used to asses the cell
viability. A549 and NCTC 1469 cells were plated in 24-well plates
(3×104/well). Following incubation for 24 h, the cells
were treated with increasing concentrations of miR-122 mixed with
carrier (MPG or MPGΔNLS) at a 1:20 molar ratio range of
5–100 nM, for 48 h. MTT (0.5 mg/ml; Sigma-Aldrich) was added to
each well (200 μl/well). Following additional incubation for 4 h,
the MTT solution was discarded and 200 μl dimethylsulfoxide
(Amresco, Solon, OH, USA) was added and the plates were shaken
gently. The absorbance was measured on an enzyme-linked
immunosorbent assay (ELISA) reader at a wavelength of 490 nm.
Measurement of total cholesterol levels
in the cultured cells
Cholesterol concentrations of the cultured cells
were analyzed by HPLC according to the method by Dong et
al(31). Briefly, cholesteryl
esters were hydrolyzed with alcoholic potassium hydroxide and, in
the presence of an internal standard (stigmasterol), extracted with
hexane. The sterols were oxidized to 4-en-3,6-diones with chromic
acid and analyzed by HPLC. The low detection limit of the method
allowed the measurement of cholesterol in dilute samples and the
internal standard calibration eliminated the requirement for
volumetric reconstitution of the ultracentrifugation (UC) bottom
fractions. Peak area ratios of cholesterol to stigmasterol for the
calibrators were linearly regressed on the corresponding
cholesterol concentrations and the resulting equation was used to
calculate the cholesterol concentrations in the cultured cells.
Statistical analysis
The experiments were repeated at least three times
with a minimum sample size of three. Student’s t-test was conducted
using STATA statistical software (StataCorp LP, College Station,
TX, USA), to test for statistical differences between samples.
P<0.05 was considered to indicate a statistically significant
difference.
Results
Transfection efficiency and impact on
cellular viability of MPG in comparison with
MPGΔNLS
The transfection efficiency and impact on cellular
viability of MPG compared with that of MPGΔNLS was
determined. Peptide-based mimic and inhibitor delivery was
conducted by incubating the NCTC 1469 cells with a constant
quantity of miR-122 (50 nM) and increasing volumes of peptides.
qPCR analysis demonstrated that the expression of cellular miR-122
gradually and markedly increased in the NCTC 1469 cells transfected
with 50 nM of peptide-based miR-122 mimic (Fig. 1A). By contrast, a significant
reduction of miR-122 levels was observed in the NCTC 1469 cells
treated with peptide-based miR-122 inhibitor (Fig. 1B), indicating that silencing was
specific to miR-122. Moreover, it was demonstrated that the
expression of miR-122 varied significantly when cells were treated
with MPGΔNLS/mimic or inhibitor, particularly when the
molar ratio was higher than 10:1. However, the effective
concentration of miR-122 mimic and inhibitor used in biological
studies varies, and the concentration commonly used is <100 nM.
Therefore, the cytotoxicity of the concentrations of peptide/miRNA
complexes were determined by the MTT assay. As shown in Fig. 1C and D, the viability of NCTC 1469
and A549 cells remained unaffected when the cells were exposed to
peptide/miRNA complexes at a concentration of 100 nM. These results
demonstrate that >100 nM of peptide/miRNA complexes had no
significant cytotoxic effect on mammalian cells. Thus, neither of
the complexes were toxic at any of the concentrations tested, as
indicated by the cell viability assays.
Cellular localization of peptide/miR-122
mimic complexes
A recommended dosage for a silencing response is 50
nM miRNA. The optimal incubation time of Fam-labeled miR-122 mimic
required for detection using confocal microscopy was determined.
The confocal microscopy characterization demonstrated that,
following incubation for 10 min with MPG/miR-122 mimic and
MPGΔNLS/miR-122 mimic, punctuated fluorescence was
observed in the cytoplasm of A549 cells, indicating the entry of
the complexes into the cells. Additionally, with increasing
incubation time, the fluorescence appeared to aggregate to more
discrete areas in the cytoplasm (data not shown).
MPGΔNLS, a sequence variant of MPG, contains a single
mutation of the second lysine residue to serine (KSKRKV) in the NLS
motif. This mutation has previously been observed to markedly
increase the import of NLS-containing siRNA into the cytoplasm
(17). As shown in Fig. 2A, green fluorescence was observed
in the cytoplasm and nucleus, while in Fig. 2B, the complexes appeared to be
heterogeneously distributed in the cytoplasm of A549 cells.
