Introduction
Periodontitis is a common inflammatory disease that
is characterized by the destruction of tooth-supporting tissues,
including alveolar bone, and is the most common cause of tooth loss
in adults (1). For decades, one of
the key obstacles in periodontitis therapy is how to inhibit
alveolar bone resorption and promote bone formation. The core
mechanism of alveolar bone resorption in periodontitis is
osteoclast activation (2,3). Human periodontal ligament cells
(HPLCs), the predominant cellular constituents of the periodontium,
are important in maintaining the integrity of the tooth-supporting
tissues in periodontitis. In addition to exhibiting multi-potential
mesenchymal stromal cell characteristics, such as osteoblastic and
cementoblastic differentiation potential (4,5),
HPLCs have been shown to affect osteoclastogenesis. Osteoprotegerin
produced by periodontal ligament fibroblasts prevented
pre-osteoclast differentiation and function (6–8), and
the conditioned media of periodontal ligament fibroblasts inhibited
osteoclast formation in a mouse bone marrow culture (6).
Icariin is the predominant active compound of the
total flavonoid extracted from the stem and leaves of Epimedium
species in traditional Chinese medicine. In addition, it has been
demonstrated to stimulate the proliferation and osteogenic
differentiation of human bone mesenchymal stem cells, promotes bone
formation and inhibits cell apoptosis, osteoclast differentiation
and bone resorption (9–12). Icariin is suggested to enhance bone
healing and reduce the occurrence of osteoporosis and had been
considered as a candidate osteogenic compound for use in bone
tissue engineering (10,13–15).
With respect to the diverse pharmacological
activities of icariin, in the present study, it was investigated
whether icariin promoted HPLC proliferation and regulated the
expression of biomarkers associated with bone formation or
resorption, such as RANKL, OPG, Cbfa1 and OC in HPLCs. The results
of the study thereby offer an insight into its use as a therapy for
periodontitis and periodontal tissue regeneration.
Materials and methods
Reagents
Icariin was obtained from the National Institute for
the Control of Pharmaceutical and Biological Products (NICPBP;
Beijing, China) with a purity of 99%. Stock solutions of icariin
were prepared in dimethylsulfoxide (DMSO; Sigma-Aldrich, St. Louis,
MO, USA) and stored at −20°C. The final concentrations of icariin
used in the culture were 0.001, 0.01, 0.1 and 1 μg/ml. Dulbecco’s
modified Eagle’s medium (DMEM), trypsin and TRIzol reagent were
purchased from Gibco-BRL (St. Louis, MO, USA); fetal bovine serum
(FBS) was purchased from the Thermo Scientific HyClone (South
Logan, UT, USA); polyclonal rabbit anti-osteoprotegerin (OPG) and
polyclonal rabbit anti-receptor activator of nuclear factor-κB
ligand (RANKL) antibodies were obtained from Santa Cruz
Biotechnology (Santa Cruz, Santa Cruz, CA, USA); polyclonal rabbit
anti-core binding factor α1 (Cbfa1) and polyclonal rabbit
anti-osteocalcin (OC) antibodies were obtained from Abcam
(Cambridge, UK).
Cell culture
HPLCs were obtained from the mid-roots of
periodontally healthy permanent premolars and the third molar teeth
extracted from 6 patients (age, 18–22 years), when informed consent
had been signed. The study was approved by the Ethics Committee of
Capital Medical University, School of Stomatology, Beijing, China.
Briefly, the periodontal ligaments were minced, dispersed and
incubated with DMEM containing 15% (v/v) FBS supplemented with
antibiotics (100 U/ml penicillin and 100 μg/ml streptomycin). All
cells were cultured at 37°C in a humidified 5% CO2
atmosphere. When a confluent monolayer was achieved, cells were
harvested with 0.05% trypsin and 0.02% EDTA, transferred to a 25
cm2 diameter plastic culture bottle (Corning Inc.,
Corningy, NY, USA) and subcultured at a 1:2 split ratio with the
initial confluent monolayer designated as one population doubling
level. HPLCs at the third passage were used for the experiments
described below.
Cell proliferation assay
The effect of icariin on cell proliferation was
analyzed using an MTT assay. HPLCs were plated in flat-bottomed
96-well plates (2×103 cells/well) in advance. Following
incubation for 24 h, the culture medium was replaced with fresh 5%
FBS-DMEM medium containing icariin (0.001, 0.01, 0.1 and 1 μg/ml).
