Introduction
The high mobility group box (HMGB) gene family, is
the most abundant type of non-histone chromatin binding protein in
eukaryotes and is comprised of four members: HMGB1, HMGB2, HMGB3
and HMGB4 (1). Mammalian HMGBs are
characterized by two highly conserved and tandem DNA binding
domains, HMG boxes A and B, followed by a long acidic C-terminal
tail (2). Proteins in this family
may bind noncanonical DNA structures, including single-stranded DNA
with their own HMG-box and act as a chromatin chaperone through
distorting DNA (3). HMGBs also
take part in nucleosome-remodeling, enhanceosome organization and
transcription regulation by interacting directly with nucleosomes
and enhancesomes, as well as transcription factors (1,4). To
date, three members of the HMGB family, including HMGB1, HMGB2 and
HMGB3, have been studied. In adult vertebrates, HMGB1 is
ubiquitously expressed in all cell types and its expression level
is found to be correlated with the differentiation stage of cells
(5). HMGB2 is abundant in the
thymus, lymphoid organs and testes in adult mice and it is also
found in all human and mouse immortalized cell lines (5,6),
whereas HMGB3 is primarily present in primitive hematopoietic cells
(7). HMGB4, as a novel member of
the HMGB family, also contains two highly conserved and tandem DNA
binding domains, HMG boxes A and B, however lacks the acidic
C-terminal tail, which exists in all other HMGB proteins (8). As a transcriptional repressor, HMGB4
is highly expressed in adult mouse testis, lowly in brain and not
expressed in other tissues (8).
However, the distribution and function of HMGB4 in various human
tissue remains unknown. In the present study, the development of a
specific anti-hHMGB4 polyclonal antibody was reported. Moreover,
the characteristics of the prepared antibody were evaluated by
ELISA, western blotting and immunohistochemical techniques.
Materials and methods
Construction of the recombinant
expression plasmid
The hHMGB4 fragment was amplified from the plasmid
pBluescriptR/hHMGB4 harboring full-length hHMGB4 cDNA (purchased
from Gene Copoeia, Rockville, MD, USA) by PCR. Gene-specific primer
pairs were designed as follows: forward, 5′-GCGCGAATTCATGGGAAAAGAAATCCAG-3′
(the EcoRI recognition site underlined); reverse,
5′-CTAGTCGAC
GCTCTGCCTGACTCTTTTCCC-3′ (the SalI recognition site
underlined). The amplified PCR product was purified, digested with
EcoRI and SalI and ligated to the pET28a(+) vector
(Novagen, Darmstadt, Germany), which was digested by the same
restriction enzymes to produce the expression plasmid
pET28a(+)/hHMGB4. Next, the expression plasmid was transformed into
E.coli DH5α-competent cells and plated on Luria Broth (LB)
plates containing 50 μg kanamycin/ml. Ten single colonies of
E.coli DH5α-pET28a(+)/HMGB4 cells were inoculated into the
LB medium, including 50 μg kanamycin/ml at 37ºC overnight. The
plasmid DNA was extracted using Plasmid Mini Preparation kit
(Tiangen Biotech Beijing Co., Beijing, China) and the insert
fragment was verified by EcoRI and SalI double
restrictive digestion and confirmed by DNA sequencing.
Expression and purification of the
recombinant hHMGB4 protein. E.coli
BL21 (DE3) was transformed with HMGB4 expression
vector pET28a(+)/HMGB4 to produce the expression bacterial strain
BL21-pET28a(+)/hHMGB4. Next, BL21-pET28a(+)/hHMGB4 cells were
induced with 1 mM isopropylthio-β-D-galactoside (IPTG), harvested
and resuspended with TE buffer (20 mM Tris-HCl and 1 mM EDTA, pH
8.0) supplemented with 1 mM phenylmethylsulfonyl fluoride (PMSF).
Next, the expression of the recombinant hHMGB4 (rhHMGB4) in the
supernatant and the pellets was evaluated by SDS-PAGE followed by
Coomassie brilliant blue staining. The procedures are subsequently
described in detail.
