Introduction
Prostate cancer (PCa) is the most frequently
diagnosed cancer in men and it was estimated that new cases of PCa
would account for 29% of all cancers in men in the United States
during 2012 (1). Moreover, the
estimated number of mortalities caused by PCa is up to 9% of the
total number of cancer-associated mortalities in males, which is
second only to lung and bronchus cancers (1). Bone is a major site of metastasis and
the incidence of bone metastasis by PCa is 68% (2). Bone metastasis is associated with
severe pain, hypercalcemia and pathological fractures. Although
numerous methods, including surgical management and nonsurgical
modalities, have been proposed (3), bone metastasis is associated with
increased morbidity and a poor outcome for patients. However, the
detailed mechanisms of bone-specific metastasis remain unclear.
Clarification of the molecular mechanisms underlying bone
metastasis is of primary importance for targeted therapeutic
strategies in patients with PCa (4).
Bone metastasis of PCa requires a series of specific
interactions between cancer and host cells, such as human bone
marrow stromal cells (hBMSCs), at metastatic sites. The
well-accepted ‘seed and soil hypothesis’ proposes that the bone
matrix and abundant growth factors secreted by the bone marrow
result in the bone microenvironment being fertile ‘soil’ for cancer
cell ingrowth (5). In addition,
there are chemotactic factors in the bone microenvironment that
attract PCa cells. However, the mechanisms underlying PCa cell
metastasis to bone remain unknown.
Wnt proteins constitute a large family of at least
19 secreted glycoproteins that are important during development and
in cell fate, growth and migration (6). Wnt signaling occurs via canonical and
non-canonical pathways. The canonical pathway is known as the
β-catenin-dependent pathway and it promotes β-catenin accumulation
and translocation to the nucleus for the stimulation of target gene
expression. The non-canonical pathway activates the
β-catenin-independent pathway through planar cell polarity and the
Ca2+ signaling pathway. Wnt5a is an important member of
the Wnt family and acts as a tumor suppressor or promoter (7–9).
Moreover, Wnt5a is a regulator of structural plasticity and cell
motility in PCa (10). The present
study analyzed the ability of Wnt5a to regulate the migration of
PCa cells toward hBMSC-conditioned medium (CM).
Materials and methods
Cell isolation and culture
hBMSCs were isolated and expanded as described
previously by Li et al(11). Once ethical approval from the
ethics committee of Shanghai Jiaotong University School of Medicine
(Shanghai, China) and written informed consent from the donors was
obtained, bone marrow aspirates were acquired from healthy donors
during routine orthopedic surgical procedures. Approximately 10 ml
volumes of the bone marrow were harvested through a bone marrow
biopsy needle inserted through the iliac crest. The bone marrow
aspirates were immediately seeded onto 100-mm culture dishes and
cultured in complete medium consisting of α-modified minimum
essential medium (HyClone, Logan, UT, USA) with 10% fetal bovine
serum (HyClone, Tauranga, New Zealand), 100 IU/ml penicillin and
100 mg/ml streptomycin (HyClone, Logan, UT, USA) in a humidified
37°C/5% CO2 incubator. After three days, non-adherent
cells were discarded by three washes with phosphate-buffered saline
(PBS), and the adherent cells were cultured further until 80–90%
confluence with medium changes every three days. The obtained
hBMSCs were digested with trypsin (0.25%; HyClone, Logan, UT, USA)
and third passage cells were used in the subsequent
experiments.
Three PCa cell lines derived from different
metastatic sites were analyzed in this study, namely PC3 (derived
from bone), LNCaP (derived from lymph nodes) and DU145 (derived
from the brain). These cell lines were purchased from the Chinese
Academy of Sciences Cell Bank (Shanghai, China) and cultured in the
complete medium in a humidified 37°C/5% CO2
incubator.
Conditioned medium preparation
The PC3 cells and hBMSCs were cultured separately in
100-mm culture dishes in complete medium, as described above, until
confluence. Subsequently, cells were rinsed with PBS and incubated
in 10 ml of serum-free (SF) medium. After 24 h, the CM was
harvested and centrifuged at 0.3 × g for 5 min to remove cell
debris. The CM was stored at −80°C until use and was combined with
10% FBS prior to use.
