Introduction
Diabetes mellitus (DM) and its complications have
become one of the most important health problems worldwide.
Diabetic nephropathy (DN) is the predominant cause of end-stage
renal disease that requires renal transplantation (1,2).
Formation and accumulation of advanced glycation end products
(AGEs) is closely linked with the aging process and is accelerated
in DM. The pathogenic role of AGEs in vascular diabetic
complications is widely recognized. AGEs and RAGE (the receptor for
AGEs) elicit inflammatory reactions leading to vascular damage
(2–4). Oxidative stress and inflammatory
molecules including inflammatory cytokines, chemokines and adhesion
molecules are intimately involved in the development and
progression of DN (5,6). There is an increasing number of
studies suggesting that kidney macrophage recruitment is related to
hyperglycemia and the accumulation of AGEs, and that
macrophage-mediated injury is fundamental in the onset of DN
(7–9).
Chemokines that are detected in the circulation and
kidney of humans and animals are generally increased following the
onset and further progress of DM, which is frequently accompanied
by the promotion of macrophage recruitment in kidney (10). Fractalkine is one of the two
chemokines which is expressed in soluble (s-fractalkine) and
transmembrane/mucin hybrid forms, which therefore combines
chemoattractant and adhesiveness functions (11). The fractalkine/CX3CR1 (receptor of
fractalkine) axis has been implicated in the pathogenesis of
atherosclerosis, DM and streptozotocin-induced DN along the
glomerular capillary lumen and peritubular capillaries (12–15).
Matrix metalloproteinase 2 (MMP2) belongs to a
subgroup of MMPs termed gelatinases and it has been implicated in
the pathological processes that contribute to fibrotic diseases,
tumor progression and inflammation (16–18).
It predominantly contributes to the breakdown and turnover of
extracellular matrix (ECM), which is manifested in mesangial
expansion, glomerulosclerosis and tubulointerstitial fibrosis in
the kidney (19–21). MMPs cleave proteins, solubilize
pericellular matrix and shed cellular ectodomains (22).
The present study aimed to investigate the function
of fractalkine and MMP2 as well as the interaction between the two
proteins using a human renal mesangial cell (HRMC)-monocyte
co-culture system. It was demonstrated that AGEs upregulated
fractalkine and downregulated MMP2 in the monocytes-HRMC co-culture
system. Thus, it was hypothesized that there is crosstalk between
monocytes and HRMCs via the interaction of MMP2 and fractalkine,
which may be important in the AGE-mediated development of DN.
Materials and methods
Materials
All experiments were conducted in serum-free
RPMI-1640 medium containing 0.2% bovine serum albumin (BSA), 2
mmol/l glutamine, 100 U/ml penicillin and 100 μg/ml streptomycin.
All reagents for cell culture were obtained from Sigma-Aldrich (St.
Louis, MO, USA). Recombinant MMP2 was obtained from PeproTech
(Rocky Hill, NJ, USA; lot no. 057106). Anti-fractalkine polyclonal
antibody was obtained from R&D Systems (Minneapolis, MN, USA;
lot no. EKC10).
Preparation of AGEs
BSA (50 g/l) was incubated with 0.5 mmol/l D-glucose
in 0.2 mmol/l phosphate-buffered saline (PBS, pH 7.4) at 37°C for
90 days. The control sample of BSA was also incubated under the
same conditions but without glucose. Following incubation, AGE-BSA
was purified by Sephadex G-200 (Sigma; Lot 271233). The Bradford
method was used for the quantification of proteins (23,24).
Cell culture
An established stable HRMC (donated by Dr XZ Ruan,
University College London, London, UK) was cultured in RPMI-1640
medium with 10% fetal calf serum (FCS), 2 mmol/l glutamine, 100
U/ml penicillin and 100 μg/ml streptomycin.
The U937 monocyte-like cell line cells (obtained
from Professor BC Liu, Southeast University, Nanjing, Jiangsu,
China) were grown in suspension in the RPMI-1640 culture medium
containing 5% FCS and were split by 1:5, twice per week. The study
was approved by the Ethics Committee of Southeast University.