The cultures were incubated for 2, 4 or 6 days. Cells treated with
medium alone were used as a negative control and wells with medium
alone were used as a blank control. Four hours prior to the end of
the incubation, the cells were washed twice with phosphate-buffered
saline (pH 7.2) and incubated with 5 mg/ml MTT (20 μl) for the last
4 h. The medium was then decanted, formazan salts were dissolved in
200 μl DMSO and the absorbance was determined at 490 nm using a
high-throughput microplate spectrophotometer SpectraMax
Plus384 (Molecular Devices, Sunnyvale, CA, USA).
qPCR
HPLCs were seeded on a 25 cm2 culture
bottle at a density of 5×103 cells/cm2. The
RANKL, OPG, Cbfa1 and OC mRNA expression levels in the cells was
analyzed 4 days following treatment with icariin (0.001, 0.01, 0.1
and 1 μg/ml μg/ml) by qPCR. Cells treated with medium alone were
considered as control cells. Briefly, the total cellular RNA was
isolated using a Total RNA Extraction kit (Sunbio, Beijing, China)
according to the manufacturer’s instructions. Complementary DNA
(cDNA) was synthesized using total RNA primed with Oligo(dT)12–18
Primer as described in the SuperScript™ III Reverse Transcriptase
kit (Invitrogen Life Sciences, Carlsbad, CA, USA). The cDNA
specimens were analyzed using SYBR-Green qPCR. Definitive primers
are listed in Table I. qPCR was
conducted using an ABI Prism 7700 system (Applied Biosystems,
Carlsbad, CA, USA) under the following conditions: 2 min at 95°C,
45 cycles of 20 sec at 95°C, 25 sec at 58°C and 30 sec at 72°C.
Following PCR, a dissociation curve (melting curve) was constructed
in the range of 65 to 95°C.
 | Table IOligonucleotide primers used for
qPCR. |
Table I
Oligonucleotide primers used for
qPCR.
| Primer | Sequence | Amplicon size
(bp) |
|---|
| β-actin | Forward:
5′-TGACGTGGACATCCGCAAAG-3′ | |
| Reverse:
5′-CTGGAAGGTGGACAGCGAGG-3′ | 205 |
| RANKL | Forward:
5′-TGGATGGCTCATGGTTAGAT-3′ | |
| Reverse:
5′-GTCATGTTGGAGATCTTGGC-3′ | 160 |
| OPG | Forward:
5′-GAAAGTGGGAGCAGAAGACA-3′ | |
| Reverse:
5′-GAAGCTGTGAAGGAACCTGA-3′ | 211 |
| Cbfa1 | Forward:
5′-CTATCAGTTTCCCATGGTGC-3′ | |
| Reverse:
5′-CACCATCATTCTGGTTAGGC-3′ | 122 |
| OC | Forward:
5′-ATGAGAGCCCTCACACTCCT-3′ | |
| Reverse:
5′-TGGGTCTCTTCACTACCTCG-3′ | 148 |
Western blot analysis
Untreated control and treated HPLCs were rinsed with
phosphate-buffered saline and solubilized with 1X radio
immunoprecipitation assay buffer. The protein concentrations of
RANKL, OPG, Cbfa1 and OC were determined using a Micro
bicinchoninic acid (BCA) Protein Assay Reagent kit (Pierce
Biotechnology Inc., Rockford, IL, USA). Equalized protein
concentrations from each lysate were combined with 5X sodium lauryl
sulfate (SDS) sample buffer and electrophoresed on a 10% SDS
polyacrylamide gel and transferred to a polyvinylidene fluoride
transfer membrane. The membrane was blocked with 5% non-fat milk in
Tris buffer solution containing 0.05% Tween-20 (TBST) for 1 h. The
membranes were incubated overnight with the appropriate dilutions
of primary antibodies in blocking buffer at 4°C. The following day,
the membranes were washed and incubated for 2–3 h with alkaline
phosphatase-conjugated secondary antibody solution in blocking
buffer and washed three times with TBST. The proteins were
visualized by enhanced chemiluminescence according to the
manufacturer’s instructions (Amersham Biosciences, Piscataway, NJ,
USA). Western blot analysis results were quantified by densitometry
(Software Labworks 4.6; UVP, Cambridge, UK).