RNA interference
Small interfering RNA (siRNA) and DharmaFECT 2
transfection reagent were obtained from Dharmacon, Inc. (Lafayette,
CO, USA). Wnt5a expression was knocked down in the PC3 cells and
hBMSCs according to the manufacturer’s instructions. Confluent
cells (40%) were seeded onto 96-well plates for proliferation
analysis and into six-well plates for other studies. A final
concentration of 50 nM siRNA and DharmaFECT 2 transfection reagent
were used for in vitro transfection. Non-targeting siRNA
(siScramble) was used as a siRNA control. Wnt5a gene expression
levels were determined by quantitative PCR (qPCR) at 24 h
post-transfection and the protein levels were detected by western
blotting at 72 h post-transfection. To prepare the CM, the medium
was replaced with SF medium at 48 h post-transfection and collected
following a further 24 h.
Cell proliferation
For cell proliferation analysis, cells were seeded
in 96-well plates in complete medium with or without recombinant
mouse Wnt5a (rmWnt5a; R&D Systems, Minneapolis, MN, USA). An
alamarBlue assay® (Biosource, Camarillo, CA, USA) was
carried out at 24, 48, 72 and 96 h in accordance with the
manufacturer’s instructions. The absorbance of the culture medium
containing alamarBlue was monitored with a spectrophotometer
(ELx800; BioTek, Winooski, VT, USA) at 570 and 600 nm.
Migration assay
Cell migration assays were performed in 24-well
transwell chambers with 8-μm pore polycarbonate membranes (Corning
Inc., Lowell, MA, USA). PC3 cells were suspended at a density of
1×105 cells/ml in SF medium, and then 100 μl of the cell
suspension was added to the upper chamber of the transwell
chambers. The lower chamber contained 500 μl of SF medium or
hBMSC-CM with various concentrations of rmWnt5a (0.1, 0.2, 0.3 and
0.5 μg/ml). Following 16 h of culture, the cells were fixed with 4%
paraformaldehyde and washed under flowing water. Cells on the upper
surface of the membrane were scraped off with a cotton swab and
cells on the lower surface were stained with crystal violet. The
cells were then counted in five fields of each well under a light
microscope (IX71; Leica, Wetzlar, Germany). Experiments were
performed in triplicate.
qPCR
Total RNA was isolated from cells using
TRIzol® reagent (Invitrogen Life Technologies, Carlsbad,
CA, USA) according to the manufacturer’s instructions. Equal
amounts of RNA (1 μg) were converted into cDNA with a PrimeScript™
RT reagent kit (Takara, Dalian, China). Subsequently, qPCR was
performed with SYBR® Premix Ex Taq™ (Takara) using an
ABI 7500 Real-Time PCR system (Applied Biosystems, Foster City, CA,
USA). PCR conditions were as follows: 40 cycles at 94°C for 5 sec
and 60°C for 34 sec. The glyceraldehyde-3-phosphate dehydrogenase
gene was used as an internal control. The primer sequences used in
this study are shown in Table
I.
 | Table IPrimer oligonucleotide sequences used
for qPCR. |
Table I
Primer oligonucleotide sequences used
