Introduction
Smooth muscle cells (SMCs) comprise the muscular
wall of the blood vasculature, where they provide a contractile
function and are critical in predominant human diseases, such as
atherosclerosis, hypertension and asthma (1). As a result, SMCs are critical for
blood vessel construction during tissue engineering (2). Although it is possible to isolate
SMCs from existing blood vessels, the process is invasive, requires
major surgery and injures the donor site (3). In addition, the limited replicative
life span of human adult SMCs and their slow rate of collagenous
matrix production in vitro present important hurdles in the
engineering of mechanically robust and biologically functional
grafts (4,5). Due to recent advances in the stem
cell field, studies now have access to numerous novel cell source
alternatives for vascular engineering, which exhibit the potential
to provide large numbers of autologous cells with vast
differentiation capacity (6).
Although bone marrow (7–9) and
adipose tissues (10–12) have been extensively studied as
sources of mesenchymal stem cells (MSCs), recent studies have
indicated that the hair follicle is a rich source of multipotent
adult stem cells and may be an easily accessible alternative source
of autologous SMCs (13,14). Hair follicle stem cells (HFSCs)
exhibit a broad potential to differentiate into adipogenic,
osteogenic, chondrogenic, neurogenic and myogenic lineages under
appropriate conditions (15–18).
When compared with bone marrow-derived stem cells, HFSCs are easier
to obtain as acquisition is less invasive with a lower risk of
morbidity at the donor sites and provide a higher yield at harvest
(14). Thus, HFSCs may be a
preferred novel cell source for blood vessel engineering.
TGF-β1 is an important cytokine that is involved in
vessel formation and assembly. TGF-β1 knockout was fatal in mice as
a result of defective haematopoiesis and vasculogenesis due to a
decrease in endothelial-mesenchymal cell contact (19). TGF-β1 has also been shown to affect
SMC differentiation in vitro(20) and in a rat artery injury model
in vivo(21). In previous
studies, several cells were induced by TGF-β1 to differentiate into
cells with an SMC-like phenotype and function (22–24).
The platelet-derived growth factor (PDGF) family is
composed of disulphide-bonded homodimers of four polypeptide
chains, PDGF-AA, -BB, -CC and -DD, and one heterodimeric protein,
PDGF-AB (25). PDGF-BB is critical
for the proliferation and migration of vascular smooth muscle
cells. Genetic studies in mice showed that the loss of PDGF-BB
resulted in a severe deficiency in vascular cell recruitment,
leading to vascular leakage and haemorrhage (26,27).
The aim of this study was to investigate the
potential of human HFSCs (hHFSCs) to differentiate into the SMC
phenotype upon induction by TGF-β1 and PDGF-BB in low-serum medium.
The expression of characteristic SMC markers in the resulting
hHFSCs was analysed at the protein and gene levels. Moreover, the
contractile function of induced hHFSCs, represented by the ability
of cells to contract collagen gel, was also demonstrated.
Materials and methods
Isolation and culture of hHFSCs
hHFSCs were obtained from human scalp tissues from
healthy adult patients (average age, 30 years) undergoing cosmetic
plastic surgery. All protocols of human tissue handling were
approved by the Research Ethical Committee of the Shanghai 9th
People’s Hospital (Shanghai, China) and written informed consent
was obtained from the patients. Briefly, two to three pieces of
scalp debris (4–9 mm) were postoperatively collected and washed two
to three times with phosphate-buffered saline (PBS). The fat tissue
was carefully removed and the scalp was cut into smaller sections.
The scalp sections were then transferred to a 15-ml centrifuge tube
and washed three times to remove any remaining hair or blood clots.
Washed tissue pieces were digested with 1 mg/ml collagenase type I
(Invitrogen Life Technologies, Carlsbad, CA, USA) at 37°C with
occasional agitation. After 2 h of digestion, single-hair follicles
were released from the full-thickness skin, filtered through a
40-mm cell strainer (BD Biosciences, San Jose, CA, USA) and washed
extensively in PBS. Subsequent to this, each hair follicle was
placed in one well of a 96-well plate (BD Biosciences), to allow
for cell migration onto the tissue culture plastic, and was
cultured in 100 μl low-glucose Dulbecco’s modified Eagle’s medium
(LG-DMEM; Gibco, Grand Island, NY, USA) containing 10% foetal
bovine serum (FBS; HyClone, Logan, UT, USA). Cells that originated
from the bulge region were visually identified as epidermal
keratinocytes, while cells migrating from the dermal sheath or
papilla exhibited the morphological appearance of mesenchymal
cells. The wells populated with cells originating from the dermal
sheath or papilla were selected, pooled and expanded. The cells
were then plated in 100-mm culture dishes (Falcon, Oxnard, CA, USA)
at a density of 4×104 cells/cm2 and cultured
in LG-DMEM (Gibco) supplemented with 10% FBS, 100 U/ml penicillin
(Sigma-Aldrich, St. Louis, MO, USA), and 100 mg/ml streptomycin
(Sigma-Aldrich) (defined as growth medium). The medium was changed
twice a week. Cells were passaged when they had reached 70–80%
confluence and second passage hHFSCs were used in the subsequent
experiments. The characterisation of hHFSCs was determined by the
CD marker profile and the ability to differentiate into osteogenic,
adipogenic and chondrogenic lineages, as previously described (data
not shown) (15,16).