RT-PCR
Total RNA was isolated from cells using TRIzol
reagent [from the RevertAid™ First Strand cDNA synthesis kit
(Invitrogen, Carlsbad, CA, USA; Lot 1382739)]. An aliquot of 2 μg
total RNA from each sample was reverse transcribed to cDNA using
the reverse transcript system (RevertAid First Strand cDNA
synthesis kit) according to the manufacturer's instructions. The
mRNA levels of the analyzed molecules were normalized to β-actin or
glyceraldehyde 3-phosphate dehydrogenase (GAPDH) mRNA levels. The
following primers were used for the amplification of mRNA: Forward:
5′-AGCCACAGGCGAAAGCAGTA-3′ and reverse: 5′-TTCAGACGGAGCATTCTCCT-3′
for fractalkine; and forward: 5′-ACAAAGAGTTGGCAGTGCAAT-3′ and
reverse: 5′-GGGTCACATCGCTCCAGACTTGG-3′ for MMP2. Amplification of
cDNA was conducted with the following primers: Forward:
5′-CGCCGCGCTCGTCGTCGACA-3′ and reverse: 5′-GTCACGCACGATTTCCCGCT-3′
for β-actin; and forward: 5′-GGAGTCAACGGATTTGGT-3′ and reverse:
5′-GTGATGGGATTTCCATTGAT-3′ for GAPDH. The sizes of the expected PCR
products for the fractalkine, MMP2, β-actin and GAPDH primers were
340, 330, 619 and 206 bp, respectively.
Western blot analysis
The cell lysates were prepared with lysis buffer,
radio immunoprecipitation assay (RIPA) and mammalian protease
inhibitor mixture (Shenerg Biocolor, Shanghai, China). Whole cell
lysates were centrifuged at 15,000 × g for 20 min at 4°C to remove
the insoluble material. The protein concentrations were determined
by the Bradford method using a BCA Protein Micro Assay kit (Shenerg
Biocolor). The cell lysates were loaded onto sodium dodecyl
sulfate-polyacrylamide gels and transferred onto polyvinylidene
difluoride membranes. The membranes were blocked and incubated with
the primary anti-fractalkine antibody (dilution, 1:400; R&D
Systems, Minneapolis, MN, USA) or anti-β-actin antibody (dilution,
1:2,000; R&D Systems) in PBS-Tween, followed by incubation with
horseradish peroxidase-conjugated secondary antibodies. The
proteins were visualized with 3,3′-diaminobenzidine
tetrahydrochloride dihydrate (DAB; Sigma-Aldrich). The membrane was
scanned and quantitated using a GelDoc-It TS Imaging System (UVP,
Upland, CA, USA). Results were expressed in arbitrary units as
folds of control.
Gelatin zymography assay
Conditioned medium collected from cultured HRMCs was
electrophoresed under non-reducing conditions on 10% polyacrylamide
gels containing 1 mg/ml gelatin as the substrate. Following
electrophoresis, the gels were re-natured in 2.5% Triton X-100
(2×30 min) and then incubated (18 h, 37°C) in a buffer containing
50 mM Tris-HCl, pH 7.4, 5 mM CaCl2 and 150 mM NaCl.
Subsequent to this, the gels were stained with 0.25% Coomassie
brilliant blue R-250 and de-stained with 10% acetic acid and 40%
methanol. The white bands against the blue background indicated the
presence of gelatinolytic activity. Image acquisition was conducted
with Image Master VDS and LisCap software (Amersham Pharmacia
Biotech, Amersham, UK). Computerized densitometry was used to
analyze the relative enzymatic activity.
Monocyte chemotaxis assay
HRMCs were harvested and washed in serum-free
RPMI-1640. Cells were then incubated with MMP2 for 6 h and washed
three times. A transwell system was used in the medium with U937
cells inside and incubated (3 h, 37°C). Following staining with
hematoxylin for 10 min, cells with purple staining were
counted.