MPGΔNLS-mediated delivery of
Cy3-labeled miR-122 mimic in mouse primary hepatocytes
To identify the cellular location of the miRNA, the
intracellular delivery of MPGΔNLS in mouse primary
hepatocytes and NCTC 1469 cells was investigated. An alternate
fluorescent probe (Cy3) was used in order to avoid any artifacts
which may have been associated with the nature of the probe. The
majority of the Cy3-labeled miR-122 mimic (red dots) was localized
in the cytoplasm, demonstrating that MPGΔNLS may also be
used for the efficient delivery of the miR-122 mimic into the NCTC
1469 cells (Fig. 3A) and mouse
primary hepatocytes (Fig. 3B).
MPGΔNLS-mediated miR-122 mimic
delivery in model organisms
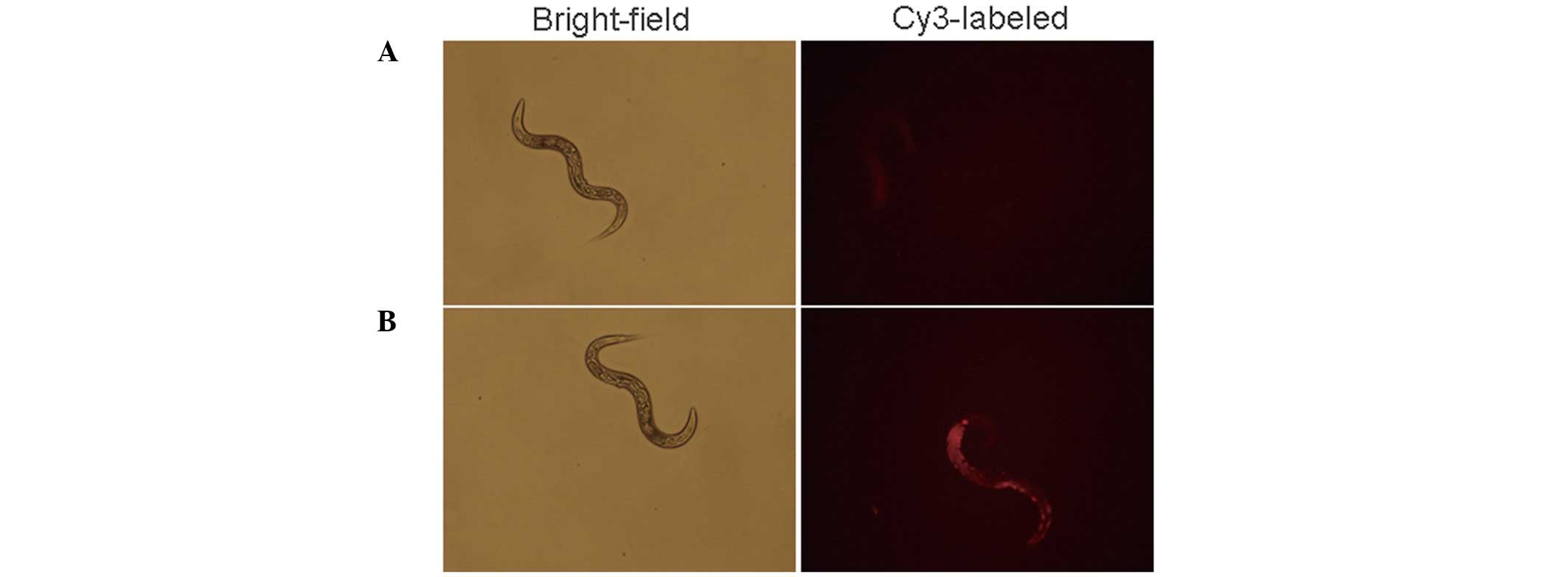
To assess the MPGΔNLS-mediated miR-122
mimic delivery in model organisms, the fluorescence concentration
in model organisms soaked with Cy3 labeled
MPGΔNLS-mediated miR 122 mimic was determined using a
fluorescence microscope. As shown in Fig. 4, a markedly higher concentration of
fluorescence was detected following treatment with
MPGΔNLS (Fig. 4B),
while fluorescence in the naked Cy3-labeled miR-122 mimic was faint
(Fig. 4A).