Induction of SM differentiation
To evaluate the effects of TGF-β1 and PDGF-BB on the
differentiation of hHFSCs into SMCs, hHFSCs reaching subconfluence
were cultured in DMEM supplemented with 5 ng/ml recombinant human
TGF-β1 (R&D Systems, Minneapolis, MN, USA) and 10 ng/ml
recombinant human PDGF-BB with 1% FBS. DMEM supplemented with 1%
FBS was defined as the basal medium (BM). Human umbilical artery
SMCs (hUASMCs) served as a positive control. The culture media were
changed every two days. Cell characterisation and functional
evaluation were performed subsequent to eight days of culture.
Immunofluorescence staining
The hHFSCs were harvested, resuspended in PBS, fixed
with 4% paraformaldehyde (Sigma-Aldrich) for 15 min and
permeabilised with 0.1% Triton X-100 (Sigma-Aldrich) for 10 min.
Subsequent to washing with PBS, the cells were blocked with 3%
bovine serum albumin (BSA, Sigma-Aldrich) for 30 min and incubated
with the following primary antibodies: Mouse monoclonal
anti-α-smooth muscle actin (α-SMA, C6198, Sigma-Aldrich), rabbit
monoclonal anti-α-calponin (ab46794; Abcam, Cambridge, UK) and
mouse monoclonal anti-smooth muscle myosin heavy chain (SM-MHC,
M7786, Sigma-Aldrich). Following incubation with the primary
antibodies for 60 min at room temperature (RT), the cultures were
washed with PBS three times. Fluorescein isothiocyanate
(FITC)-conjugated goat anti-rabbit secondary antibody (Millipore,
Billerica, MA, USA) was used to detect the localisation of
anti-α-calponin antibodies. FITC-conjugated goat anti-mouse
secondary antibody (Millipore) was used to detect the localisation
of anti-α-SMA and anti-SM-MHC antibodies. In addition, cell nuclei
were stained with propidium iodide. Control samples consisted of
cells without primary antibody and were used to assess background
fluorescence. The images were viewed by a fluorescence microscope
(Eclipse E400 epi-fluorescence microscope; Nikon, Tokyo,
Japan).
Flow cytometry analysis
Cells were trypsinised, centrifuged at 500 × g for 5
min (Allegra 64R; Beckman Coulter, Brea, CA, USA), resuspended in
PBS/1% BSA and incubated with anti-α-SMA (dilution, 1:100;
Sigma-Aldrich), anti-α-calponin (dilution, 1:100; Abcam) and
anti-SM-MHC (dilution 1:100; Sigma-Aldrich) for 30 min at RT on a
shaking plate (Lab Rotors; Thermo Scientific, Logan UT, USA). The
cells were then washed, resuspended in PBS/1% BSA with
FITC-conjugated secondary antibody and incubated for 30 min at RT
on a shaking plate. Subsequent to this, cells were washed and
fixed. FITC-conjugated isotype-matching immunoglobulins were used
to determine non-specific staining. Fluorescence was determined
using a flow cytometer (FACSCalibur; Becton-Dickinson, San Jose,
CA, USA) and the data were analysed using CellQuest software
(Becton-Dickinson).
RNA isolation and reverse
transcription-polymerase chain reaction (RT-PCR)
Expression levels of SMC-specific markers (α-SMA,
α-calponin and SM-MHC) were identified by isolating the total RNA
from cells using the RNeasy total RNA isolation kit (Qiagen, Inc.,
Valencia, CA, USA) and cDNA was synthesised using the SuperScript
First-strand Synthesis system (Life Technologies, Barcelona,
Spain). Specific genes were amplified by PCR using the Fast-Run Taq
Master kit (Protech Technologies Inc., Taipei, Taiwan). The primer
sequences designed using the Primer Express software are listed in
Table I. The cDNA product was
amplified by PCR using standard methods (35 cycles of 95°C for 30
sec, 56°C for 30 sec and 72°C for 60 sec). Gel electrophoresis was
then performed on a 2% agarose gel treated with ethidium bromide
and bands were visualised using a UV light box. hUASMCs and human
chondrocyte cells (hCs) served as positive and negative controls,
respectively.
 | Table IPrimers for polymerase chain reaction
analysis. |
Table I
Primers for polymerase chain reaction
analysis.
| RNA | Primer sequences
(nucleotides) | Fragment size
(bp) |
|---|
| α-SMA | Forward:
GGTGATGGTGGGAATGGG (18)
Reverse: GCAGGGTGGGATGCTCTT (18) | 188 |
| Calponin | Forward:
GGCGAAGACGAAAAGGAAA (19)
Reverse: GGGTACTCGGGAGTCAGACAG (21) | 447 |
| SM-MHC | Forward:
TGCTTTCGCTCGTCTTCC (18)
Reverse: CGGCAACTCGTGTCCAAC (18) | 516 |
Collagen gel lattice contraction
assay
The contractility of cells was measured as
previously described (28).