Statistical analysis
All values were presented as the mean ± standard
deviation. Statistical analysis was conducted using one-way
analysis of variance and Wilcoxon’s signed-rank test. P<0.05 was
considered to indicate a statistically significant difference.
Results
Cell proliferation
The effect of icariin (0.001, 0.01, 0.1 and 1 μg/ml)
on the proliferation of HPLCs was analyzed. Fig. 1 shows that compared with the
control, icariin promoted cell proliferation in a dose- and
time-dependent manner. The highest stimulatory activity was
observed with an icariin concentration of 0.01 μg/ml at 4 days and
declined thereafter.
Effect of icariin on RANKL and OPG mRNA
and protein expression in HPLCs
The mRNA levels of RANKL and OPG expressed by HPLCs
exposed to icariin at 4 days were analyzed by qPCR. β-actin was
used as a reference gene. Fig. 2
shows that HPLCs expressed RANKL and OPG genes under physiological
conditions. Low-dose icariin with a concentration of 0.001 μg/ml
did not exhibit a significant effect on RANKL and OPG expression in
HPLCs. However, at a concentration of 0.01 μg/ml, icariin
significantly increased OPG while decreasing RANKL expression and
resulted in the lowest RANKL/OPG expression ratio compared with the
control and other treatment groups. At concentrations of 0.1 and 1
μg/ml, treatment with icariin resulted in a decrease of OPG
expression, an increase in RANKL expression and a higher RANKL/OPG
expression ratio.
Western blot analysis was conducted to analyze RANKL
and OPG protein production. HPLCs expressed RANKL and OPG under
physiological conditions. Icariin exhibited a dose-dependent effect
on decreasing RANKL and increasing OPG production in HPLCs. This
was greatest in the 0.01 μg/ml icariin group, which exhibited the
lowest RANKL/OPG production ratio (Fig. 3).
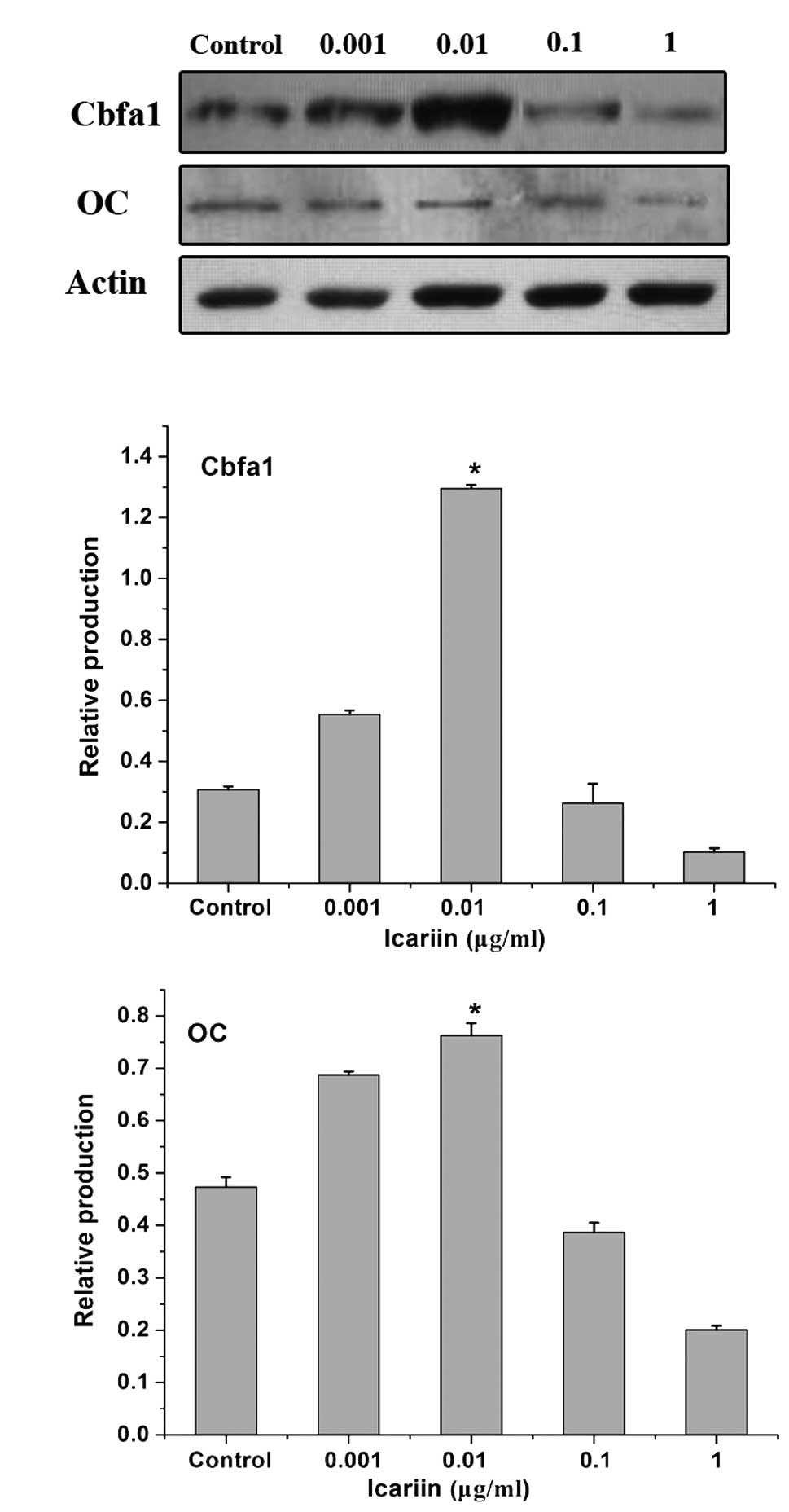
Effect of icariin on Cbfa1 and OC mRNA
and protein expression in HPLCs
To investigate the effects of icariin on the
regulation of osteogenic differentiation of periodontal ligament
cells, Cbfa1 and OC expression in HPLCs was analyzed. β-actin was
used in the same samples as a reference gene. HPLCs expressed Cbfa1
and OC under physiological conditions. Icariin exhibited a
dose-dependent effect on the induction of Cbfa1 and OC mRNA
expression in HPLCs. Cbfa1 and OC expression was induced in the
0.001 μg/ml icariin group. The highest expression value was
observed in the 0.01 μg/ml icariin group. At concentrations of 0.1
and 1 μg/ml, treatment with icariin resulted in the decrease in
Cbfa1 and OC expression (Fig.
4).
In addition, icariin also exhibited a dose-dependent
effect on the induction of Cbfa1 and OC protein production in
HPLCs. The most marked effect was observed in the 0.01 μg/ml
icariin group (Fig. 5).
Discussion