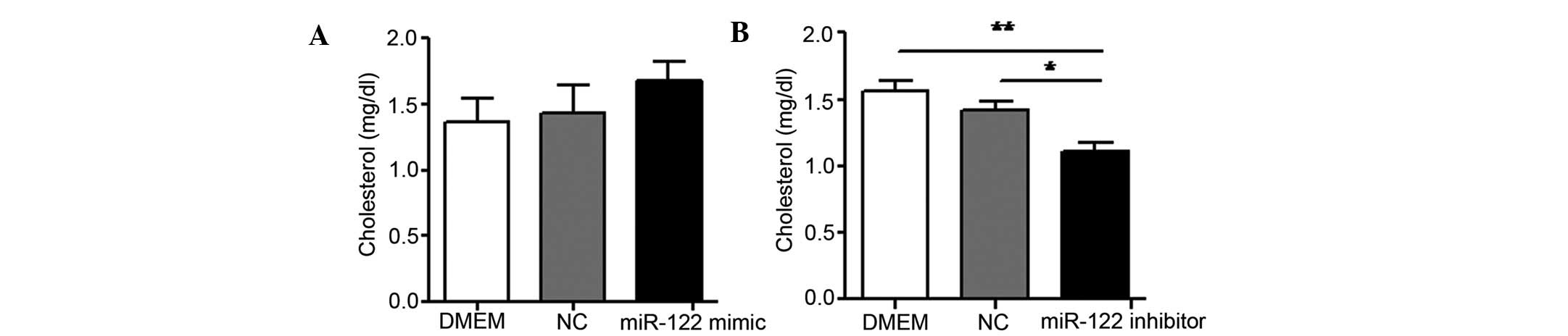
Effect of MPGΔNLS/miR-122
mimic and inhibitor on cholesterol levels
As miR-122 regulates cholesterol levels (31), the levels of cholesterol were
determined following transfection with MPGΔNLS/miR-122
mimic and MPGΔNLS/miR-122 inhibitor in NCTC 1469 cells.
It was demonstrated that cholesterol levels were increased
following transfection with the MPGΔNLS/miR-122 mimic
(Fig. 5A). Conversely,
transfection with the MPGΔNLS/miR-122 inhibitor
decreased cholesterol levels in NCTC 1469 cells (Fig. 5B). This suggested the efficient
delivery of miR-122 regulated cholesterol metabolism through the
non-covalent peptide-based strategy.
Discussion
For the past two decades, studies have investigated
the application of oligonucleotides as therapeutic agents.
Theoretically, the administration of an miRNA mimic compound would
replenish miRNA activity and concomitantly restore negative
regulation of multiple target genes. By contrast, an miRNA
inhibitor compound would antagonize miRNA activity and thereby
relieve the inhibition of target genes, effectively adjusting the
function of cells. However, as oligonucleotides are negatively
charged hydrophilic macromolecules, their delivery is problematic.
MPG is a 27-residue amphipathic peptide, which forms stable
particles with siRNA and improves siRNA delivery ex vivo and
in vivo without activating the innate immune response
(13,32,33).
siRNA and miRNA exhibit similar chemical properties and therefore,
MPG strategies that are used for siRNA delivery may also be used
for miRNA delivery.
In the present study, MPG family-mediated miR-122
mimic and inhibitor delivery was developed in vitro and in
C. elegans. Various technical factors were optimized, such
as the molar ratio of peptides/oligonucleotide complexes, and the
efficiency of MPG in modulating miR-122 compared with that of
MPGΔNLS was determined. Using miRNA-specific qPCR
measurements, the levels of miRNA-122 were observed in NCTC 1469
cells. The results demonstrated that the miR-122 levels increased
dose-dependently up to 4000-fold compared with that of the delivery
of the negative control when NCTC 1469 cells were treated with 50
nM peptide/miR-122 mimic complexes at a molar ratio of 20:1
(Fig. 1A). A 60% reduction of
miR-122 levels was obtained in the cells transfected with
peptide/miR-122 inhibitor complexes at a molar ratio of 20:1
(Fig. 1B). However, further
increase of the peptide/oligonucleotide to a molar ratio of 50:1
demonstrated only a marginal change in the miR-122 levels
(P>0.05), which may be explained by a net increase in the size
of particles. At a lower ratio (1:1), the efficiency of MPG- and
MPGΔNLS-based miR-122 mimic delivery was 10-fold lower
than that of peptides at a 20:1 ratio and similar results were
observed in the delivery of miR-122 inhibitor at 1:1 ratio. These
results are in agreement with those of Crombez et
al(34), which may indicate
that the complexes were unstable and poorly taken up. By contrast,
there was a limited adjustment of the miR-122 levels when the cells
were treated with MPG/miR-122 complexes compared with that of
MPGΔNLS at the same peptide/miR-122 ratio. A higher
efficiency of MPGΔNLS/miR-122 is associated with a
single mutation in the NLS sequence that induces rapid release of
the miR-122 into the cytoplasm, and this correlates with an
increased biological response. Therefore, to ensure optimal
biological conditions for miR-122 delivery,
MPGΔNLS/miR-122 particles were used and systematically
prepared at a 20:1 ratio.