Briefly, cells were trypsinised by treatment with
trypsin-ethylenediaminetetraacetic acid, counted and resuspended in
serum-free DMEM at a density of 1×106 cells/ml. The
prepared cell suspension was added to a collagen gel solution to
achieve a final concentration of 3 mg collagen/ml and a density of
4×105 cells/ml. The cell collagen mixture was poured
into 12-well culture plates for 1 h to polymerise the collagen cell
lattices. The lattices were mechanically released from the bottom
of the tissue culture dishes by gently pipetting medium at the
lattice-dish interface to initiate collagen gel contraction. The
extent of gel contraction of each group at different time points
was calculated by measuring the dimensions of the gel lattices
viewed by a digital camera. The area of the gel lattices was
analysed using NIH ImageJ software (National Institutes of Health,
Bethesda, MD, USA). The relative lattice area was obtained by
dividing the area at each time point by the initial area of the
lattice.
Statistical analysis
Each experiment was repeated at least three times.
The results are presented as the mean ± SD. Comparisons between
groups were performed by a paired Student’s t-test. P<0.05 was
considered to indicate a statistically significant difference.
Results
Culture of hHFSCs
The total quantity of cells isolated from each scalp
tissue sample ranged from 5×104 to 1×105
cells. Approximately 0.5–2% of the isolated tissue cells were
identified to be the adherent stem cells. Subsequent to 1–2 days of
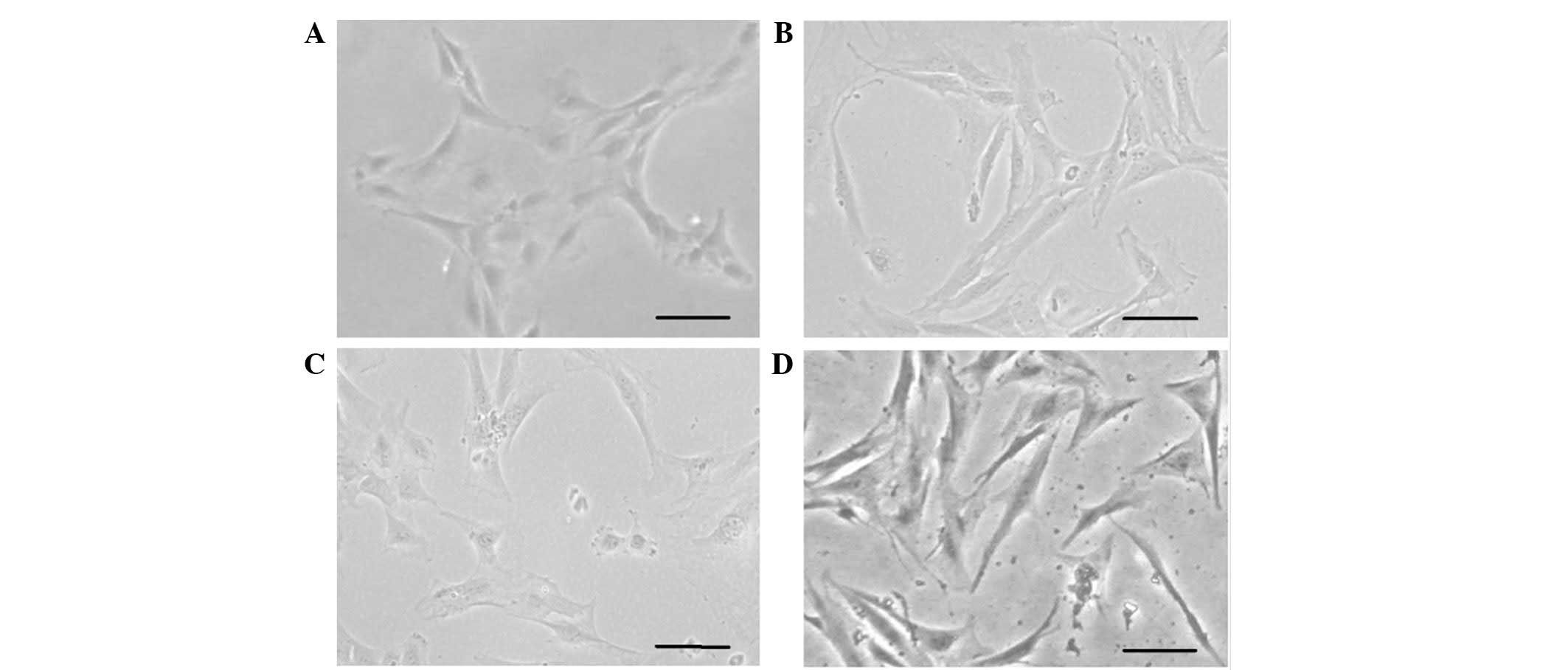
culture, the hHFSCs were observed to be elongated (Fig. 1A). Within another 3–4 days, the
cells reached confluency and were subsequently passaged onto a
novel plate.
TGF-β1 and PDGF-BB induce differentiation
of hHFSCs into SMCs
At the second passage, 5 ng/ml TGF-β1 and 10 ng/ml
PDGF-BB were selected to induce the differentiation of hHFSCs into
the SMC lineage. hHFSCs treated with TGF-β1 and PDGF-BB for eight
days acquired a spindle morphology and grew in a hill and valley
pattern similar to that previously observed in primary isolated
hUASMCs. No obvious change was identified in the hHFSCs cultured in
BM (Fig. 1B–D). Fifth passage
hHFSCs appeared to be a relatively homogenous population that
exhibited a fibroblast-like spindle morphology.