for qPCR.
| Gene | Forward primer
(5′-3′) | Reverse primer
(5′-3′) | Product size
(bp) |
|---|
| BMP2 |
ACCCGCTGTCTTCTAGCGT |
TTTCAGGCCGAACATGCTGAG | 180 |
| BMP4 |
AAAGTCGCCGAGATTCAGGG |
GACGGCACTCTTGCTAGGC | 135 |
| BMP7 |
TCGGCACCCATGTTCATGC |
GAGGAAATGGCTATCTTGCAGG | 150 |
| CXCR4 |
ACTACACCGAGGAAATGGGCT |
TTCTTCACGGAAACAGGGTTC | 65 |
| IGF2 |
GGAGACGTACTGTGCTACCC |
CTGCTTCCAGGTGTCATATTGG | 124 |
| IGF1 |
GCTCTTCAGTTCGTGTGTGGA |
CGACTGCTGGAGCCATACC | 71 |
| IL11 |
CGAGCGGACCTACTGTCCTA |
GCCCAGTCAAGTGTCAGGTG | 272 |
| MMP1 |
GGGGCTTTGATGTACCCTAGC |
TGTCACACGCTTTTGGGGTTT | 142 |
| MMP7 |
GAGTGAGCTACAGTGGGAACA |
CTATGACGCGGGAGTTTAACAT | 158 |
| OPG |
GCGCTCGTGTTTCTGGACA |
AGTATAGACACTCGTCACTGGTG | 226 |
| OPN |
CTCCATTGACTCGAACGACTC |
CAGGTCTGCGAAACTTCTTAGAT | 230 |
| RANKL |
CAACATATCGTTGGATCACAGCA |
GACAGACTCACTTTATGGGAACC | 161 |
| TGFB2 |
CAGCACACTCGATATGGACCA |
CCTCGGGCTCAGGATAGTCT | 113 |
| WNT10B |
CATCCAGGCACGAATGCGA |
CGGTTGTGGGTATCAATGAAGA | 204 |
| WNT2 |
ATGTGCGATAATGTGCCAGG |
AGATTCCCGACTACTTCGGAG | 207 |
| WNT3A |
CCTGGCTTTGGAATGCTC |
CCTCTGCGAAGTCCCTGT | 172 |
| WNT5A |
TTGGTGGTCGCTAGGTATGAA |
AGTGGCACAGTTTCTT | 120 |
| WNT7B |
GAAGCAGGGCTACTACAACCA |
CGGCCTCATTGTTATGCAGGT | 155 |
| bFGF |
AGAAGAGCGACCCTCACATCA |
CGGTTAGCACACACTCCTTTG | 82 |
| IGF1R |
AGGATATTGGGCTTTACAACCTG |
ACAGAGGTCAGCATTTTTCTCAA | 74 |
| MMP9 |
TGTACCGCTATGGTTACACTCG |
GGCAGGGACAGTTGCTTCT | 97 |
| VEGF |
CGCAGCTACTGCCATCCAAT |
GTGAGGTTTGATCCGCATAATCT | 192 |
| GAPDH |
TCACCATCTTCCAGGAGCGA |
CACAATGCCGAAGTGGTCGT | 293 |
Western blot analysis
Cells were lysed for 30 min on ice in
radioimmunoprecipitation assay lysis buffer containing 1%
phenylmethylsulfonyl fluoride. Proteins were fractionated by sodium
dodecyl sulfate-polyacrylamide gel electrophoresis, and then
electrotransferred onto nitrocellulose membranes. Blocked membranes
were incubated overnight with antibodies against Wnt5a (Abcam,
Cambridge, UK) and β-actin (Cell Signaling Technology, Inc.,
Danvers, MA, USA). Following incubation with the appropriate
secondary antibodies (IRDye 800CW-Conjugated Goat Anti-Rabbit IgG;
LI-COR Biosciences, Lincoln, NE, USA) for 1 h, the membranes were
scanned with an Odyssey® CLx system (LI-COR
Biosciences).
Statistical analysis
The results are expressed as the mean ± standard
deviation. An unpaired t-test was used to compare single groups.
Analysis of variance was used to test for significant differences
between >2 groups. The significance level for all tests was
P<0.05.
Results
Expression profiles of bone
metastasis-associated genes in three PCa cell lines
Three PCa cell lines (PC3, LNCaP, and DU145) were
analyzed in this study. The expression of 22 genes associated with
bone metastasis was measured by qPCR (Fig. 1). Significantly higher levels of
Wnt5a mRNA expression were observed in the PC3 cells, at 10- and
6-fold higher than those in the LNCaP (P<0.01) and DU145 cells
(P<0.01), respectively.