Monocyte adhesive assay
HRMCs were harvested and washed in serum-free
RPMI-1640. Cells were then incubated with MMP2 for 6 h and washed
with PBS. U937 cells were stained with Calcein-AM (30 min, 37°C)
and co-cultured with HRMCs (3 h, 37°C). Subsequent to washing,
cells exhibiting blue staining were counted.
Data analysis
Data are expressed as the mean ± SD. In all
experiments, groups of data were evaluated for significance using
one-way analysis of variance or a student-Newman-Keuls test.
P<0.05 was considered to indicate a statistically significant
difference. SPSS 13.0 software (SPSS, Inc., Chicago, IL, USA) was
used to determine significance.
Results
Effects of AGEs on MMP2 in HRMCs
The expression of MMP2 in HRMCs was assessed
following incubation with various concentrations of AGEs (0, 50,
100, 200, 400 and 800 mg/ml) and BSA, by mRNA and protein analysis.
As shown in Fig. 1A and B, MMP2
mRNA and protein levels as assessed in HRMCs decreased dose
dependently. There was also a time-dependent reduction of MMP2 mRNA
and protein expression while treated with 200 mg/ml AGEs (0, 8, 16,
24, 48 and 72 h) and BSA (Fig. 1C and
D). There appeared to be a greater reduction in the mRNA than
the protein level, suggesting potential post-transcriptional
regulation of MMP2 in response to AGE-BSA treatment.
Effects of AGEs on fractalkine in
HRMCs
In contrast to the significant reduction of MMP2