Expression of SMC-specific markers in
hHFSCs treated with TGF-β1 and PDGF-BB
To determine whether TGF-β1 and PDGF-BB induced the
differentiation of hHFSCs into the SMC phenotype, SM-specific
proteins (α-SMA, α-calponin and SM-MHC) were detected by
immunofluorescence staining. In parallel, the three markers in
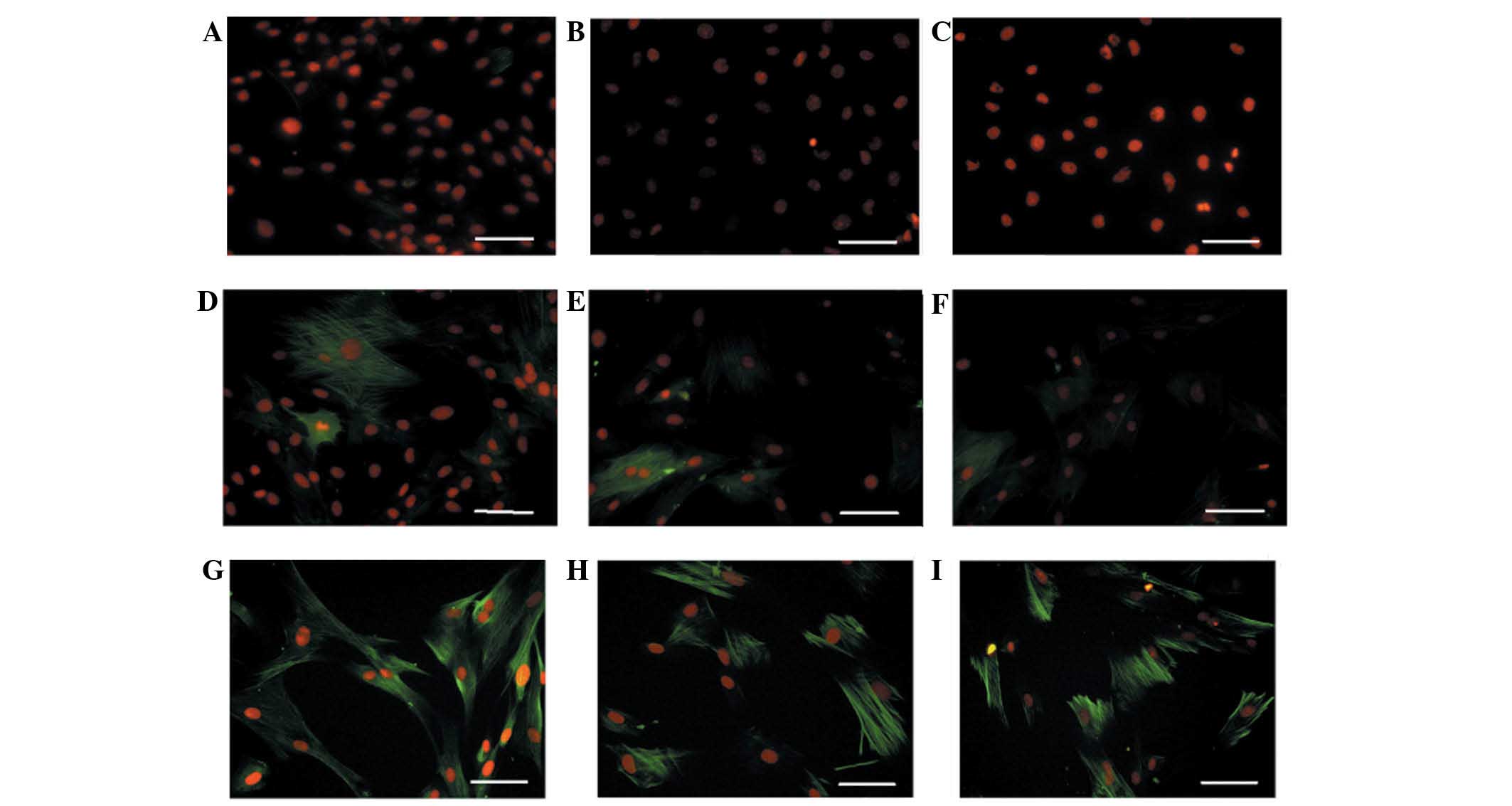
hUASMCs were also analysed as a positive control. As shown in
Fig. 2, there was a baseline
expression of α-SMA, but marginal expression of α-calponin and
SM-MHC in undifferentiated hHFSCs cultured in BM. When cultured in
DMEM supplemented with TGF-β1 and PDGF-BB, expression levels of
α-SMA and α-calponin were identified to be enhanced. Furthermore,
expression of SM-MHC was markedly increased, reaching a similar
level to that in the hUASMCs.
To determine the percentage of SM differentiated
cells in the hHFSCs, the expression levels of α-SMA, α-calponin and
SM-MHC were analysed by flow cytometry. As shown in Fig. 3, the α-SMA was detected in
17.41±0.78%, 45.32±1.21% and 89.46±0.83% of undifferentiated
hHFSCs, induced hHFSCs and hUASMCs, respectively. By comparison,
marginal expression of α-calponin (3.47±0.82%) and SM-MHC
(2.78±0.63%) was observed in the undifferentiated hHFSCs, while
their expression levels reached 57.24±2.08% and 70.71±1.78%,
respectively, in the induced hHFSCs, which was closer to the
expression observed in hUASMCs.