The pET28a(+)/HMGB4 was transformed into E.
coli BL21 (DE3)-competent cells to produce the expression
bacterial strain BL21-pET28a(+)/hHMGB4. Small scale testing of
antibody expression was performed prior to scaling up the
expression procedure. A single colony of E.coli
BL21-pET28a(+)/HMGB4 cells was inoculated into 2 ml LB medium
containing 50 μg kanamycin/ml and incubated at 37ºC with agitation
at 200 rpm overnight in a shaking incubator with a rotational
radius of 10 cm. The cells were inoculated into 20 ml fresh LB
medium with 50 μg kanamycin/ml and grown under the same conditions
until the OD600 reached 0.6–1.0. Next, 1 mM of final
concentration of IPTG was added for an additional 4 h, the cells
were harvested by centrifugation at 10,000 × g at 4ºC for 5 min.
Pellet fractions were suspended in 200 μl TE buffer with 1 mM PMSF
and lysed by sonication on ice at 300 W for 20 cycles (5 sec on and
10 sec off). The resulting cell lysate was centrifuged at 12,000 ×
g for 30 min at 4ºC. The supernatant and pellets were analyzed by
15% (v/v) SDS-PAGE followed by Coomassie brilliant blue R250
staining.
Inclusion bodies were purified as described
elsewhere (9). Briefly, pellets
containing inclusion bodies were washed three times with the
washing buffer (50 mM Tris-HCl, pH 8.0, 10 mM Triton X-100, 2 M
urea and 10 mM EDTA) and resuspended in inclusion body
solubilization buffer (50 mM Tris-HCl, pH 8.0, 8 M urea, 0.5 M NaCl
and 5 mM imidazole) by stirring for 30 min at room temperature. The
supernatant was collected by centrifugation at 12,000 × g for 30
min at 4ºC and then refolded by urea gradient dialysis (from 8 to 0
M urea). The refolded recombinant protein solution was concentrated
and collected to run SDS-PAGE. Finally, the predicted protein band
was excised from the gel. rhHMGB4 was retrieved using a Model 422
Electro-Eluter (Bio-Rad, Hercules, CA, USA). The electroelution
process was performed at 24 mA for 2 h at 4ºC. Finally, the protein
concentration was determined by the Bradford assay (10).
Identification of the recombinant hHMGB4
protein by tandem mass spectrometery
The recombinant protein was digested by
carboxypeptidase Y (CPY) and the resulting peptide mixture was
analyzed by matrix-assisted laser desorption/ionization
time-of-flight mass spectrometry (MALDI-TOF)-TOF-MS/MS (4700
Proteomics Analyzer, Applied Biosystems China, Beijing, China). The
procedure was performed as follows: Briefly, the rhHMGB4 protein
was digested with CPY. Then, the digested sample was admixed with
0.5 μl matrix solution containing 5 mg CHCA/ml. The mixture was
vortexed and removed to the sample target, which was allowed to dry
in the air at room temperature. The peptide mass fingerprint (PMF)
was analyzed in the reflex model and four peptide peaks with the
highest ion intensity were measured by tandem mass spectrometery.
The MS/MS data were analyzed using De novo Explorer software for
de novo sequencing.
Preparation and purification of the
anti-hHMGB4 polyclonal antibody
The purified recombinant hHMGB4 protein was
emulsified with an equal volume of Freund’s complete adjuvant and
was injected subcutaneously at six sites in the back of New Zealand
rabbits. The study was approved by the Ethics Committee of Shanghai
Institute of Planned Parenthood Research. Two weeks later, the
rabbits were injected with the hHMGB4 protein with Freund’s
incomplete adjuvant as a booster immunization. A total of three
booster injections were performed at 2-week intervals. Following
the second immunization, serum was separated from the blood
collected from the rabbit’s ear vein at 2-week intervals to test
the antibody titer. The control group was administered with an
equal volume of PBS. One week following the final immunization, the
antiserum was collected and stored at −20ºC. The purification of
antibodies was performed using affinity chromatography according to
the following instructions: The purification of antibodies was
performed using affinity chromatography. Serum was mixed with 20 mM
Na-phosphate buffer (pH 7.0) and the mixture was applied to a
HiTrap Protein G column (1 ml), equilibrated with 20 mM phosphate
buffer (pH 7.0). Following washing of the column with 10 column
volume of phosphate buffer, the antibody was eluted by 0.15 M
Gly-HCl (pH 2.5). The resulting elution was collected and
neutralized with 1 M Tris-HCl (pH 9.0) and then stored at
−20ºC.