expression in response to AGE-BSA in HRMCs, a marked increase of
mRNA and protein levels of fractalkine in HRMCs was readily
detected in an AGE-BSA concentration and time-dependent manner. The
initial concentration that was sufficient to induce this regulation
was 50 mg/l, consistent with the observation in the MMP2 experiment
(Fig. 2).
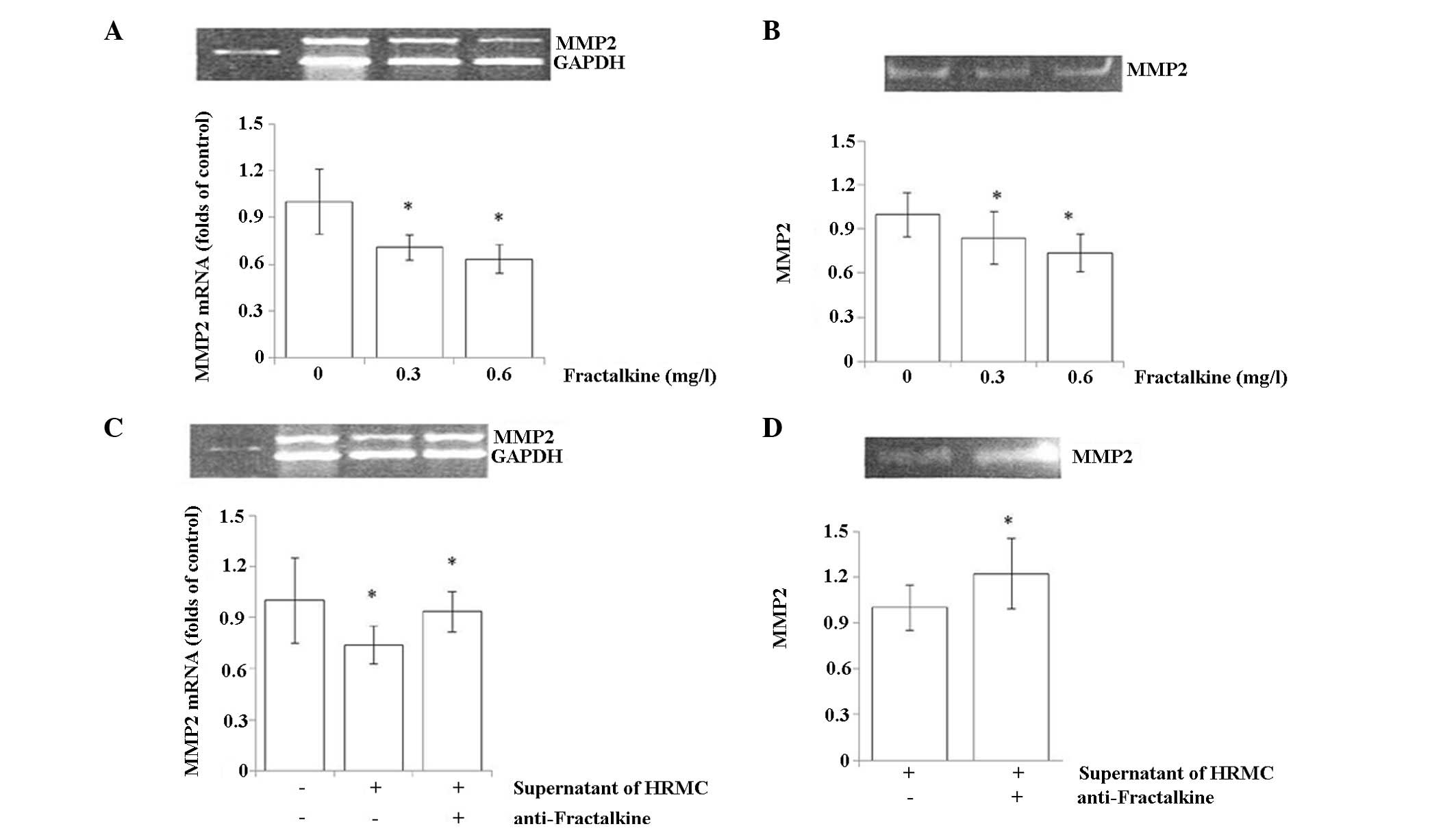
Regulation of MMP2 in U937 by
fractalkine
The present study investigated how MMP2 expression
in U937 cells is regulated by fractalkine in two experiments. U937
cells were treated with increasing concentrations of fractalkine
and the levels of MMP2 were measured. A significant reduction of
MMP2 mRNA and protein was observed upon treatment with fractalkine
in U937 cells. A U937 and HRMC co-culture experiment was also
conducted to investigate the same question. Co-culture of U937
cells with HRMCs led to a significant reduction of the MMP2 mRNA
level in U937 cells (Fig. 3A).
This regulation was blocked when a monoclonal antibody against
fractalkine was added to the culture medium (Fig. 3C). Consistent with the observation
at the mRNA level, the measurement of the MMP2 activity using a
gelatin zymography assay also demonstrated that the reduced MMP2
activity was reversed with the administration of the fractalkine
antibody (Fig. 3B and D).
Collectively, these studies indicated that fractalkine regulates
MMP2 expression in U937 cells.