Proliferation and migration of PC3 cells
transfected with siRNA against Wnt5a
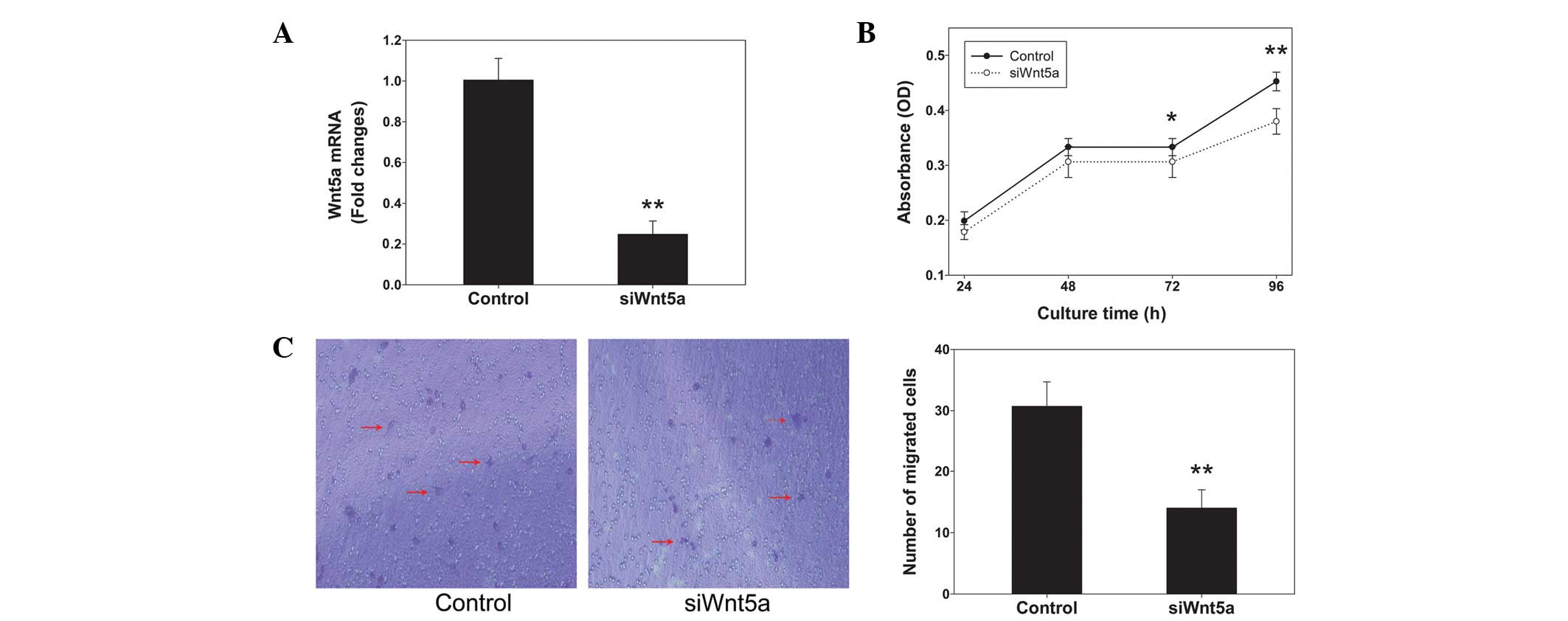
siRNA against Wnt5a expression was employed to
investigate the role of Wnt5a in the proliferation and migration of
PC3 cells. Wnt5a siRNA reduced the levels of Wnt5a mRNA by ~75%
compared with those in the control (Fig. 2A). The alamarBlue assays indicated
that Wnt5a siRNA significantly decreased the proliferation rate of
cells cultured for 72 and 96 h (P<0.05 and P<0.01,
respectively; Fig. 2B). Transwell
chambers were used to assess cell migration. Representative images
of migrated cells stained with crystal violet are shown in Fig. 2C. The inhibition of Wnt5a
expression by siRNA knockdown significantly reduced the PC3 cell
migration by 50% compared with that in the control (P<0.01).
Proliferation and migration of PC3 cells
treated with rmWnt5a
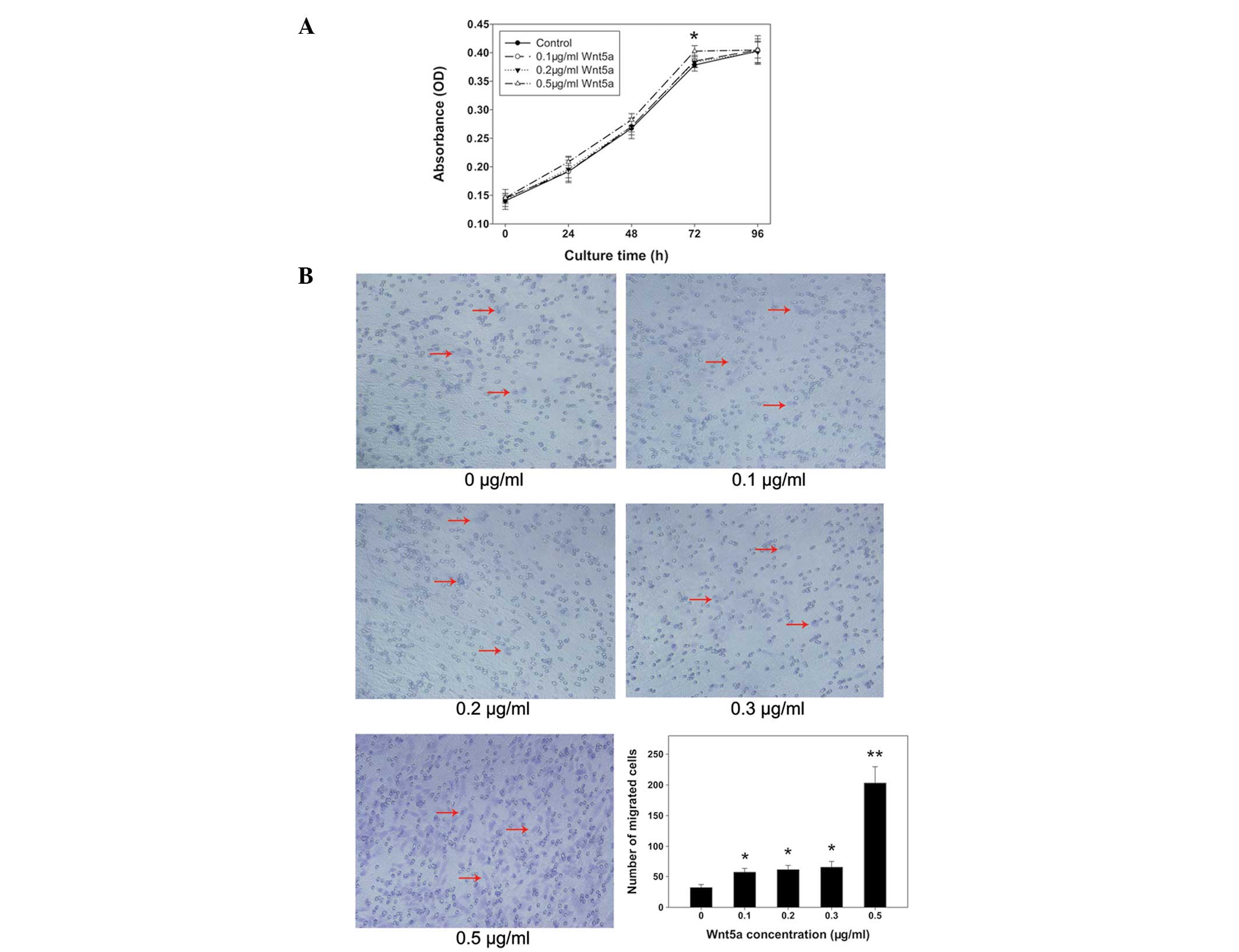
PC3 cells were treated with various concentrations
of rmWnt5a and then alamarBlue assays were performed. Proliferation
rates were equal among groups with the exception of 0.5 μg/ml
rmWnt5a, which showed a higher cell proliferation rate than that in
the other groups at 72 h (P<0.05) (Fig. 3A). Results from the transwell