Identification of the antibody
specificity by western blotting
Proteins were separated by 15% (v/v) SDS-PAGE and
transferred onto polyvinylidene fluoride membrane. Following
blocking with 5% (w/v) non-fat milk, the membrane was incubated
with the hHMGB4 polyclonal antibody (1:500) at 4ºC overnight,
washed with PBST, incubated with HRP-conjugated goat anti-rabbit
IgG for 1 h. The bound antibody complexes were detected using
SuperSignal® West Pico Chemiluminescent Substrate
(Pierce, Rockford, IL, USA) on X-ray films. The preparation of
protein samples used is subsequently described: Briefly, the
E.coli BL21 cells transformed with the pET28a(+) vector or
the recombinant pET28a(+)/HMGB4 plasmid were incubated at 37ºC to
overexpress the recombinant protein. Next, an equal number of
bacterial cells were pelleted and lysed for SDS-PAGE and western
blotting, as described previously. The proteins were extracted from
human prostate cancer cell lines DU145 and LNCaP cells with the
RIPA buffer (25 mM Tris-HCl pH 7.6, 150 mM NaCl, 1% NP-40, 1%
sodium deoxycholate and 0.1% SDS) added with a cocktail of
proteinase inhibitors (Sigma-Aldrich, St. Louis, MO, USA).
Determination of antibody titer by
ELISA
Briefly, the purified hHMGB4 protein (5 μg/ml) was
coated to a microtiter plate and incubated at 4ºC overnight.
Following blocking with 1% BSA, hHMGB4 antisera with serial
dilutions (1/200–1/204,800) were pipetted into the wells and
incubated for 1 h at 37ºC. Following thorough washing, the
HRP-conjugated goat anti-rabbit IgG was added for 1 h at 37ºC.
Color development was performed with the substrate solution