Previous studies have demonstrated that icariin
stimulates bone marrow stromal cells (MSCs), osteoblast
proliferation and osteogenic differentiation in a time- and
dose-dependent manner (9,16,17).
The results of the present study were in concordance with previous
studies and demonstrated that icariin enhanced human periodontal
ligament cell proliferation. In the present study, treatment with
0.01 μg/ml icariin at 4 days showed the greatest stimulatory effect
on HPLCs proliferation. This suggested icariin may exhibit an
osteogenic function by stimulating periodontal ligament cell
proliferation. Compared with previous reports, the differences in
the effects of the icariin concentration may be due to cell type,
inoculation number and culture conditions.
It was determined that icariin promoted HPLC
proliferation, and thus, the next aim was to investigate the
regulation of certain biomarkers involved in alveolar bone
resorption or formation. Numerous studies have demonstrated that
RANKL and its decoy receptor, OPG, were essential regulatory
molecules in osteoclastogenesis and bone resorption, in
physiological and pathological conditions. Significantly higher
levels of RANKL and lower osteoprotegerin protein levels are
expressed in the periodontitis tissue. The RANKL/OPG ratio in the
gingival crevice fluid was significantly increased in periodontal
disease patients compared with healthy subjects (18). Osteoblasts as well as other cells,
such as periodontal ligament cells participated in the regulation
of RANKL and OPG in periodontal tissue. OPG synthesized locally by
periodontal ligament cells regulated the resorption of alveolar
bone via cytokines, such as interleukin-1β and tumor necrosis
factor-α (19). Inactivation of
OPG is involved in osteoclast formation by periodontal ligament
fibroblasts (20). Icariin was
shown to be a regulatory agent in the upregulation of OPG and
downregulation of RANKL gene expression in osteoblasts and UMR 106
cells (13). In the present study,
icariin at a concentration of 0.01 μg/ml significantly increased
OPG while decreasing RANKL mRNA expression and protein production,
and obtained the lowest RANKL/OPG expression ratio. However, a
high-dose icariin (0.1 μg/ml and 1 μg/ml) resulted in an increased
RANKL/OPG expression ratio, possibly due to cell cytotoxicity. A
previous study by Fan et al, demonstrated that icariin
exhibited a dose-dependent effect on the proliferation and
osteogenic differentiation of human bone mesenchymal stem cells at
a suitable concentration range; however, when the concentration was
>10−5 M, cytotoxicity limited its effect (9). It was suggested that icariin may
inhibit osteoclast differentiation and alveolar bone resorption by
regulating RANKL and OPG levels in periodontal ligament cells.