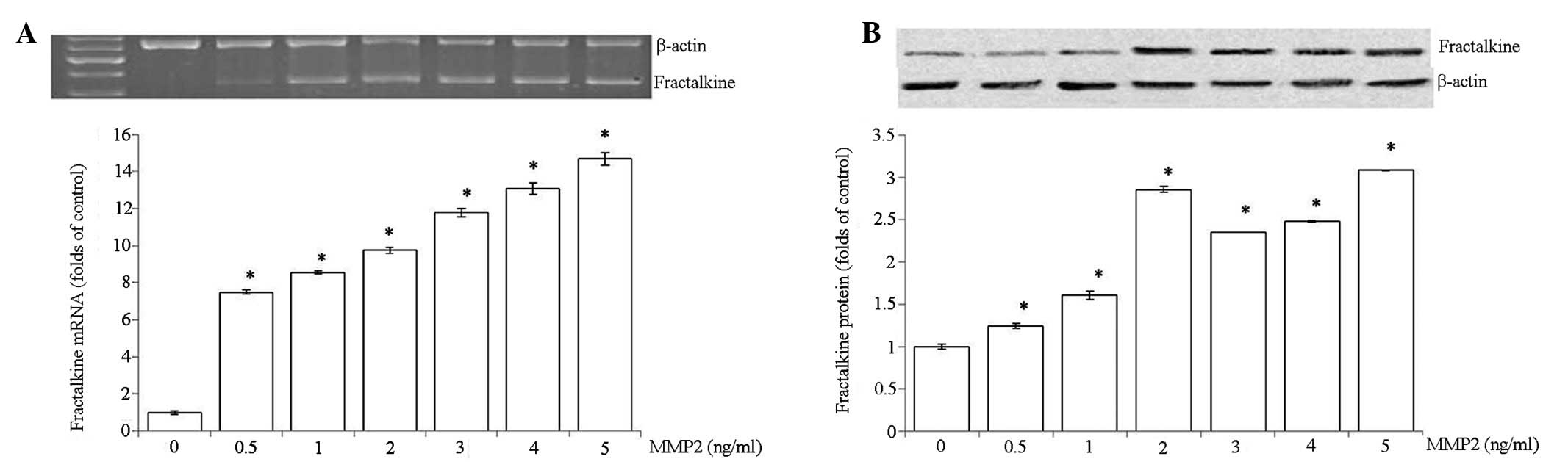
Regulation of fractalkine in HRMCs by
MMP2
To determine whether MMP2 regulates fractalkine
expression in HRMCs, HRMCs were treated with increasing
concentrations of MMP2 (0, 0.5, 1, 2, 3, 4 and 5 ng/ml). As shown
in Fig. 4, a marked increase of
fractalkine was observed in a dose-dependent manner.
Effects of fractalkine in HRMCs treated
with MMP2
Fractalkine exists in soluble and membrane bound
forms that exhibit differential involvement in chemotaxis and cell
adhesion. Soluble fractalkine is critical in mediating the
chemotaxis effect of monocytes, while the membrane bound form
primarily regulates cell adhesion. The functional consequence of
fractalkine regulation in HRMCs was thus investigated in a
transwell system, in which HRMCs and U937 cells were co-cultured in
different compartments and U937 cell transmigration and adhesion
were analyzed as described in the Materials and methods. The number
of U937 cells that transmigrated to HRMCs treated with MMP2
increased dose dependently (0, 0.625, 1.25, 2.5 and 5 ng/ml)
compared with the control, but the number that adhered decreased
(Fig. 5). These results suggested
that MMP2 may largely produce the soluble form of fractalkine that
primarily mediates cell transmigration, instead of the membrane
bound form that mediates cell adhesion.
Discussion
Chronic hyperglycemia, a necessary prerequisite for
the development of DN leads to the formation of long-lived,
non-enzymatically glycated proteins referred to as AGEs (2). As a consequence of increased
substrate (glucose) availability, AGEs accumulate at an accelerated
rate in diabetic patients, and they have been postulated to be
essential in the pathogenesis of the microvascular complications of
DM. In the present study, it was demonstrated that MMP2 expression
was diminished by AGE treatment, which may lead to the accumulation
of ECM. In addition, fractalkine mRNA and protein levels in HRMCs
were demonstrated to be significantly upregulated by AGEs in a
dose-dependent manner. It is known that s-fractalkine exhibits
chemoattractant activity for T cells and monocytes, whereas cell
surface-bound fractalkine promotes strong adhesion of those
leukocytes via its receptor, CX3CR1. The results demonstrating that
AGEs regulate MMP2 and fractalkine expression, suggest the
potential involvement of AGEs in DN through macrophage recruitment
and ECM accumulation in the kidney.