containing 3′,3′,5′,5′-tetramethylbenzidine (TMB) and halted by the
addition of 1 M H2SO4. The absorbance at 450
nm was measured. The antibody titer was defined as the highest
antiserum dilution when the P/N value (division of the positive
serum OD450 value by the negative control
OD450 value) was >2 (P/N >2).
Immunohistochemical staining
Paraffin-embedded tissue of the normal human
prostate and endometrium was purchased from Shanxi Chaoying Co.
These tissues were cut into 5 μm thick sections and deparaffinized
in xylene, then rehydrated with graded ethanol. Antigen retrieval
was performed by microwaving the slides using 0.01 M citrate
buffers (pH 6.0). Endogenous peroxidase activity was removed with
3% H2O2 and tissues were blocked with 10%
(v/v) normal goat serum at 37ºC for 1 h. The slides were incubated
with 1:200 diluted hHMGB4 polyclonal antibodies overnight at 4ºC.
Following washing with PBST, slides were incubated with
biotinylated goat anti-rabbit secondary antibody, followed by
incubation with streptavidin-HRP complex. Color reaction was
detected using diaminobenzidine tetrahydrochloride (DAB).
Replacement of the hHMGB4 antibody with PBS was conducted as a
negative control to confirm specificity. The slides were
counterstained with hematoxylin and mounted with neutral resin.
Results
Construction of the pET28a(+)/hHMGB4
expression plasmid
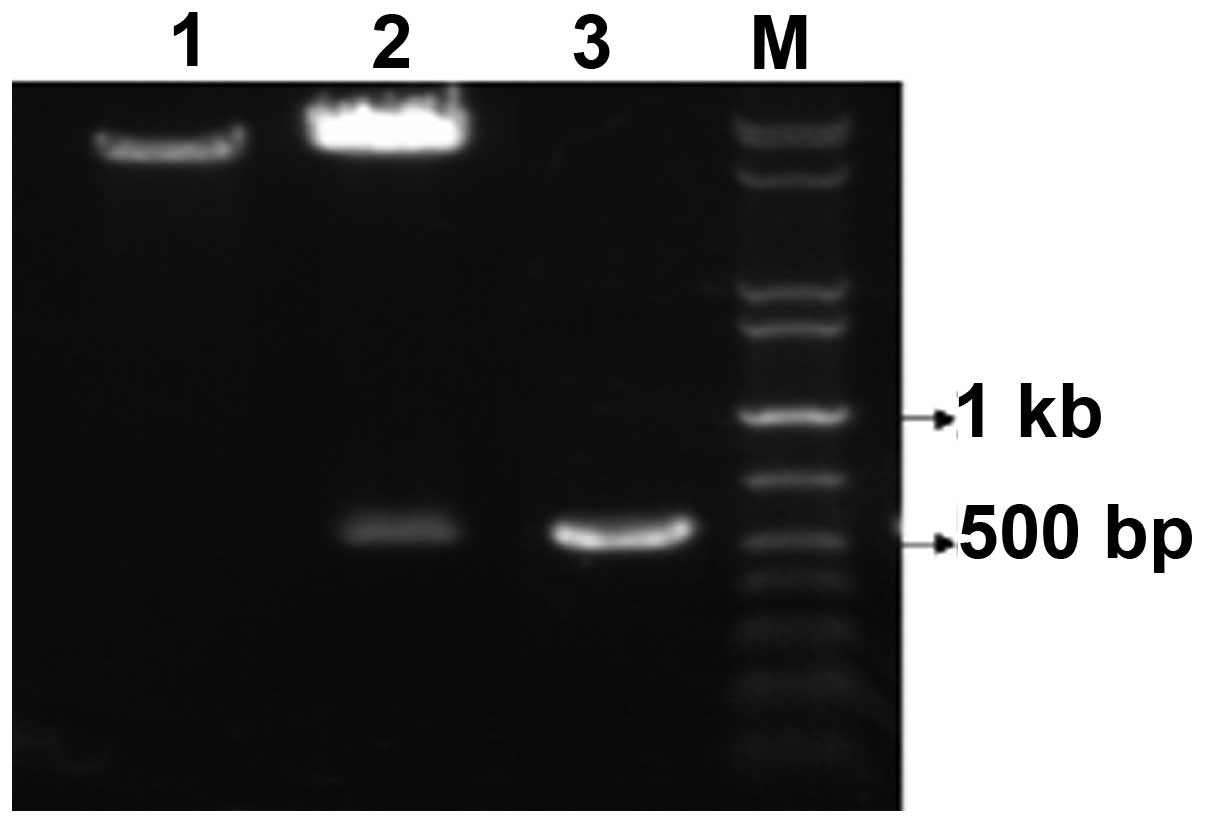
The 560 bp DNA fragment encoding the full-length
hHMGB4 was amplified from plasmid pBluescriptR/hHMGB4 and cloned
into the pET28a(+) expression vector between the EcoRI and
SalI restriction sites. The recombinant plasmid