assays demonstrated that the migration of PC3 cells was
significantly promoted by increasing concentrations of rmWnt5a
(P<0.05) (Fig. 3B). The number
of cells treated with 0.5 μg/ml rmWnt5a that migrated was ~6-fold
higher than that in the control (P<0.01). In addition, the
numbers of migrated cells in the other groups (0.1, 0.2 and 0.3
μg/ml rmWnt5a) were nearly two-fold higher than that in the control
(P<0.05).
Enhancement of PC3 cell migration in
hBMSC-CM
Bone metastasis depends on complex interactions
between tumor cells and cells in the bone microenvironment, such as
hBMSCs. Thus, hBMSC-CM was prepared to investigate the indirect
interactions of PC3 cells with hBMSCs in vitro. The results
from the transwell assays demonstrated that the migration of PC3
cells was significantly promoted in hBMSC-CM compared with that in
SF medium (P<0.01) (Fig. 4).
When rmWnt5a was added to the hBMSC-CM, the number of migrated
cells increased further. The number of migrated cells in hBMSC-CM
containing 0.2 μg/ml rmWnt5a was three-fold higher than that in
hBMSC-CM without rmWnt5a (P<0.01) and five-fold higher than that
in SF medium containing 0.2 μg/ml rmWnt5a (P<0.01).