In order to use MPG derivatives as delivery vehicles
for miRNA therapeutics, the degree of cytotoxicity of
peptides/miRNA complexes is critical. Thus, the cytotoxicity of MPG
and MPGΔNLS with miR-122 mimic complexes was determined
by an MTT assay in NCTC 1469 and A549 cells (Fig. 1C and D). Following transfection for
48 h, no toxicity was detected up to a concentration of 100 nM in
the cell lines and only 4% of cell death was observed with 100 nM
of MPG/miR-122 particles in NCTC 1469 cells. Therefore,
peptide/miRNA was considered to be relatively non-toxic for
cells.
Fam-labeled miR-122 mimic was distributed in the
cytoplasm and nucleus within 10 min when the cells were transfected
with MPG, but remained predominantly in the cytoplasm of the cells
transfected with MPGΔNLS, which is consistent with the
results of a previous study (17)
and contributed to the higher efficiency of downregulation.
Moreover, further studies demonstrated that MPG- and
MPGΔNLS-mediated miR-122 mimic and inhibitor delivery
was independent of the presence of serum (data not shown). In order
to avoid any artifacts that may have been associated with the
nature of the probe, an alternate fluorescent probe (Cy3) was used
to label miR-122 mimic in NCTC 1469 cells. Furthermore, while
primary cells remained relatively refractory to transfection,
MPGΔNLS mediated Cy3-labeled miR-122 mimic transfection
within 10 min at a 95% efficiency. These results collectively
demonstrated that MPGΔNLS may be able to transfect
different cell types without affecting the cell phenotype, which
opens novel possibilities for large-scale miRNA therapy in
disease-relevant primary cells. Meanwhile, it was demonstrated that
the fluorescence was markedly low in certain cells (Fig. 3, indicated by an arrow), which may
be due to the variation in the membrane components. However, Jones
and Howl (35) suggested that CPP
induced non-specific membrane perturbations, thus leading to cell
death by necrotic mechanisms, particularly at higher
concentrations.
In addition to microinjection, soaking C.
elegans in dsRNA is also an effective method of delivery
(36). To investigate the delivery
efficiency of MPGΔNLS in model organisms, C.
elegans were soaked in the MPGΔNLS and Cy3-labeled
miR-122 mimic complexes for varying time periods (10 min-1 day). To
avoid artifacts from the ingestion of the fluorescent miR-122, the
fluorescence was observed within 10 min after soaking. Greater
fluorescence was detected in the C. elegans treated with
MPGΔNLS compared with those without peptide treatment.
Moreover, fluorescence localization in the C. elegans was
monitored after 24 h, which accumulated mainly inside the
intestines (data not shown).
To investigate the physiological effects of miR-122
silencing on cholesterol metabolism, the total cholesterol levels
in NCTC 1469 cells 48 h following coculture with
MPGΔNLS/miR-122 mimic and inhibitor were determined. To
the best of our knowledge, this is the first time that cholesterol
levels have been regulated by MPG-mediated miRNA delivery. Using
the novel delivery method, the levels of miR-122 in the NCTC 1469
cells were regulated, accompanied by 15–20% cholesterol changes
(Fig. 5). In a previous study,
total cholesterol reduced by a similar extent (26–28%) in mice
treated with miR-122 ASO as compared with saline-treated mice
(25). In the present study, there
were no chemical modifications that were associated with lower
stability of the complexes and lower pharmacological
efficiency.