pET28a(+)/hHMGB4 was confirmed by PCR and restriction enzyme
digestion. As shown in Fig. 1, two
DNA electrophoretic bands of ~560 and ~8000 bp were produced
following the EcoRI and SalI restrictive digestion.
The target fragment of ~560 bp, which was generated following PCR
amplification using pET28a(+)/hHMGB4 as the template, is also shown
in Fig. 1. DNA sequencing verified
that the inserted DNA fragment sequence was as expected (GenBank
Accession no. BC021180).
Expression and purification of the
recombinant hHMGB4
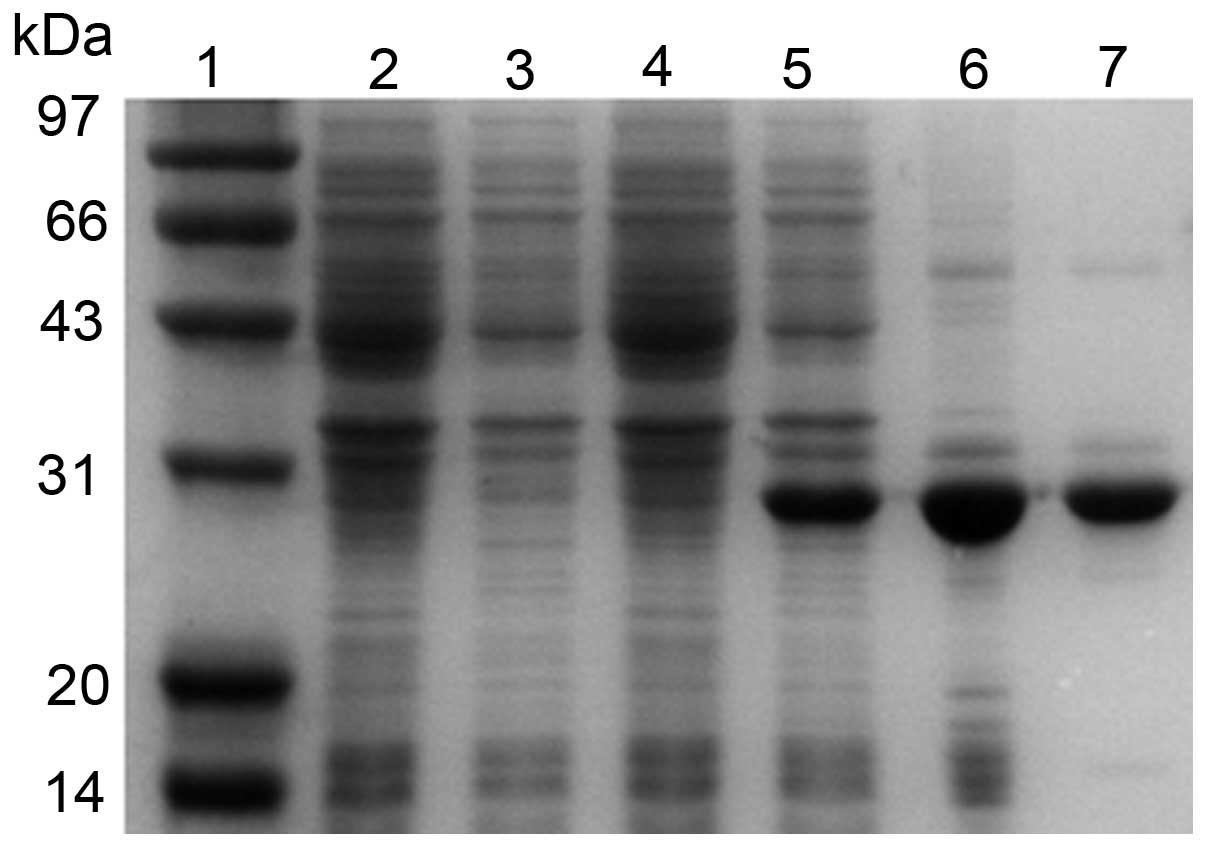
SDS-PAGE analysis revealed that the recombinant
hHMGB4 protein was expressed in E.coli BL21 (DE3) cells at
an expected band of 28 kDa (Fig.
2). rhHMGB4 was found to be primarily expressed as inclusion
bodies composed of insoluble aggregates. More than 80% soluble
recombinant hHMGB4 protein was obtained following extraction with 8
M urea from inclusion bodies and dialyzed (Fig. 2). The purity of the rhHMGB4 reached
>95% following dialysis and electroelution process.
MS/MS analysis of the recombinant hHMGB4
protein
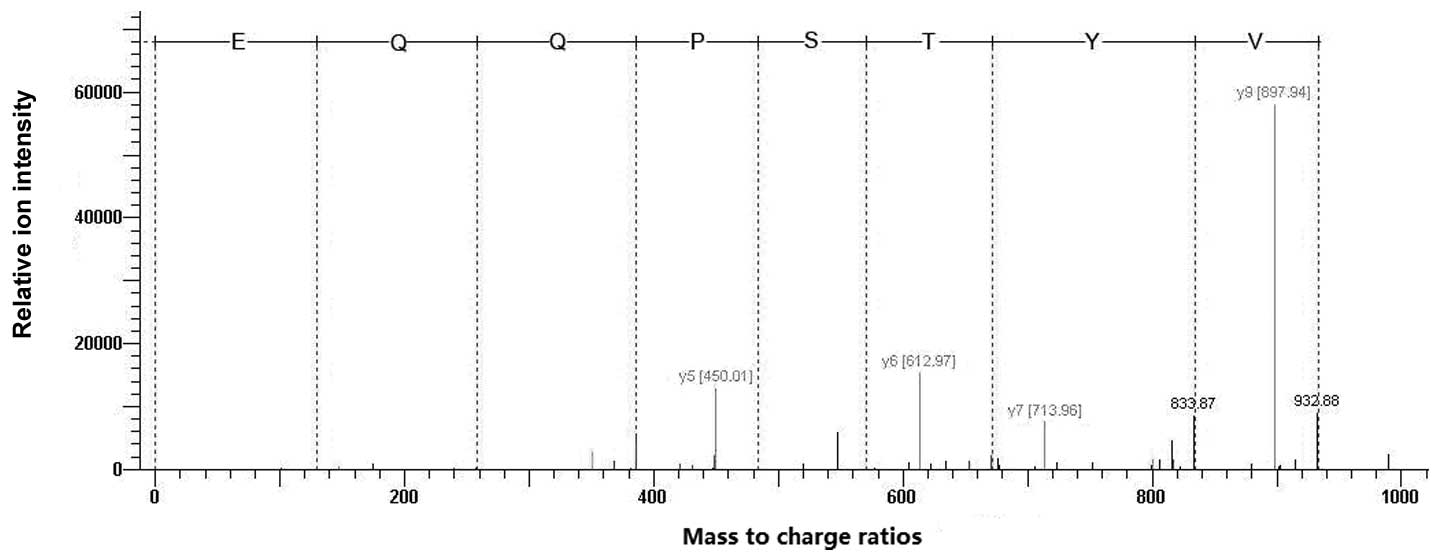
MALDI-TOF was used to analyze the digested hHMGB4
proteins. Four precursor ion peaks at m/z 967.24, 1261.23, 1283.21
and 1370.26 were identified, respectively, and PMF analysis
suggested that the protein is HMGB4. In addition, 1283.21 was
selected for tandem MS analysis. As shown in Fig. 3, the amino acid sequence was
exactly matched with the N-terminal sequence of HMGB4 among 29–36
amino acid residues, re-confirming that the purified recombinant
protein is rhHMGB4.
