Introduction
Retroperitoneal lymph nodes and lung metastases are
an important route of dissemination and a significant prognostic
factor for various types of gynecologic cancer, such as ovarian
cancer (1,2). Retroperitoneal lymph node metastases
are difficult to treat surgically since these lymph nodes are
located close to the great vessels of the abdominopelvic cavity.
Surgery becomes particularly difficult when the great vessels are
involved. Current treatments for retroperitoneal lymphatic node and
lung metastases include systemic chemotherapy, reduction in visible
tumor burden and palliative therapy. Progress in the treatment of
ovarian and other types of gynecologic cancer has been limited by
difficulties in the removal of the retroperitoneal lymph nodes and
the lack of studies focusing on suitable animal models for
retroperitoneal lymphatic and lung metastasis.
Only a few experimental animal models of lung and
lymph node metastasis have been developed that involve the use of
mice, rats and rabbits (3–5). In rat and mouse models of lymph node
metastases, cancer cells are injected directly into the arch
cushion resulting in metastasis to inguinal lymph nodes (3,4). Fu
and Hoffman (6) previously
reported that human ovarian carcinoma metastatic models were
constructed in nude mice by the orthotopic transplantation of
histologically intact patient specimens. Due to limitations in body
size, blood supply and tolerance to major surgical interventions
and local chemotherapy, these models were suitable only for studies
of treatment with systemic or intraperitoneal chemotherapy
(3,4). Use of larger animals improves the
ability to evaluate the efficacy of chemotherapy or other medically
relevant procedures for treating metastatic cancer. Over the past
two decades, rabbit animal models have been used to investigate
lymph node metastasis (7–16), however, a number of these studies
did not specifically characterize retroperitoneal lymph node
metastasis. Characterization of metastasis to the retroperitoneal
lymph nodes in a tumor-bearing rabbit have been previously reported
only once and this involved injection of cancer cells into the
endometrium (7).
The development of a large animal model of the
metastasis of various types of gynecologic cancer to the
retroperitoneal lymph nodes and lungs is highly desirable. Such
models may be established by inoculation of the ovary or cervix
with VX2 squamous cell carcinoma tissues. Rabbit VX2 carcinoma,
which is a transplantable squamous cell carcinoma, was selected for
establishment of this animal model since it is characterized by
rapid growth and early metastasis. VXT rabbit models of cancer have
previously been used to study types of renal, liver, lung, head and
neck, brain and uterine cancer (7–9,16).
The current study assessed the initial characterization of a rabbit
VX2 carcinoma model designed to investigate metastasis to the
retroperitoneal lymph nodes and lungs.
Materials and methods
Animals
In total, 41 female New Zealand white rabbits were
obtained from the Animal Experimental Center of Sun Yat-sen
University (Guangzhou, Guangdong, China) and kept in the Animal
Biosafety Level 3 Laboratory of the Animal Experimental Center of
Sun Yat-sen University (Animal Study Certificate 0027164). One
animal (the carrier) had previously been implanted with VX2 cells
and the other animals were healthy. Rabbits were 8–9-weeks-old and
had body weights of 1.7–2.2 kg (median, 1.85 kg). The animals were
individually housed, allowed free access to standard laboratory
food and water and were subjected to daily 12-h light/dark cycles.
The animal protocol used was approved by the Animal Welfare
Committee of the State Key Laboratory of Oncology in South
China.
Preparation of the animal model
Construction of the retroperitoneal lymph node
animal model required two transplantation steps. First, it was
necessary to transfer tumor cells from the carrier rabbit to a
healthy rabbit (referred to as the transfer animal) to ensure that
the VX2 tumor cells, which were to be used in the model system, had
optimal viability. Secondly, tumor tissue from the transfer animal
was transplanted into the experimental rabbits (n=39).
To generate the transfer animal, the carrier animal
was anesthetized by injection of ketamine (10 mg/kg) into the ear
vein. The volume of the tumor in the carrier animal was ~283.5
cm3 at 45 days post-transplantation. Following the
preparation and disinfection of the skin in the area of the tumor,
fresh tumor tissue was excised, flushed with 0.9% sodium chloride
solution, and placed in RPMI 1640 medium (Gibco, Life Technologies,
Carlsbad, CA, USA). Excised tumor tissue was minced into fragments
of about 0.5 to 1.0 mm3, suspended in RPMI 1640 medium (Gibco).
Following suspension, the tumor cell concentration was adjusted to
20% (~1×1010 cells/ml), drawn into a 2 ml lumbar
puncture needle and injected into the left gastrocnemius of a
healthy rabbit that served as the transferring animal. On
post-inoculation day 30, the transfer rabbit was sacrificed by
aeroembolism (injection of air into the ear vein) and the tumor was
removed for analysis and construction of the retroperitoneal lymph
node animal model.