PC3 cell migration decreases in CM of
Wnt5a siRNA-transfected hBMSCs
To obtain further evidence that Wnt5a is important
in the interaction between PC3 cells and hBMSCs, Wnt5a gene
expression in hBMSCs was knocked down using siRNA prior to
collection of CM. Subsequently, the CM obtained from these cells
was applied to PC3 cells in transwell assays. The results from qPCR
and western blot analyses demonstrated that Wnt5a gene expression
levels were reduced by 50% (Fig. 5A
and B). Fig. 5C shows
representative crystal violet staining of migrated cells in
transwell assays. CM from Wnt5a siRNA-transfected hBMSCs
significantly reduced PC3 cell migration compared with that of CM
from siScramble-transfected hBMSCs (P<0.05). These results
suggest that the migration of PC3 cells toward hBMSCs is, at least
in part, dependent on Wnt5a secreted from hBMSCs.
Discussion
Tumor metastasis indicates a poor prognosis and bone
is a prime target for PCa metastasis. When PCa metastasizes to
bone, the five-year survival rate drops to ~30% from virtually 100%
for PCa that remains confined to the prostate (12). However, the mechanisms of PCa bone
metastasis are unclear. Thus, the expression of 22 genes associated
with bone metastasis was analyzed in PCa cell lines. The present
study identified that Wnt5a was highly expressed in PC3 cells that
were derived from a bone metastasis site. Therefore, the study
focused on this potential candidate gene.
Wnt5a regulates a variety of cellular functions such
as adhesion, proliferation, differentiation and migration (13,14).
A number of important roles of Wnt5a have been demonstrated in
organ development (15). In
addition, Wnt5a participates in tumor progression. Wnt5a has either
a tumor-suppressing or -promoting function depending on the type of
cancer (16). A number of studies
have indicated increased expression levels of Wnt5a in melanoma
(9,17,18),
breast cancer cells (13), gastric
cancer (19), pancreatic cancer
(20) and non-small cell lung
cancer (16). Wnt5a expression is
correlated with aggressiveness and a poor prognosis of gastric
cancer (19,21). It is a mediator of chemoresistance
in ovarian cancer (22) and
correlates with the clinical grade (23). Although there is firm evidence that
Wnt5a has an oncogenic role, certain studies have indicated that
Wnt5a has a suppressive role in tumors arising from a variety of
tissues. Wnt5a is downregulated in certain malignancies, including
colorectal cancer (24),
neuroblastoma (25), invasive
ductal breast carcinomas (26) and
leukemias (27), indicating a
tumor-suppressive effect (24).
In PCa, Wang et al identified that the Wnt5a
protein levels are increased compared with those in benign tissue
(10). However, Syed Khaja et
al demonstrated that elevated levels of Wnt5a protein in
patients with localized PCa predict a favorable outcome following
surgery (28). In the present
study, the addition of rmWnt5a enhanced PC3 cell migration in a
concentration-dependent manner. Furthermore, when Wnt5a gene
expression was silenced by siRNA, PC3 cell migration was reduced by
50%. Yamamoto et al have also demonstrated that knockdown of
Wnt5a in human PCa cell lines reduces the cells’ invasive
activities, indicating that Wnt5a promotes the aggressiveness of
PCa and is involved in relapses following prostatectomy (29).
The role of Wnt5a in PCa cell proliferation was also
assessed. Although decreased proliferation rates were observed when
PC3 cells were cultured for 72 and 96 h following Wnt5a gene
knockdown, the addition of rmWnt5a did not significantly affect PC3
cell proliferation. Thus, the role of Wnt5a in PCa cells is mainly
associated with cell migration. Wnt5a activates the
Wnt/Ca2+ pathway in PCa cells, which causes a major
reorganization of the cytoskeleton in cancer cells by decreasing
the length and frequency of fine filopodia-like actin structures
and results in an increase in cell motility (10).
The role of Wnt5a in mediating PCa bone metastasis
is unclear. Bone metastasis is a multistep process. The ‘seed and
soil’ hypothesis suggests that there are chemotactic factors in the
bone microenvironment that attract PCa cells (2,5).
Cell-cell interactions between PCa cells and cells in the bone
microenvironment are important and contribute to metastatic cell
behavior (30,31). Previous studies have shown that
BMSCs are significant in PCa cell metastasis (32,33).
Therefore, in the present study hBMSC-CM was collected for further
study and it was demonstrated that the number of PC3 cells that
migrated toward hBMSC-CM was ~3-fold higher than that toward SF
medium. To determine whether the enhanced migration of PC3 cells
toward hBMSC-CM was due to Wnt5a protein expression in hBMSCs,
Wnt5a expression in hBMSCs was knocked down and PC3 cell migration
toward hBMSC-CM was reduced by 30%. The migration of PC3 cells
toward hBMSC-CM was confirmed to be at least partly dependent on
Wnt5a expression in hBMSCs. In addition to Wnt5a, other factors
participate in PCa cell migration toward hBMSCs. The interaction
between the stromal-derived factor-1 and the CXCR4 ligand-receptor
system is the most studied and is also involved in the activation
of PCa cell migration (34,35).