The gene expression profile analysed by RT-PCR
further confirmed the differentiation of hHFSCs into SMCs. As shown
in Fig. 4, undifferentiated hHFSCs
expressed low levels of α-SMA mRNA and did not express α-calponin
or SM-MHC, which was similar to that observed in the hCs. By
contrast, these factors were upregulated in induced hHFSCs at a
similar level to that in hUASMCs. These data support the hypothesis
that hHFSCs are capable of differentiating into SMCs when induced
by TGF-β1 and PDGF-BB.
Characterisation of contractility of SMCs
differentiated from hHFSCs
To evaluate whether SMCs, differentiated from hHFSCs
by TGF-β1 and PDGF-BB induction, exhibit the ability to contract
and generate force, the contractility of the smooth muscle-like
cells was analysed using a collagen lattice gel contraction assay.
The shrinkage of gels embedded with differentiated SMCs occurred in
a time-dependent manner. After 48 h, the area of gel lattice was
markedly decreased. However, collagen gel matrix containing
undifferentiated hHFSCs contracted to a lesser extent. Compared
with the gel containing undifferentiated cells, the area of the
collagen lattice with differentiated hHFSCs was significantly
diminished after 6 h (Fig. 5).
Thus, these results showed that with the combined induction by
TGF-β1 and PDGF-BB, the hHFSCs differentiated into the SMC
phenotype and acquired the contractile ability observed in
hUASMCs.
Discussion
Although there has been promising progress towards
the development of biological vascular grafts in treating vascular
diseases, the cell source remains a predominant obstacle in
vascular tissue engineering. Adult somatic SMCs have been shown to
exhibit limited replicative capacity and, therefore, are not an
optimal source of seed cells for constructing tissue-engineered
blood vessels, as previously suggested (4,29–32).
Although SMCs have been derived from multipotent adult stem cells
(9,10,16,19,
33–35), HFSCs have been investigated less
frequently.
In the present study, the effect of TGF-β1 and
PDGF-BB on the differentiation of hHFSCs toward the SMC lineage was
investigated. Considerable variation was observed in the expression
of the SMC markers, α-SMA, α-calponin and SM-MHC, at the gene and
protein levels. α-SMA is often considered to be an early marker of
developing smooth muscle cells (12). SM-MHC and α-calponin are widely
accepted to be late markers of SMC differentiation and are more
specific to an SMC lineage (36).
It was demonstrated that TGF-β1 and PDGF-BB induced the expression
of these three proteins in hHFSCs. Furthermore, the induction time
was extended to ≥21 days and it was demonstrated that the
expression of the three markers remained detectable in
differentiated hHFSCs by immunofluorescence staining (data not
shown). These results suggest that the combination of TGF-β1 and
PDGF-BB fully stimulates the differentiation of hHFSCs towards the
SMC phenotype.
TGF-β1 is important in regulating SMC proliferation
and differentiation, and in the maturation of blood vessels in
vasculogenesis (37). It has been
demonstrated that TGF-β1 enhances the expression and organisation
of α-SMA, SM-MHC and smooth muscle protein 22-α in SMC lines as
well as primary rat and human SMC cultures (38–42).
Furthermore, in rodent models, the level of TGF-β1 in the neointima
and damaged media of injured vessels is decreased and is correlated
with a decrease in α-SMA, type IV collagen and SM-MHC (24). In addition, TGF-β1 is shown to
stabilise elastin mRNA (43,44);
increase the mRNA level and enzymatic activity of lysyl oxidase
(45); enhance the mechanical
strength (30,46) and vascular reactivity of
fibrin-based V-SMC tissue equivalents (47); and promote the differentiation of
embryonic stem cells (48), bone
marrow mesenchymal stem cells (49,50),
and bone marrow multipotent adult progenitor cells (34) into mature contractile SMCs.
PDGF-BB released by endothelial cells (ECs) has been
demonstrated to stimulate the proliferation of cocultured
mesenchymal cells (51). PDGF-BB
mediates mesenchymal differentiation and acts as a mitogen and
chemoattractant for mesenchymal cells (52). PDGF-BB specifically exerts a
paracrine effect on undifferentiated mesenchymal cells. Holmgren
et al(53) observed the
expression of PDGF ligand and receptors in forming blood vessels
within the human placenta. It was demonstrated that ECs of
developing blood vessels express the PDGF-BB mRNA and protein but
not the PDGF-β receptor, whereas mRNA of the PDGF-β receptor was
detectable in the SMCs surrounding intermediate and large blood
vessels.
The mechanism of SMC differentiation of hHFSCs
induced by PDGF-BB and TGF-β1 remains unclear. In vivo,
TGF-β1 is secreted as a complex with latency-associated propeptide
and is targeted to specific locations in the extracellular matrix
through binding to latent TGF-β binding protein. TGF-β modulates
SMC differentiation by directly binding to the type-I receptor and
thereafter activating downstream signals of Smad proteins (54–56).
In its C-terminal domain, PDGF-BB contains a stretch of basic amino
acids that confer binding to heparan sulphate proteoglycans
(HSPGs). It is assumed that HSPG binding generates growth-factor
gradients that provide positional information and spatial control
of cellular responses (57).