However, numerous challenges remain in the
development of MPG derivative-based RNA therapeutics. Future
studies are required to calculate and analyze 3D models of the
non-covalent MPG/RNA complexes in order to understand their
formation and stabilization. Further toxicity studies of the
chemically modified miRNA inhibitor in animal models are also
required.
In conclusion, the non-covalent peptide-based
strategy was used for the efficient delivery of miR-122 mimic and
inhibitor into mouse liver cell lines, as well as mouse primary
hepatocytes and C. elegans, without any associated
cytotoxicity. This method is valuable in the study of the
biological functions of individual miRNAs and miRNA-associated
gene-regulatory networks, and in evaluating miRNA targets. This
method is significantly faster and simpler than comparable
genetics-based approaches.
Acknowledgements
This study was supported in part by the open funding
of the National Key Laboratory of Crop Genetic Improvement (grant
no. ZK201201) and by funding from the Guangdong Provincial
Population and Family Planning Commission of scientific research
project (grant no. 20110223). The authors would like to thank
Professor Jian-Ping Cai and Dr. Xiaoyang Zhou (The Key Laboratory
of Geriatrics, Beijing Hospital and Beijing Institute of
Geriatrics) for providing the nematode Caenorhabditis
elegans (C. elegans) and Dr. Jun Dong (The Peking Union
Medical College and Chinese Academy of Medical Sciences) for
assistance with the HPLC analysis.
References
|
1
|
Bartel DP: MicroRNAs: genomics,
biogenesis, mechanism, and function. Cell. 116:281–297. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Lee Y, Ahn C, Han J, et al: The nuclear
RNase III Drosha initiates microRNA processing. Nature.
425:415–419. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Yi R, Qin Y, Macara IG and Cullen BR:
Exportin-5 mediates the nuclear export of pre-microRNAs and short
hairpin RNAs. Genes Dev. 17:3011–3016. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Ambros V: The functions of animal
microRNAs. Nature. 431:350–355. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Yekta S, Shih IH and Bartel DP:
MicroRNA-directed cleavage of HOXB8 mRNA. Science. 304:594–596.
2004. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Hildebrandt-Eriksen ES, Aarup V, Persson
R, Hansen HF, Munk ME and Orum H: A locked nucleic acid
oligonucleotide targeting microRNA 122 is well-tolerated in
cynomolgus monkeys. Nucleic Acid Ther. 22:152–161. 2012.PubMed/NCBI
|
|
7
|
Elmén J, Lindow M, Schutz S, et al:
LNA-mediated microRNA silencing in non-human primates. Nature.
452:896–899. 2008.PubMed/NCBI
|
|
8
|
Heitz F, Morris MC and Divita G: Twenty
years of cell-penetrating peptides: from molecular mechanisms to
therapeutics. Br J Pharmacol. 157:195–206. 2009.PubMed/NCBI
|
|
9
|
Zatsepin TS, Turner JJ, Oretskaya TS and
Gait MJ: Conjugates of oligonucleotides and analogues with cell
penetrating peptides as gene silencing agents. Curr Pharm Des.
11:3639–3654. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
El-Andaloussi S, Holm T and Langel U:
Cell-penetrating peptides: mechanisms and applications. Curr Pharm
Des. 11:3597–3611. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Joliot A and Prochiantz A: Transduction
peptides: from technology to physiology. Nat Cell Biol. 6:189–196.
2004. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Torchilin VP: Tat peptide-mediated
intracellular delivery of pharmaceutical nanocarriers. Adv Drug
Deliv Rev. 60:548–558. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Deshayes S, Morris M, Heitz F and Divita
G: Delivery of proteins and nucleic acids using a non-covalent
peptide-based strategy. Adv Drug Deliv Rev. 60:537–547. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Deshayes S, Morris MC, Divita G and Heitz
F: Cell-penetrating peptides: tools for intracellular delivery of
therapeutics. Cell Mol Life Sci. 62:1839–1849. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Snyder EL and Dowdy SF: Recent advances in
the use of protein transduction domains for the delivery of
peptides, proteins and nucleic acids in vivo. Expert Opin Drug
Deliv. 2:43–51. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Eguchi A and Dowdy SF: siRNA delivery
using peptide transduction domains. Trends Pharmacol Sci.