Overall, the findings of the present study implicate Wnt5a in the
stimulation of PCa cell migration toward BMSC-CM. Consequently, the
inhibition of Wnt5a signaling may be an attractive therapeutic
target for the treatment of advanced PCa.
Acknowledgements
This study was supported by the National Natural
Science Foundation of China (grant no. 81172549); the Shanghai
Science and Technology Development Fund (grant no. 10410711100,
grant no. 11XD1403300); the Key Disciplines of Shanghai Municipal
Education Commission (No. J50206); the Specialized Research Fund
for the Doctoral Program of Higher Education (grant no.
20110073110075); and the Ph.D. Programs Foundation of Shanghai
Jiaotong University School of Medicine (grant no. BXJ201125).
References
|
1
|
Siegel R, Naishadham D and Jemal A: Cancer
statistics, 2012. CA Cancer J Clin. 62:10–29. 2012. View Article : Google Scholar
|
|
2
|
Suva LJ, Washam C, Nicholas RW and Griffin
RJ: Bone metastasis: mechanisms and therapeutic opportunities. Nat
Rev Endocrinol. 7:208–218. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Bickels J, Dadia S and Lidar Z: Surgical
management of metastatic bone disease. J Bone Joint Surg Am.
91:1503–1516. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Santini D, Galluzzo S, Zoccoli A, et al:
New molecular targets in bone metastases. Cancer Treat Rev.
36(Suppl 3): S6–S10. 2010. View Article : Google Scholar
|
|
5
|
Jin JK, Dayyani F and Gallick GE: Steps in
prostate cancer progression that lead to bone metastasis. Int J
Cancer. 128:2545–2561. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Reya T and Clevers H: Wnt signalling in
stem cells and cancer. Nature. 434:843–850. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Jenei V, Sherwood V, Howlin J, et al: A
t-butyloxycarbonyl-modified Wnt5a-derived hexapeptide functions as
a potent antagonist of Wnt5a-dependent melanoma cell invasion. Proc
Natl Acad Sci USA. 106:19473–19478. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Bitler BG, Nicodemus JP, Li H, et al:
Wnt5a suppresses epithelial ovarian cancer by promoting cellular
senescence. Cancer Res. 71:6184–6194. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Weeraratna AT, Jiang Y, Hostetter G, et
al: Wnt5a signaling directly affects cell motility and invasion of
metastatic melanoma. Cancer Cell. 1:279–288. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Wang Q, Symes AJ, Kane CA, et al: A novel
role for Wnt/Ca2+ signaling in actin cytoskeleton
remodeling and cell motility in prostate cancer. PLoS One.
5:e104562010.PubMed/NCBI
|
|
11
|
Li D, Dai K and Tang T: Effects of dextran
on proliferation and osteogenic differentiation of human bone
marrow-derived mesenchymal stromal cells. Cytotherapy. 10:587–596.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Jemal A, Siegel R, Ward E, Hao Y, Xu J and
Thun MJ: Cancer statistics, 2009. CA Cancer J Clin. 59:225–249.
2009. View Article : Google Scholar
|
|
13
|
Kikuchi A, Yamamoto H, Sato A and
Matsumoto S: Wnt5a: its signalling, functions and implication in
diseases. Acta Physiol (Oxf). 204:17–33. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Witze ES, Litman ES, Argast GM, Moon RT
and Ahn NG: Wnt5a control of cell polarity and directional movement
by polarized redistribution of adhesion receptors. Science.
320:365–369. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Roarty K and Serra R: Wnt5a is required
for proper mammary gland development and TGF-beta-mediated
inhibition of ductal growth. Development. 134:3929–3939. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
McDonald S and Silver A: The opposing
roles of Wnt-5a in cancer. Br J Cancer. 101:209–214. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Da Forno PD, Pringle JH, Hutchinson P, et
al: WNT5A expression increases during melanoma progression and
correlates with outcome. Clin Cancer Res. 14:5825–5832.