Therefore, it was speculated that cross-talk between PDGF-BB and
TGF-β1 pathways may contribute to the differentiation of hHFSCs
into SMCs; however, the detailed underlying mechanism requires
further investigation.
To ascertain a differentiated SMC phenotype,
demonstration of the quintessential functional property of
contraction is required. The results of the present study showed
that hHFSC differentiation was induced by TGF-β1 and PDGF-BB and
these cells elicited noteworthy contraction of the collagen gel
lattice, a characteristic of differentiated SMCs. The data suggest
that hHFSCs are capable of being induced to differentiate into an
SMC phenotype with contractile function when stimulated by a
combination of TGF-β1 and PDGF-BB. Thus, hHFSCs may be a source of
functional SMCs for tissue engineering of blood vessels, as well as
other SMC-containing tissues, including bladder, urethral and
intestinal tissues.
In conclusion, it was demonstrated that expanded
hHFSCs were able to be induced to differentiate along an SMC
pathway by TGF-β1 and PDGF-BB stimulation for eight days in
vitro, as determined by the expression of SMC-specific
transcripts and proteins, including α-SMA, SM-MHC and SM-MHC. When
embedded in a collagen lattice, the differentiated hHFSCs
contracted. These results substantiate the possibility of using
hHFSCs as a candidate for cardiovascular tissue engineering and
regenerative medicine.
Acknowledgements
This study was supported by the National Natural
Science Foundation of China (grant no. 81000842). The authors would
like to thank Hong Li, Demin Ying, Lijuan Zong and Bing Zhong for
their technical support in the laboratory.
References
|
1
|
Owens GK, Kumar MS and Wamhoff BR:
Molecular regulation of vascular smooth muscle cell differentiation
in development and disease. Physiol Rev. 84:767–801. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Sundaram S and Niklason LE: Smooth muscle
and other cell sources for human blood vessel engineering. Cells
Tissues Organs. 195:15–25. 2012.PubMed/NCBI
|
|
3
|
Xu ZC, Zhang WJ, Li H, Cui L, Cen L, Zhou
GD, Liu W and Cao Y: Engineering of an elastic large muscular
vessel wall with pulsatile stimulation in bioreactor. Biomaterials.
29:1464–1472. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Poh M, Boyer M, Solan A, Dahl SL, Pedrotty
D, Banik SS, McKee JA, Klinger RY, Counter CM and Niklason LE:
Blood vessels engineered from human cells. Lancet. 365:2122–2124.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
McKee JA, Banik SS, Boyer MJ, Hamad NM,
Lawson JH, Niklason LE and Counter CM: Human arteries engineered in
vitro. EMBO Rep. 4:633–638. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Bajpai VK and Andreadis ST: Stem cell
sources for vascular tissue engineering and regeneration. Tissue
Eng Part B Rev. 18:405–425. 2012.PubMed/NCBI
|
|
7
|
Matsumura G, Miyagawa-Tomita S, Shin’oka
T, Ikada Y and Kurosawa H: First evidence that bone marrow cells
contribute to the construction of tissue-engineered vascular
autografts in vivo. Circulation. 108:1729–1934. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Shin’oka T, Matsumura G, Hibino N, Naito
Y, Watanabe M, Konuma T, Sakamoto T, Nagatsu M and Kurosawa H:
Midterm clinical result of tissue-engineered vascular autografts
seeded with autologous bone marrow cells. J Thorac Cardiovasc Surg.
129:1330–1338. 2005.
|
|
9
|
Gong Z and Niklason LE: Small-diameter
human vessel wall engineered from bone marrow-derived mesenchymal
stem cells (hMSCs). FASEB J. 22:1635–1648. 2008. View Article : Google Scholar
|
|
10
|
Heydarkhan-Hagvall S, Schenke-Layland K,
Yang JQ, Heydarkhan S, Xu Y, Zuk PA, MacLellan WR and Beygui RE:
Human adipose stem cells: a potential cell source for
cardiovascular tissue engineering. Cells Tissues Organs.
187:263–274. 2008.PubMed/NCBI
|
|
11
|
Wang C, Cen L, Yin S, Liu Q, Liu W, Cao Y
and Cui L: A small diameter elastic blood vessel wall prepared
under pulsatile conditions from polyglycolic acid mesh and smooth
muscle cells differentiated from adipose-derived stem cells.
Biomaterials. 31:621–630. 2010. View Article : Google Scholar
|
|
12
|
Harris LJ, Abdollahi H, Zhang P, McIlhenny
S, Tulenko TN and DiMuzio PJ: Differentiation of adult stem cells
into smooth muscle for vascular tissue engineering. J Surg Res.
168:306–314. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Hsu YC, Pasolli HA and Fuchs E: Dynamics
between stem cells, niche, and progeny in the hair follicle. Cell.
144:92–105. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Mistriotis P and Andreadis ST: Hair
Follicle: a novel source of multipotent stem cells for tissue
engineering and regenerative medicine. Tissue Eng Part B Rev.