To generate the animal model, the tumor from the
transfer animal was excised, suspended and injected into the 39
healthy rabbits as described above. The rabbits bearing VX2
carcinoma cells were randomly divided into 3 groups according to
the day the rabbits were sacrificed following transplantation: 1,
day 19; 2, day 22; and 3, day 25.
Following surgery, the animals were observed daily
for food intake, activity and the presence of abnormalities, such
as diarrhea or dehydration. Implanted primary tumor (IPTu) size was
measured every 2 days following post-transplantation. Animals were
euthanized by aeroembolism and post-mortem examinations included
determination of gross tumor pathology, tumor size, tumor
distribution, and local morphological features, which were
performed by YW Huang to avoid differing judgments. The volume of
the IPTu and left and right retroperitoneal lymph nodes, as well as
the wet weights of the lungs were measured. The volume of the lymph
node was calculated using the formula: Volume = length ×
(width2)/2
Critical tissues, including IPTu, left and right
retroperitoneal lymph nodes and lungs, were stained with
hematoxylin and eosin to evaluate tissue histopathology. For
swollen lymph nodes observable by the naked eye, as well as visible
pulmonary metastases, 3 serially sectioned, 4-μM samples were
evaluated per sample. For lymph nodes without enlargement as
observed by the naked eye, a total of five sections (4 μM)
including the 5th, 10th, 15th, 20th, and 25th sections from 25
serial sections were selected for analysis. Similarly, for lung
tissue without metastases as observed by the naked eye, a total of
five sections (4 μM), including the 10th, 20th, 30th, 40th and 50th
sections from a total 50 serial sections, were chosen for analysis.
The slides were evaluated by two pathologists who were blinded to
the experiment. The rates of metastases were calculated as per
rabbit with at least one positively identified metastatic tumor.
The rates of metastases to the lymph nodes and lungs, positive
lymph node number and lung wet weights were compared among the 3
groups.
Statistical analysis
Comparability among the 3 groups was tested using