Our results are consistent with previous studies
that have demonstrated the interaction between chemokine and MMP
systems in multiple physiological and pathological processes
(25–27). Thus, the interaction between
chemokine and MMP systems in DN was investigated via the
monocyte-HRMC co-culture system. It was demonstrated that
fractalkine downregulated the mRNA expression and activity of MMP2,
which was involved in ECM degradation. Abnormal ECM deposition is
the hallmark of diabetic nephropathy and ECM is essential for
monocyte extravasation and migration into the tissues (28). Furthermore, numerous studies have
suggested that chemokines are key in the regulation of MMPs during
transmigration (29–30). Vitale et al(27) showed that s-fractalkine inhibits
the activity of MMP2 secreted by monocytes. The observation that
s-fractalkine limits monocyte migration in response to MCP-1
suggests a critical involvement of s-fractalkine in the control of
the inflammatory process (27).
While the mechanism through which MMP2 expression is inhibited by
fractalkine remains to be elucidated, the results of the present
study along with others, suggested the involvement of fractalkine
in facilitating monocyte and T cell transmigration as a chemokine,
by suppressing MMP2 secretion from monocytes.
It was also observed that MMP2 upregulated
fractalkine at the mRNA and protein levels. Further, in the
co-culture experiment, it was demonstrated that monocytic cell
transmigration was increased while cell adhesion was reduced.
Fractalkine is initially synthesized as an intracellular precursor
that undergoes glycosylation and is transported to the cell surface
as a 100-kDa glycoprotein. It then presents itself in two forms, a
membrane-anchored form which acts as an adhesion molecule (100 kDa)
or a soluble chemoattractant extracellular form (ADAM17; 85 kDa)
consisting of its mucin stalk and chemokine domain that is shed
from the cell membrane by phorbol 12-myristate 13-acetate or tumor
necrosis factor-converting enzyme. ADAMs are type I transmembrane
proteins that contain a disintegrin-like and metalloproteinase-like
domain (31,32). It has been shown that the release
of the CX3CL1 soluble form is closely associated with MMP2 activity
(33–35). Dean and Overall (36) showed the release of the chemokine
domain from the cell membrane by MMP2 cleavage specifically at
amino acid 69 AAA↑LTK. Furthermore, it was identified that an
N-terminal tetrapeptide truncation of the chemokine domain that is
deficient in chemotactic activity acted as a potent antagonist of
CX3CR (37). Thus, it was
hypothesized that the increased expression of fractalkine in the
co-culture system existed predominantly in the soluble form as
increased monocyte transmigration rather than cell adhesion was
observed.
In conclusion, AGEs increased fractalkine and
decreased MMP2 expression in HRMCs. Monocyte-HRMC co-culture
studies demonstrated that fractalkine downregulated the mRNA
expression and activity of MMP2, and MMP2 increased the expression
of fractalkine that primarily served as a chemoattractant. The
results suggest a crosstalk between monocytes and HRMCs via the
interaction of MMP2 and fractalkine, which may be involved in
abnormal ECM deposition and monocyte migration into the tissues. It
was shown that AGEs not only downregulate MMP2 directly, but also
regulate MMP2 indirectly by upregulating fractalkine. In addition,
MMP2 mediated the upregulation of AGEs to fractalkine. These
results may improve our understanding of the inflammatory
mechanisms of DN and suggest a crosstalk between monocytes and
HRMCs via the interaction of MMP2 and fractalkine, which may
represent a therapeutic target to impede the inflammatory process
associated with DN.
References
|
1
|
Kaul K, Hodgkinson A, Tarr JM, Kohner EM
and Chibber R: Is inflammation a common retinal-renal-nerve
pathogenic link in diabetes? Curr Diabetes Rev. 6:294–303. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Busch M, Franke S, Rüster C and Wolf G:
Advanced glycation end-products and the kidney. Eur J Clin Invest.
40:742–755. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Yamagishi S: Role of advanced glycation
end products (AGEs) and receptor for AGEs (RAGE) in vascular damage
in diabetes. Exp Gerontol. 46:217–224. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Navarro-González JF and Mora-Fernández C:
Inflammatory pathways. Contrib Nephrol. 170:113–123. 2011.
|
|
5
|
Yamagishi S and Imaizumi T: Diabetic
vascular complications: pathophysiology, biochemical basis and
potential therapeutic strategy. Curr Pharm Des. 11:2279–2299. 2005.