19:265–278. 2013.PubMed/NCBI
|
|
15
|
Jahoda CA, Whitehouse J, Reynolds AJ and
Hole N: Hair follicle dermal cells differentiate into adipogenic
and osteogenic lineages. Exp Dermatol. 12:849–859. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Yu H, Fang D, Kumar SM, Li L, Nguyen TK,
Acs G, Herlyn M and Xu X: Isolation of a novel population of
multipotent adult stem cells from human hair follicles. Am J
Pathol. 168:1879–1888. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Drewa T, Joachimiak R, Kaznica A, Sarafian
V and Pokrywczynska M: Hair stem cells for bladder regeneration in
rats: preliminary results. Transplant Proc. 41:4345–4351. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Lin H, Liu F, Zhang C, Zhang Z, Kong Z,
Zhang X and Hoffman RM: Characterization of nerve conduits seeded
with neurons and Schwann cells derived from hair follicle neural
crest stem cells. Tissue Eng Part A. 17:1691–1698. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Dickson MC, Martin JS, Cousins FM,
Kulkarni AB, Karlsson S and Akhurst RJ: Defective haematopoiesis
and vasculogenesis in transforming growth factor-beta 1 knock out
mice. Development. 121:1845–1854. 1995.PubMed/NCBI
|
|
20
|
Shah NM, Groves AK and Anderson DJ:
Alternative neural crest cell fates are instructively promoted by
TGFbeta superfamily members. Cell. 85:331–343. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Grainger DJ, Metcalfe JC, Grace AA and
Mosedale DE: Transforming growth factor-beta dynamically regulates
vascular smooth muscle differentiation in vivo. J Cell Sci.
111:2977–2988. 1998.PubMed/NCBI
|
|
22
|
Hirschi KK, Rohovsky SA and D’Amore PA:
PDGF, TGF-beta, and heterotypic cell-cell interactions mediate
endothelial cell-induced recruitment of 10T1/2 cells and their
differentiation to a smooth muscle fate. J Cell Biol. 141:805–814.
1998. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Chen S and Lechleider RJ: Transforming
growth factor-beta-induced differentiation of smooth muscle from a
neural crest stem cell line. Circ Res. 94:1195–1202. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Hirschi KK, Burt JM, Hirschi KD and Dai C:
Gap junction communication mediates transforming growth factor-beta
activation and endothelial-induced mural cell differentiation. Circ
Res. 93:429–437. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Ross JJ, Hong Z, Willenbring B, Zeng L,
Isenberg B, Lee EH, Reyes M, Keirstead SA, Weir EK, Tranquillo RT
and Verfaillie CM: Cytokine-induced differentiation of multipotent
adult progenitor cells into functional smooth muscle cells. J Clin
Invest. 116:3139–3149. 2006. View
Article : Google Scholar : PubMed/NCBI
|
|
26
|
Lindahl P, Johansson BR, Levéen P and
Betsholtz C: Pericyte loss and microaneurysm formation in
PDGF-B-deficient mice. Science. 277:242–245. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Hellström M, Kalén M, Lindahl P, Abramsson
A and Betsholtz C: Role of PDGF-B and PDGFR-beta in recruitment of
vascular smooth muscle cells and pericytes during embryonic blood
vessel formation in the mouse. Development. 126:3047–3055.
1999.PubMed/NCBI
|
|
28
|
Kim YM, Jeon ES, Kim MR, Jho SK, Ryu SW
and Kim JH: Angiotensin II-induced differentiation of adipose
tissue-derived mesenchymal stem cells to smooth muscle-like cells.
Int J Biochem Cell Biol. 40:2482–2491. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
McKee JA, Banik SS, Boyer MJ, Hamad NM,
Lawson JH, Niklason LE and Counter CM: Human arteries engineered in
vitro. EMBO Rep. 4:633–638. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Long JL and Tranquillo RT: Elastic fiber
production in cardiovascular tissue-equivalents. Matrix Biol.
22:339–350. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Gong Z and Niklason LE: Blood vessels
engineered from human cells. Trends Cardiovasc Med. 16:153–156.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Isenberg BC, Williams C and Tranquillo RT:
Small-diameter artificial arteries engineered in vitro. Circ Res.
98:25–35. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Cho SW, Lim SH, Kim IK, Hong YS, Kim SS,
Yoo KJ, Park HY, Jang Y, Chang BC, Choi CY, et al: Small-diameter
blood vessels engineered with bone marrow-derived cells. Ann Surg.
241:506–515. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Rodríguez LV, Alfonso Z, Zhang R, Leung J,
Wu B and Ignarro LJ: Clonogenic multipotent stem cells in human
adipose tissue differentiate into functional smooth muscle cells.
Proc Natl Acad Sci USA. 103:12167–12172. 2006.PubMed/NCBI
|
|
35
|
Beltrami AP, Cesselli D, Bergamin N,
Marcon P, Rigo S, Puppato E, D’Aurizio F, Verardo R, Piazza S,
Pignatelli A, et al: Multipotent cells can be generated in vitro
from several adult human organs (heart, liver, and bone marrow).
Blood. 110:3438–3446. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Miano JM: Mammalian smooth muscle
differentiation: origins, markers and transcriptional control.
Results Probl Cell Differ. 38:39–59. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Grainger DJ: Transforming growth factor
beta and atherosclerosis: so far, so good for the protective
cytokine hypothesis. Arterioscler Thromb Vasc Biol. 24:399–404.