Generation of polyclonal antibodies
against rhHMGB4 protein
Polyclonal antibodies were generated in rabbits by
using the purified rhHMGB4 protein. The rhHMGB4 antigen may be
detected with the antisera at the dilution of 1:102,400 by ELISA
(as described in Materials and methods). In addition, no immune
reactivity was detected with pre-immune serum.
Analysis of the antibody specificity
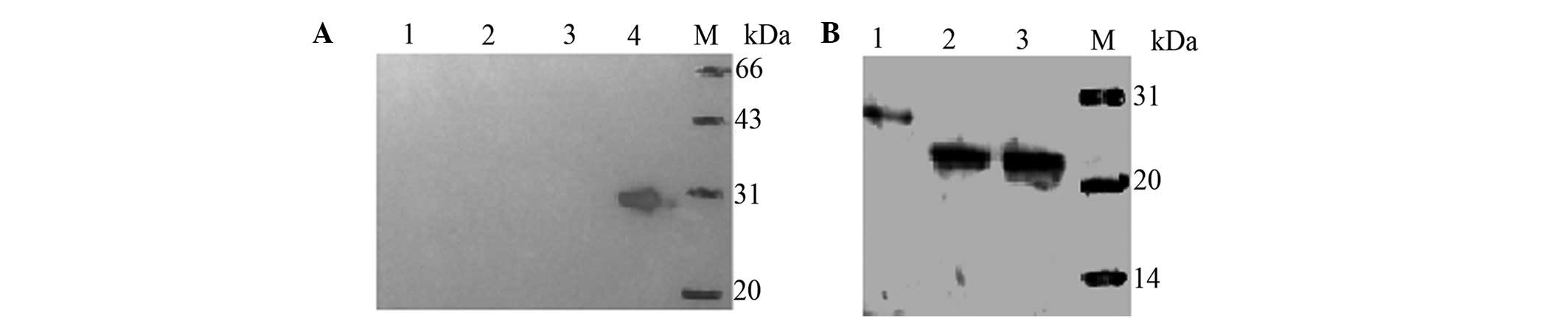
To analyze the specificity of the prepared HMGB4
polyclonal antibody, the expression of the recombinant hHMGB4
protein was measured using the lysate prepared from the transformed
E.coli BL21 cells by western blot analysis. As shown in
Fig. 4A, there is a distinguished
band of 28 kDa as expected in the IPTG-treated BL21-pET28a(+)/HMGB4
cells (lane 4), while no band was detected in the BL21-pET28a(+)
cells untreated (lane 1) and treated with IPTG (lane 2), as well as
the BL21-pET28a(+)/HMGB4 cells treated with IPTG (lane 3).
In addition, hHMGB4 expression was measured in the
two human prostate cancer cell lines, DU145 and LNCaP, in the
immunoblotting analysis. The results showed that one single
highlighted band above 20 kDa was detected in the two cell lines
(Fig. 4B), indicating that the
antibody recognizes denatured HMGB4 prepared from human cells with
a high specificity.