View Article : Google Scholar
|
|
6
|
van Dijk C and Berl T: Pathogenesis of
diabetic nephropathy. Rev Endocr Metab Disord. 5:237–248. 2004.
|
|
7
|
Furuta T, Saito T, Ootaka T, Soma J, Obara
K, Abe K and Yoshinaga K: The role of macrophages in diabetic
glomerulosclerosis. Am J Kidney Dis. 21:480–485. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Nguyen D, Ping F, Mu W, Hill P, Atkins RC
and Chadban SJ: Macrophage accumulation in human progressive
diabetic nephropathy. Nephrology (Carlton). 11:226–231. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Yonemoto S, Machiguchi T, Nomura K,
Minakata T, Nanno M and Yoshida H: Correlations of tissue
macrophages and cytoskeletal protein expression with renal fibrosis
in patients with diabetes mellitus. Clin Exp Nephrol. 10:186–192.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Chow F, Ozols E, Nikolic-Paterson DJ,
Atkins RC and Tesch GH: Macrophages in mouse type 2 diabetic
nephropathy: correlation with diabetic state and progressive renal
injury. Kidney Int. 65:116–128. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Braunersreuther V, Mach F and Steffens S:
The specific role of chemokines in atherosclerosis. Thromb Haemost.
97:714–721. 2007.PubMed/NCBI
|
|
12
|
Bazan JF, Bacon KB, Hardiman G, Wang W,
Soo K, Rossi D, Greaves DR, Zlotnik A and Schall TJ: A new class of
membrane-bound chemokine with a CX3C motif. Nature. 385:640–644.
1997. View
Article : Google Scholar : PubMed/NCBI
|
|
13
|
Wong BW, Wong D and McManus BM:
Characterization of fractalkine (CX3CL1) and CX3CR1 in human
coronary arteries with native atherosclerosis, diabetes mellitus,
and transplant vascular disease. Cardiovasc Pathol. 11:332–338.
2002. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Kikuchi Y, Ikee R, Hemmi N, Hyodo N,
Saigusa T, Namikoshi T, Yamada M, Suzuki S and Miura S: Fractalkine
and its receptor, CX3CR1, upregulation in streptozotocin-induced
diabetic kidneys. Nephron Exp Nephrol. 97:e17–e25. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Lesnik P, Haskell CA and Charo IF:
Decreased atherosclerosis in CX3CR1−/− mice reveals a
role for fractalkine in atherogenesis. J Clin Invest. 111:333–340.
2003.PubMed/NCBI
|
|
16
|
Visse R and Nagase H: Matrix
metalloproteinases and tissue inhibitors of metalloproteinases:
structure, function, and biochemistry. Circ Res. 92:827–839. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Corbel M, Belleguic C, Boichot E and
Lagente V: Involvement of gelatinases (MMP-2 and MMP-9) in the
development of airway inflammation and pulmonary fibrosis. Cell
Biol Toxicol. 18:51–61. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
John A and Tuszynski G: The role of matrix
metalloproteinases in tumor angiogenesis and tumor metastasis.
Pathol Oncol Res. 7:14–23. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Brosius FC III: New insights into the
mechanisms of fibrosis and sclerosis in diabetic nephropathy. Rev
Endocr Metab Disord. 9:245–254. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Alsaad KO and Herzenberg AM:
Distinguishing diabetic nephropathy from other causes of
glomerulosclerosis: an update. J Clin Pathol. 60:18–26. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Mason RM and Wahab NA: Extracellular
matrix metabolism in diabetic nephropathy. J Am Soc Nephrol.