one-way analysis of variance (ANOVA) for continuous variables and
χ2/Fisher's exact test for categorical variables. The
differences among the groups for the number of positive left
retroperitoneal lymph nodes was determined by Kruskal-Wallis
one-way analysis of variance. When a significant difference among
the groups was detected, multiple comparison tests were performed
using the Bonferroni method with type-I error adjustment. The
difference between the left and right retroperitoneal lymph nodes
within each group was tested using the paired t-test. Continuous
variables were presented as mean ± SD and categorical data were
represented by number (n) and percentage (%). In addition, the
number of positive left retroperitoneal lymph nodes was presented
as the median (range). The point biserial correlation coefficient
was used to test the correlation between a continuous and a
categorical variable with two levels. All statistical assessments
were two-sided and P<0.05 was considered to indicate a
statistically significant difference. Statistical analyses were
performed using SPSS 15.0 statistics software (SPSS Inc., Chicago,
IL, USA).
Results
Survival, implantation and growth of the
primary tumor
Of the 39 animals that were transplanted with VX2
tumor cells, 38 survived prior to sacrifice; one rabbit in group 1
died of diarrhea of unknown cause on day 5. In all the surviving
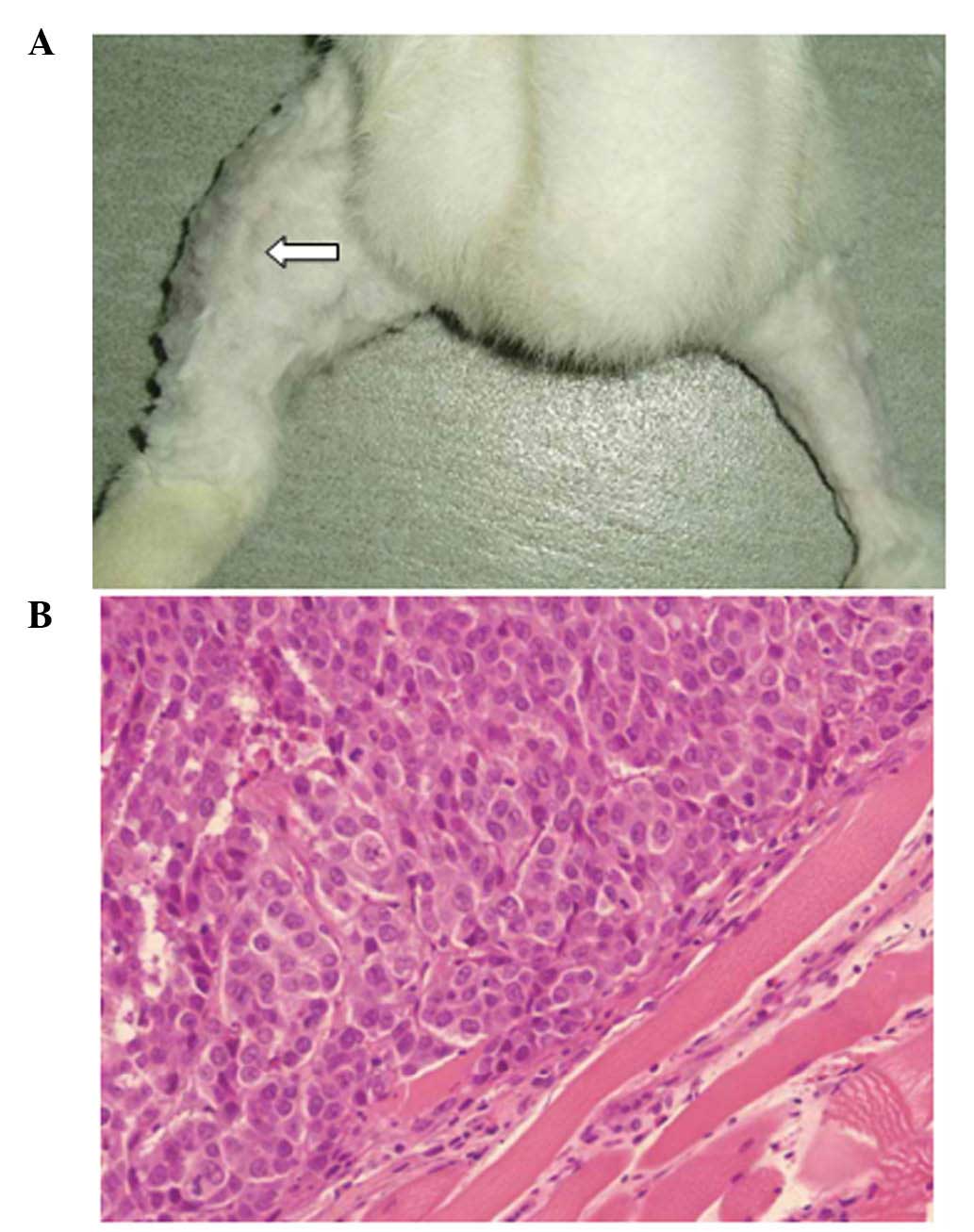
animals, a primary tumor was determined in the injected
gastrocnemius muscle (Fig. 1A and
B).
A significant difference was identified in the
volume of the IPTu among the 3 groups (all P<0.001; Table I). In addition, a significant
difference was identified in the volume of the IPTu between groups
1 and 3 (P<0.001) and groups 1 and 2 (P=0.041).
 | Table IIPTu, LRLN and RRLN volumes among the
3 groups. |
Table I
IPTu, LRLN and RRLN volumes among the
3 groups.
| Variable | Group 1 (n=12) | Group 2 (n=13) | Group 3 (n=13) | P-value |
|---|
| Mean ± SD IPTu
volume, cm3 | 24.62±4.97... | 31.64±5.52.. |
57.65±8.95f,g |
<0.001a,h |
| Mean ± SD LRLN
volumes, cm3 | 0.54±0.12. | 0.65±0.15 |
1.84±0.47f,g |
<0.001a,h |
| Mean ± SD RRLN
volumes, cm3 |
0.011±0.004e |
0.012±0.009e |
0.020±0.007e–g |
<0.001a,h |