Immunostaining analysis of HMGB4 in human
tissue of the prostate and uterus
Immunohistochemical studies were performed in human
prostate tissues as well as decidual tissues to further
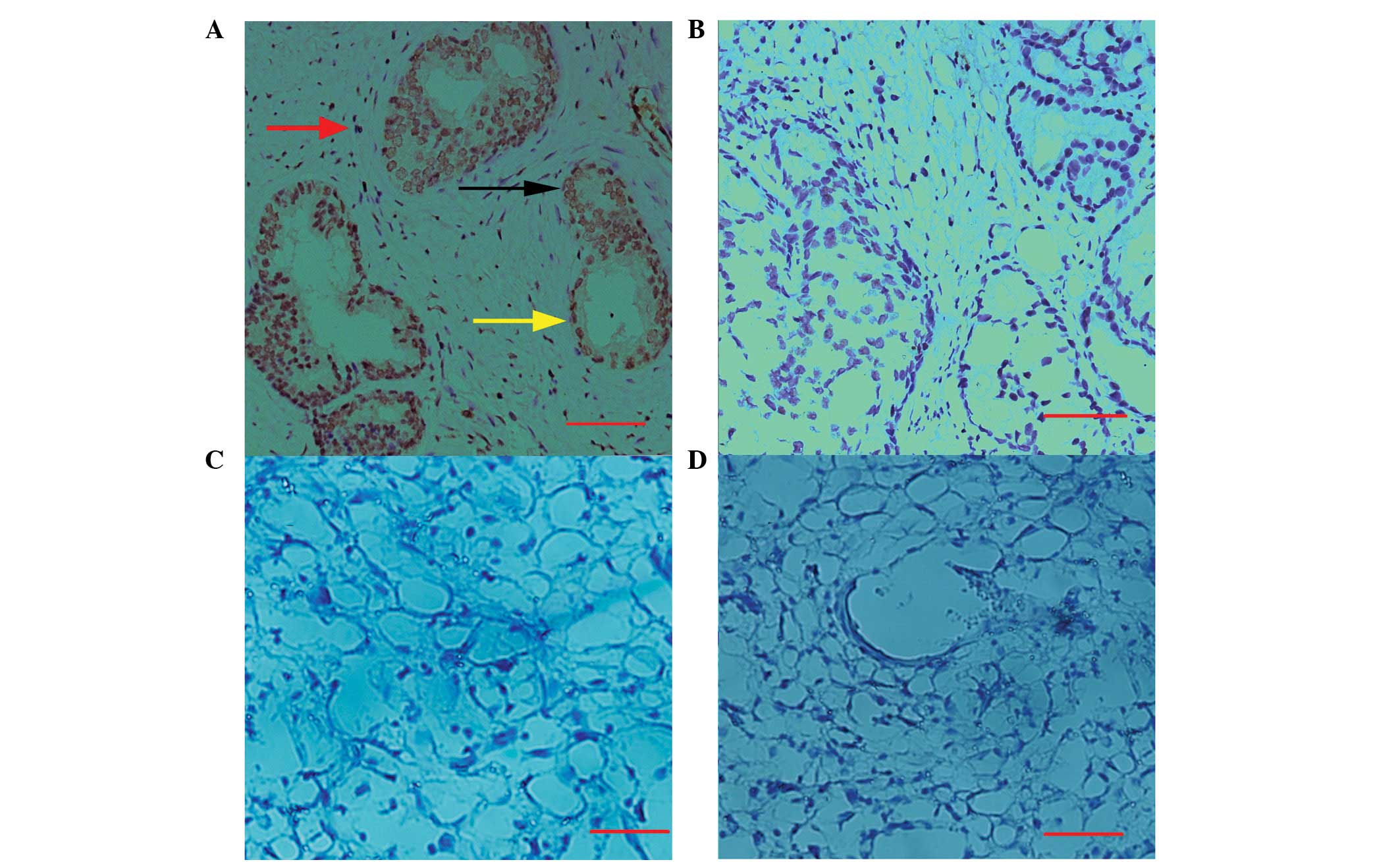
characterize the anti-hHMGB4 antibody. The results in Fig. 5A show that HMGB4 is localized
predominantly in the nuclei of epithelial, basal and stromal cells
of the prostate, while cytosolic staining in these cells was
extremely weak (Fig. 5A). However,
no immunostaining of hHMGB4 was detected in the decidual areas of
the uterus (Fig. 5C). In addition,
no significant staining was observed in the prostate (Fig. 5B) and decidual tissues (Fig. 5D) when the preimmunized serum was
used.
Discussion
The current study reports the successful generation
of a polyclonal antibody against the human HMGB4. For production of
its antigen, the prokaryotic expression vector pET28a(+) was used
to express a fusion protein with the full length human HMGB4 and
His6-tags and thrombin at the terminal of the protein.