2004. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Björkerud S: Effects of transforming
growth factor-beta 1 on human arterial smooth muscle cells in
vitro. Arterioscler Thromb. 11:892–902. 1991.PubMed/NCBI
|
|
39
|
Deaton RA, Su C, Valencia TG and Grant SR:
Transforming growth factor-beta 1-induced expression of smooth
muscle marker genes involves activation of PKN and p38 MAPK. J Biol
Chem. 280:31172–31181. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Hautmann MB, Madsen CS and Owens GK: A
transforming growth factor beta (TGFbeta) control element drives
TGFbeta-induced stimulation of smooth muscle alpha-actin gene
expression in concert with two CarG elements. J Biol Chem.
272:10948–10956. 1997. View Article : Google Scholar
|
|
41
|
Kawai-Kowase K, Sato H, Oyama Y, Kanai H,
Sato M, Doi H and Kurabayashi M: Basic fibroblast growth factor
antagonizes transforming growth factor-beta1-induced smooth muscle
gene expression through extracellular signal-regulated kinase 1/2
signaling pathway activation. Arterioscler Thromb Vasc Biol.
24:1384–1390. 2004. View Article : Google Scholar
|
|
42
|
Papetti M, Shujath J, Riley KN and Herman
IM: FGF-2 antagonizes the TGF-beta1-mediated induction of pericyte
alpha-smooth muscle actin expression: a role for myf-5 and
Smad-mediated signaling pathways. Invest Ophthalmol Vis Sci.
44:4994–5005. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Kucich U, Rosenbloom JC, Abrams WR, Bashir
MM and Rosenbloom J: Stabilization of elastin mRNA by TGF-beta:
initial characterization of signaling pathway. Am J Respir Cell Mol
Biol. 17:10–16. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Kucich U, Rosenbloom JC, Abrams WR and
Rosenbloom J: Transforming growth factor-beta stabilizes elastin
mRNA by a pathway requiring active Smads, protein kinase C-delta,
and p38. Am J Respir Cell Mol Biol. 26:183–188. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Hong HH, Uzel MI, Duan C, Sheff MC and
Trackman PC: Regulation of lysyl oxidase, collagen, and connective
tissue growth factor by TGF-beta1 and detection in human gingiva.
Lab Invest. 79:1655–1667. 1999.PubMed/NCBI
|
|
46
|
Ross JJ and Tranquillo RT: ECM gene
expression correlates with in vitro tissue growth and development
in fibrin gel remodeled by neonatal smooth muscle cells. Matrix
Biol. 22:477–490. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Yao L, Swartz DD, Gugino SF, Russell JA
and Andreadis ST: Fibrin-based tissue-engineered blood vessels:
differential effects of biomaterial and culture parameters on
mechanical strength and vascular reactivity. Tissue Eng.
11:991–1003. 2005. View Article : Google Scholar
|
|
48
|
Sinha S, Hoofnagle MH, Kingston PA,
McCanna ME and Owens GK: Transforming growth factor-beta1 signaling
contributes to development of smooth muscle cells from embryonic
stem cells. Am J Physiol Cell Physiol. 287:C1560–C1568. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Kinner B, Zaleskas JM and Spector M:
Regulation of smooth muscle actin expression and contraction in
adult human mesenchymal stem cells. Exp Cell Res. 278:72–83. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Wang D, Park JS, Chu JS, Krakowski A, Luo
K, Chen DJ and Li S: Proteomic profiling of bone marrow mesenchymal
stem cells upon transforming growth factor beta1 stimulation. J
Biol Chem. 279:43725–43734. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Collins T, Pober JS, Grimbone MA Jr,
Hammacher A, Betsholtz C, Westermark B and Heldin CH: Cultured
human endothelial cells express platelet-derived growth factor A
chain. Am J Pathol. 126:7–12. 1987.PubMed/NCBI
|
|
52
|
Westermark B, Siegbahn A, Heldin CH and
Claesson-Welsh L: B-type receptor for platelet-derived growth
factor mediates a chemotactic response by means of ligand-induced
activation of the receptor protein-tyrosine kinase. Proc Natl Acad
Sci USA. 87:128–132. 1990. View Article : Google Scholar
|
|
53
|
Holmgren L, Glaser A, Pfeifer-Ohlsson S
and Ohlsson R: Angiogenesis during human extraembryonic development
involves the spatiotemporal control of PDGF ligand and receptor
gene expression. Development. 113:749–754. 1991.PubMed/NCBI
|
|
54
|
Hyytiäinen M, Penttinen C and Keski-Oja J:
Latent TGF-beta binding proteins: extracellular matrix association
and roles in TGF-beta activation. Crit Rev Clin Lab Sci.
41:233–264. 2004.PubMed/NCBI
|
|
55
|
Derynck R and Akhurst RJ: Differentiation
plasticity regulated by TGF-beta family proteins in development and
disease. Nat Cell Biol. 9:1000–1004. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
56
|
Liang MS and Andreadis ST: Engineering
fibrin-binding TGF-β1 for sustained signaling and contractile
function of MSC based vascular constructs. Biomaterials.
32:8684–8693. 2011.PubMed/NCBI
|
|
57
|
Gallagher JT: Heparan sulfate: growth
control with a restricted menu. J Clin Invest. 108:357–361. 2001.
View Article : Google Scholar : PubMed/NCBI
|