14:1358–1373. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Nagase H and Woessner JF Jr: Matrix
metalloproteinases. J Biol Chem. 274:21491–21494. 1999. View Article : Google Scholar
|
|
23
|
Sun Z, Liu N and Liu B: The preparation
and application of antiserum against advanced glycated end
products. Chin J Lab Med. 5:293–295. 1999.(In Chinese).
|
|
24
|
Zilin S, Naifeng L, Bicheng L and Jiping
W: The determination of AGE-peptides by flow injection assay, a
practical marker of diabetic nephropathy. Clin Chim Acta.
313:69–75. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Kenig S, Alonso MB, Mueller MM and Lah TT:
Glioblastoma and endothelial cells cross-talk, mediated by SDF-1,
enhances tumour invasion and endothelial proliferation by
increasing expression of cathepsins B, S, and MMP-9. Cancer Lett.
289:53–61. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Tang CH, Tan TW, Fu WM and Yang RS:
Involvement of matrix metalloproteinase-9 in stromal cell-derived
factor-1/CXCR4 pathway of lung cancer metastasis. Carcinogenesis.
29:35–43. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Vitale S, Schmid-Alliana A, Breuil V,
Pomeranz M, Millet MA, Rossi B and Schmid-Antomarchi H: Soluble
fractalkine prevents monocyte chemoattractant protein-1-induced
monocyte migration via inhibition of stress-activated protein
kinase 2/p38 and matrix metalloproteinase activities. J Immunol.
172:585–592. 2004. View Article : Google Scholar
|
|
28
|
Klier CM, Nelson EL, Cohen CD, Horuk R,
Schlöndorff D and Nelson PJ: Chemokine-induced secretion of
gelatinase B in primary human monocytes. Biol Chem. 382:1405–1410.
2001. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Cross AK and Woodroofe MN: Chemokine
modulation of matrix metalloproteinase and TIMP production in adult
rat brain microglia and a human microglial cell line in vitro.
Glia. 28:183–189. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Madri JA and Graesser D: Cell migration in
the immune system: the evolving inter-related roles of adhesion
molecules and proteinases. Dev Immunol. 7:103–116. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Garton KJ, Gough PJ, Blobel CP, Murphy G,
Greaves DR, Dempsey PJ and Raines EW: Tumor necrosis
factor-alpha-converting enzyme (ADAM17) mediates the cleavage and
shedding of fractalkine (CX3CL1). J Biol Chem. 276:37993–38001.
2001.PubMed/NCBI
|
|
32
|
Tsou CL, Haskell CA and Charo IF: Tumor
necrosis factor-alpha-converting enzyme mediates the inducible
cleavage of fractalkine. J Biol Chem. 276:44622–44626. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Théret N, Musso O, L'Helgoualc'h A and
Clément B: Activation of matrix metalloproteinase-2 from hepatic
stellate cells requires interactions with hepatocytes. Am J Pathol.
150:51–58. 1997.PubMed/NCBI
|
|
34
|
Théret N, Lehti K, Musso O and Clément B:
MMP2 activation by collagen I and concanavalin A in cultured human
hepatic stellate cells. Hepatology. 30:462–468. 1999.PubMed/NCBI
|
|
35
|
Bourd-Boittin K, Basset L, Bonnier D,
L'Helgoualc'h A, Samson M and Théret N: CX3CL1/fractalkine shedding
by human hepatic stellate cells: contribution to chronic
inflammation in the liver. J Cell Mol Med. 13:1526–1535. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Dean RA and Overall CM: Proteomics
discovery of metalloproteinase substrates in the cellular context
by iTRAQ labeling reveals a diverse MMP-2 substrate degradome. Mol
Cell Proteomics. 6:611–623. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Inoue A, Hasegawa H, Kohno M, Ito MR,
Terada M, Imai T, Yoshie O, Nose M and Fujita S: Antagonist of
fractalkine (CX3CL1) delays the initiation and ameliorates the
progression of lupus nephritis in MRL/lpr mice. Arthritis Rheum.
52:1522–1533. 2005. View Article : Google Scholar : PubMed/NCBI
|