Therefore, the molecular weight of the fusion protein is ~28 kDa,
detected by SDS-PAGE analysis, higher than the theoretical
molecular weight (~22.4 kDa) of hHMGB4. In addition, the 28 kDa
protein was further validated to be hHMGB4 via MS/MS analysis.
Following rabbit immunization with the purified
recombinant hHMGB4, the polyclonal antibody against hHMGB4 was
produced with a high titer of antiserum (1:102,400) determined via
ELISA. Notably, only a unique band was detected in the lysate
prepared from two prostate cancer cell lines analyzed via western
blotting, indicating that the antibody recognized denatured human
HMGB4 with a high specificity.
Finally, the antibody was tested for its potential
application used in immunohistochemistry. Staining was observed in
the prostate but not in uterine. In addition, expression of HMGB4
was measured in 33 human tissues by RT-PCR, showing that expression
of HMGB4 is only detectable in the testis and prostate and is
absent in others, including uterine (data not shown). The data
strongly suggest that the present antibody is a usable reagent in
immunostaining analysis.
HMGB1 is not only a nuclear protein, but also
promotes prostate cancer progression as a secreted protein
(11). However, marked
immuniostaining of HMGB4 was observed in the nuclei of epithelial,
basal and stromal cells of the prostate, but the staining was weak
in the cytoplasm of these cells, suggesting that HMGB4 function is
restricted in the nuclei. Future studies are likely to focus on
determining whether the HMGB4 antibody is useful in
immuno-precipitation assays to perform a functional study of HMGB4
in prostate cancer.
In conclusion, a rabbit polyclonal antibody highly
specific for HMGB4 was generated, which may be used for
immunostaining and immunoblotting analysis. Availability of this
HMGB4 antibody is likely to facilitate the further investigation of
human HMGB4.
Acknowledgements
This study was supported by grants from the National
Natural Science Foundation of China (grant no. 81270760), the
National Basic Research Program of China (grant no. 2009CB941704)
and Shanghai Municipal Committee of Science and Technology (grant
nos. 09140903200 and 10ZR1425500).
References
|
1
|
Ueda T and Yoshida M: HMGB proteins and
transcriptional regulation. Biochim Biophys Acta. 1799:114–118.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Knapp S, Müller S, Digilio G, et al: The
long acidic tail of high mobility group box 1 (HMGB1) protein forms
an extended and flexible structure that interacts with specific
residues within and between the HMG boxes. Biochemistry.
43:11992–11997. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Stros M: HMGB proteins: interactions with
DNA and chromatin. Biochim Biophys Acta. 1799:101–113. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Agresti A and Bianchi ME: HMGB proteins
and gene expression. Curr Opin Genet Dev. 13:170–178. 2003.
View Article : Google Scholar
|
|
5
|
Müller S, Ronfani L and Bianchi ME:
Regulated expression and subcellular localization of HMGB1, a
chromatin protein with a cytokine function. J Intern Med.
255:332–343. 2004.PubMed/NCBI
|
|
6
|
Ronfani L, Ferraguti M, Croci L, et al:
Reduced fertility and spermatogenesis defects in mice lacking
chromosomal protein Hmgb2. Development. 128:1265–1273.
2001.PubMed/NCBI
|
|
7
|
Nemeth MJ, Curtis DJ, Kirby MR, et al:
Hmgb3: an HMG-box family member expressed in primitive
hematopoietic cells that inhibits myeloid and B-cell
differentiation. Blood. 102:1298–1306. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Catena R, Escoffier E, Caron C, et al:
HMGB4, a novel member of the HMGB family, is preferentially
expressed in the mouse testis and localizes to the basal pole of
elongating spermatids. Biol Reprod. 80:358–366. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Kou G, Shi S, Wang H, et al: Preparation
and characterization of recombinant protein ScFv(CD11c)-TRP2 for
tumor therapy from inclusion bodies in Escherichia coli.
Protein Expr Purif. 52:131–138. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Carlsson N, Borde A, Wölfel S, et al:
Quantification of protein concentration by the Bradford method in
the presence of pharmaceutical polymers. Anal Biochem. 411:116–121.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Kuniyasu H, Chihara Y, Kondo H, et al:
Amphoterin induction in prostatic stromal cells by androgen
deprivation is associated with metastatic prostate cancer. Oncol
Rep. 10:1863–1868. 2003.PubMed/NCBI
|