Cbfa1 is an essential transcription factor in
osteoblast differentiation (21–23).
Mesenchymal stem cells transfected with an adenovirus encoding
Cbfa1 resulted in the increase of a number of osteoblastic markers
including osteocalcin, osteopontin and type I collagen expression,
and also induced a significantly increased bone formation and
marrow cavity rebuilding (21). OC
is a small, acidic extracellular protein synthesized by osteoblasts
during bone formation (24). Bone
cell activity is indirectly evaluated by the measurement of
specific biochemical markers of bone formation such as osteocalcin;
the greater the OC expression the more bone formed (25). Previous studies demonstrated that
icariin increased the proliferation of osteoblasts and the gene
expression of Cbfa1/Runx2 in these cells. In addition, it also
increased OPG gene expression but downregulated RANKL gene
expression (26). In the present
study, similar effects of icariin were observed and certain
concentrations markedly increased Cbfa1 and OC mRNA expression, and
protein production in periodontal ligament cells. The results
suggested that icariin may be considered as a growth factor similar
to bone morphogenetic protein-2 and may be used to promote bone
formation and periodontal regeneration.
In conclusion, the results demonstrated the
potential use of icariin in treating alveolar bone resorption and
promoting periodontal tissue regeneration in periodontitis, due to
its ability to stimulate the proliferation and osteogenic
differentiation of human periodontal ligament cells and inhibition
of osteoclast differentiation. However, the present study only
determined the effects in vitro, and thus, the effect of
icariin in periodontal tissue regeneration in vivo requires
further investigation.
Acknowledgements
This study was supported by the financial support of
Beijing Natural Science Foundations (grant no. 7122077) and the
Beijing Municipal Health Bureau (grant no. JJ2009-26), China.
References
|
1
|
Dikbas I, Tanalp J, Tomruk CO and Koksal
T: Evaluation of reasons for extraction of crowned teeth: a
prospective study at a university clinic. Acta Odontol Scand.
71:848–856. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Kobayashi Y, Udagawa N and Takahashi N:
Action of RANKL and OPG for osteoclastogenesis. Crit Rev Eukaryot
Gene Expr. 19:61–72. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Boyce BF and Xing L: Functions of
RANKL/RANK/OPG in bone modeling and remodeling. Arch Biochem
Biophys. 473:139–146. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Seo BM, Miura M, Gronthos S, et al:
Investigation of multipotent postnatal stem cells from human
periodontal ligament. Lancet. 364:149–155. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Gronthos S, Mrozik K, Shi S and Bartold
PM: Ovine periodontal ligament stem cells: isolation,
characterization, and differentiation potential. Calcif Tissue Int.
79:310–317. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Wada N, Maeda H, Tanabe K, et al:
Periodontal ligament cells secrete the factor that inhibits
osteoclastic differentiation and function: the factor is
osteoprotegerin/osteoclastogenesis inhibitory factor. J Periodontal
Res. 36:56–63. 2001. View Article : Google Scholar
|
|
7
|
Hasegawa T, Yoshimura Y, Kikuiri T, et al:
Expression of receptor activator of NF-kappa B ligand and
osteoprotegerin in culture of human periodontal ligament cells. J
Periodontal Res. 37:405–411. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Kanzaki H, Chiba M, Shimizu Y and Mitani
H: Dual regulation of osteoclast differentiation by periodontal
ligament cells through RANKL stimulation and OPG inhibition. J Dent
Res. 80:887–891. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Fan JJ, Cao LG, Wu T, et al: The
dose-effect of icariin on the proliferation and osteogenic
differentiation of human bone mesenchymal stem cells. Molecules.