2008.PubMed/NCBI
|
|
18
|
O’Connell MP, Fiori JL, Xu M, et al: The
orphan tyrosine kinase receptor, ROR2, mediates Wnt5A signaling in
metastatic melanoma. Oncogene. 29:34–44. 2010.PubMed/NCBI
|
|
19
|
Kurayoshi M, Oue N, Yamamoto H, et al:
Expression of Wnt-5a is correlated with aggressiveness of gastric
cancer by stimulating cell migration and invasion. Cancer Res.
66:10439–10448. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Ripka S, König A, Buchholz M, et al:
WNT5A-target of CUTL1 and potent modulator of tumor cell migration
and invasion in pancreatic cancer. Carcinogenesis. 28:1178–1187.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Hanaki H, Yamamoto H, Sakane H, et al: An
anti-Wnt5a antibody suppresses metastasis of gastric cancer cells
in vivo by inhibiting receptor-mediated endocytosis. Mol Cancer
Ther. 11:298–307. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Peng C, Zhang X, Yu H, Wu D and Zheng J:
Wnt5a as a predictor in poor clinical outcome of patients and a
mediator in chemoresistance of ovarian cancer. Int J Gynecol
Cancer. 21:280–288. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Kamino M, Kishida M, Kibe T, et al: Wnt-5a
signaling is correlated with infiltrative activity in human glioma
by inducing cellular migration and MMP-2. Cancer Sci. 102:540–548.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Ying J, Li H, Yu J, et al: WNT5A exhibits
tumor-suppressive activity through antagonizing the
Wnt/beta-catenin signaling, and is frequently methylated in
colorectal cancer. Clin Cancer Res. 14:55–61. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Blanc E, Roux GL, Bénard J and Raguénez G:
Low expression of Wnt-5a gene is associated with high-risk
neuroblastoma. Oncogene. 24:1277–1283. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Jönsson M, Dejmek J, Bendahl PO and
Andersson T: Loss of Wnt-5a protein is associated with early
relapse in invasive ductal breast carcinomas. Cancer Res.
62:409–416. 2002.PubMed/NCBI
|
|
27
|
Deng G, Li ZQ, Zhao C, et al: WNT5A
expression is regulated by the status of its promoter methylation
in leukaemia and can inhibit leukemic cell malignant proliferation.
Oncol Rep. 25:367–376. 2011.PubMed/NCBI
|
|
28
|
Syed Khaja AS, Helczynski L, Edsjö A, et
al: Elevated level of Wnt5a protein in localized prostate cancer
tissue is associated with better outcome. PLoS One.
6:e265392011.PubMed/NCBI
|
|
29
|
Yamamoto H, Oue N, Sato A, et al: Wnt5a
signaling is involved in the aggressiveness of prostate cancer and
expression of metalloproteinase. Oncogene. 29:2036–2046. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Zhang S, Wang J, Bilen MA, Lin SH, Stupp
SI and Satcher RL: Modulation of prostate cancer cell gene
expression by cell-to-cell contact with bone marrow stromal cells
or osteoblasts. Clin Exp Metastasis. 26:993–1004. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Zhau HE, He H, Wang CY, et al: Human
prostate cancer harbors the stem cell properties of bone marrow
mesenchymal stem cells. Clin Cancer Res. 17:2159–2169. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Hart CA, Brown M, Bagley S, Sharrard M and
Clarke NW: Invasive characteristics of human prostatic epithelial
cells: understanding the metastatic process. Br J Cancer.
92:503–512. 2005.PubMed/NCBI
|
|
33
|
van den Hoogen C, van der Horst G, Cheung
H, Buijs JT, Pelger RC and van der Pluijm G: Integrin αv expression
is required for the acquisition of a metastatic stem/progenitor
cell phenotype in human prostate cancer. Am J Pathol.
179:2559–2568. 2011.
|
|
34
|
Mochizuki H, Matsubara A, Teishima J, et
al: Interaction of ligand-receptor system between
stromal-cell-derived factor-1 and CXC chemokine receptor 4 in human
prostate cancer: a possible predictor of metastasis. Biochem
Biophys Res Commun. 320:656–663. 2004. View Article : Google Scholar
|
|
35
|
Taichman RS, Cooper C, Keller ET, Pienta
KJ, Taichman NS and McCauley LK: Use of the stromal cell-derived
factor-1/CXCR4 pathway in prostate cancer metastasis to bone.
Cancer Res. 62:1832–1837. 2002.PubMed/NCBI
|