30:341–345. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Simeoni F, Morris MC, Heitz F and Divita
G: Insight into the mechanism of the peptide-based gene delivery
system MPG: implications for delivery of siRNA into mammalian
cells. Nucleic Acids Res. 31:2717–2724. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Yoo JW, Hong SW, Kim S and Lee DK:
Inflammatory cytokine induction by siRNAs is cell type- and
transfection reagent-specific. Biochem Biophys Res Commun.
347:1053–1058. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Crombez L, Charnet A, Morris MC,
Aldrian-Herrada G, Heitz F and Divita G: A non-covalent
peptide-based strategy for siRNA delivery. Biochem Soc Trans.
35:44–46. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Lundberg P, El-Andaloussi S, Sutlu T,
Johansson H and Langel U: Delivery of short interfering RNA using
endosomolytic cell-penetrating peptides. FASEB J. 21:2664–2671.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Simeoni F, Morris MC, Heitz F and Divita
G: Peptide-based strategy for siRNA delivery into mammalian cells.
Methods Mol Biol. 309:251–260. 2005.PubMed/NCBI
|
|
22
|
Morris MC, Chaloin L, Méry J, Heitz F and
Divita G: A novel potent strategy for gene delivery using a single
peptide vector as a carrier. Nucleic Acids Res. 27:3510–3517. 1999.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Morris KV, Chan SW, Jacobsen SE and Looney
DJ: Small interfering RNA-induced transcriptional gene silencing in
human cells. Science. 305:1289–1292. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Nguyen QN, Chavli RV, Marques JT, et al:
Light controllable siRNAs regulate gene suppression and phenotypes
in cells. Biochim Biophys Acta. 1758:394–403. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Esau C, Davis S, Murray SF, et al: miR-122
regulation of lipid metabolism revealed by in vivo antisense
targeting. Cell Metab. 3:87–98. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Liu GT, Carrazana EJ, Macklis JD and
Mikati MA: Delayed oculogyric crises associated with
striatocapsular infarction. J Clin Neuroophthalmol. 11:198–201.
1991.PubMed/NCBI
|
|
27
|
Esau CC: Inhibition of microRNA with
antisense oligonucleotides. Methods. 44:55–60. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Lanford RE, Hildebrandt-Eriksen ES, Petri
A, et al: Therapeutic silencing of microRNA-122 in primates with
chronic hepatitis C virus infection. Science. 327:198–201. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Méry J, Granier C, Juin M and Brugidou J:
Disulfide linkage to polyacrylic resin for automated Fmoc peptide
synthesis. Immunochemical applications of peptide resins and
mercaptoamide peptides. Int J Pept Protein Res. 42:44–52.
1993.PubMed/NCBI
|
|
30
|
Salonpää P, Pelkonen O, Kojo A, Pasanen M,
Negishi M and Raunio H: Cytochrome P4502A5 expression and
inducibility by phenobarbital is modulated by cAMP in mouse primary
hepatocytes. Biochem Biophys Res Commun. 205:631–637.
1994.PubMed/NCBI
|
|
31
|
Dong J, Guo H, Yang R, et al: Serum LDL-
and HDL-cholesterol determined by ultracentrifugation and HPLC. J
Lipid Res. 52:383–388. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Krützfeldt J, Rajewsky N, Braich R, et al:
Silencing of microRNAs in vivo with ‘antagomirs’. Nature.
438:685–689. 2005.
|
|
33
|
Crombez L, Morris MC, Heitz F and Divita
G: A non-covalent peptide-based strategy for ex vivo and in vivo
oligonucleotide delivery. Methods Mol Biol. 764:59–73. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Crombez L, Morris MC, Dufort S, et al:
Targeting cyclin B1 through peptide-based delivery of siRNA
prevents tumour growth. Nucleic Acids Res. 37:4559–4569. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Jones S and Howl J: Applications of
cell-penetrating peptides as signal transduction modulators for the
selective induction of apoptosis. Methods Mol Biol. 683:291–303.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Kimber MJ, McKinney S, McMaster S, Day TA,
Fleming CC and Maule AG: flp gene disruption in a parasitic
nematode reveals motor dysfunction and unusual neuronal sensitivity
to RNA interference. FASEB J. 21:1233–1243. 2007. View Article : Google Scholar : PubMed/NCBI
|