16:10123–10133. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Hsieh TP, Sheu SY, Sun JS and Chen MH:
Icariin inhibits osteoclast differentiation and bone resorption by
suppression of MAPKs/NF-κB regulated HIF-1α and PGE(2) synthesis.
Phytomedicine. 18:176–185. 2011.PubMed/NCBI
|
|
11
|
Ma HP, Ming LG, Ge BF, et al: Icariin is
more potent than genistein in promoting osteoblast differentiation
and mineralization in vitro. J Cell Biochem. 112:916–923. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Cao H, Ke Y, Zhang Y, Zhang CJ, Qian W and
Zhang GL: Icariin stimulates MC3T3-E1 cell proliferation and
differentiation through up-regulation of bone morphogenetic
protein-2. Int J Mol Med. 29:435–439. 2012.PubMed/NCBI
|
|
13
|
Mok SK, Chen WF, Lai WP, et al: Icariin
protects against bone loss induced by oestrogen deficiency and
activates oestrogen receptor-dependent osteoblastic functions in
UMR 106 cells. Br J Pharmacol. 159:939–949. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Nian H, Ma MH, Nian SS and Xu LL:
Antiosteoporotic activity of icariin in ovariectomized rats.
Phytomedicine. 16:320–326. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Zhao J, Ohba S, Komiyama Y, Shinkai M,
Chung UI and Nagamune T: Icariin: a potential osteoinductive
compound for bone tissue engineering. Tissue Eng Part A.
16:233–243. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Chen KM, Ge BF, Ma HP, Liu XY, Bai MH and
Wang Y: Icariin, a flavonoid from the herb Epimedium enhances the
osteogenic differentiation of rat primary bone marrow stromal
cells. Pharmazie. 60:939–942. 2005.PubMed/NCBI
|
|
17
|
Yin XX, Chen ZQ, Liu ZJ, Ma QJ and Dang
GT: Icariine stimulates proliferation and differentiation of human
osteoblasts by increasing production of bone morphogenetic protein
2. Chin Med J (Engl). 120:204–210. 2007.PubMed/NCBI
|
|
18
|
Mogi M, Otogoto J, Ota N and Togari A:
Differential expression of RANKL and osteoprotegerin in gingival
crevicular fluid of patients with periodontitis. J Dent Res.
83:166–169. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Sakata M, Shiba H, Komatsuzawa H, et al:
Expression of osteoprotegerin (osteoclastogenesis inhibitory
factor) in cultures of human dental mesenchymal cells and
epithelial cells. J Bone Miner Res. 14:1486–1492. 1999. View Article : Google Scholar
|
|
20
|
Hasegawa T, Yoshimura Y, Kikuiri T, et al:
Expression of receptor activator of NF-kappa B ligand and
osteoprotegerin in culture of human periodontal ligament cells. J
Periodontal Res. 37:405–411. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Dong SW, Ying DJ, Duan XJ, et al: Bone
regeneration using an acellular extracellular matrix and bone
marrow mesenchymal stem cells expressing Cbfa1. Biosci Biotechnol
Biochem. 73:2226–2233. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Ducy P: Cbfa1: a molecular switch in
osteoblast biology. Dev Dyn. 219:461–471. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Lian JB, Stein JL, Stein GS, et al:
Runx2/Cbfa1 functions: diverse regulation of gene transcription by
chromatin remodeling and co-regulatory protein interactions.
Connect Tissue Res. 44(Suppl 1): 141–148. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Patterson-Buckendahl P: Osteocalcin is a
stress-responsive neuropeptide. Endocr Regul. 45:99–110. 2011.
View Article : Google Scholar
|
|
25
|
Maïmoun L, Fattal C and Sultan C: Bone
remodeling and calcium homeostasis in patients with spinal cord
injury: a review. Metabolism. 60:1655–1663. 2011.PubMed/NCBI
|
|
26
|
Hsieh TP, Sheu SY, Sun JS, Chen MH and Liu
MH: Icariin isolated from Epimedium pubescens regulates osteoblasts
anabolism through BMP-2, SMAD4, and Cbfa1 expression.
Phytomedicine. 17:414–423. 2010. View Article : Google Scholar : PubMed/NCBI
|