| Metastases to LRLN, n
(%) | 7 (58.3) | 11 (84.6) | 13
(100)f |
0.019b,h |
| Median positive LRLN
(range) | 3 (2–3) | 3 (3–5) | 4
(4–5)f,g |
<0.001c,h |
| Metastases to the
lung, n (%) | 4 (33.3) | 5 (38.5) | 10 (76.9) |
0.055d |
| Mean ± SD lung
weight, g | 8.52±0.65 | 8.91±1.10 | 9.04±0.76 |
0.302a |
Metastasis of retroperitoneal lymph
nodes
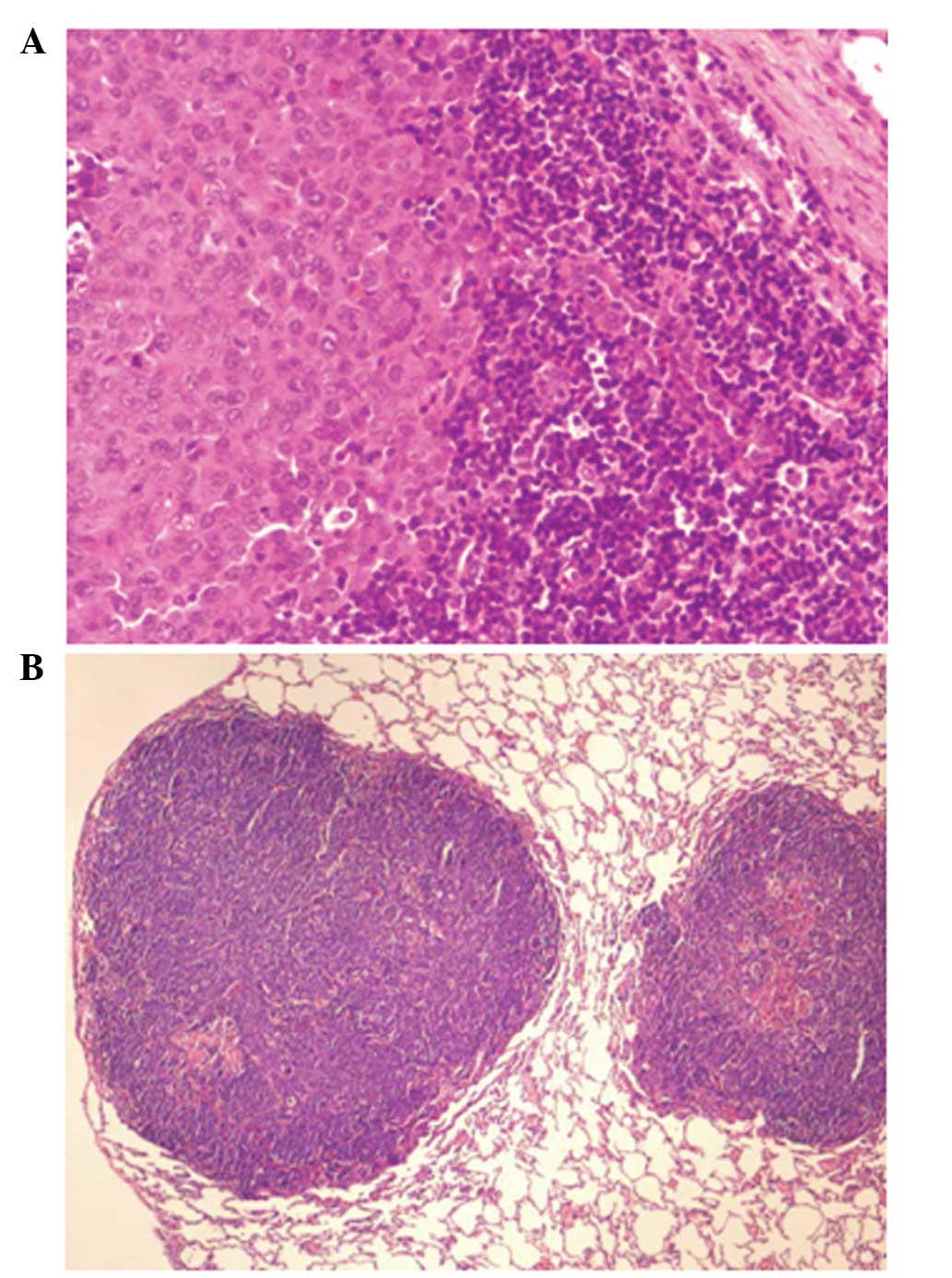
Metastasis was detected in the left retroperitoneal
lymph nodes (Fig. 2A). For these
lymph nodes the volume, rate of metastasis and number of positive
lymph nodes were found to be significantly different among the 3
groups (all P≤0.05; Table I). For
lymph node volume, group 3 was significantly different compared
with groups 1 and 2 (both P<0.001; Table I) while the volumes of groups 1 and
2 remained similar (P=1.00). A significant difference was
identified in the rates of metastasis (percentage of animals with
metastasis/day of sacrifice) to the left retroperitoneal lymph
nodes among the three groups (P=0.019). This significant difference
reflected differences between groups 1 and 3 (P=0.015), however,
the rate was found to be similar between groups 1 and 2 and groups
2 and 3 (P=0.202 and P=0.480, respectively). The differences among
the groups for the number of positive left retroperitoneal lymph
nodes resulted from a significant difference between groups 1 and 3
(P=0.001) and groups 2 and 3 (P<0.001). In addition, the number
of positive lymph nodes was found to be significantly different
between groups 1 and 2 (P=0.029). The rate of metastasis to the
left peritoneal lymph node was found to positively correlate with
lymph node volume (r=0.416; P=0.009; data not shown).
In contrast to the left retroperitoneal lymph nodes,
no metastasis was detected in the right retroperitoneal lymph
nodes, however, the volume of these lymph nodes differed among the
3 groups (P<0.001; Table I).
Significant differences were identified in the volumes between
groups 1 and 3 and groups 2 and 3 (both P<0.017), but lymph node
volumes were similar between groups 1 and 2 (P=1.000). For all 3
groups, on the day of sacrifice, the volume of the right peritoneal
lymph node was considerably smaller than that of the left (all
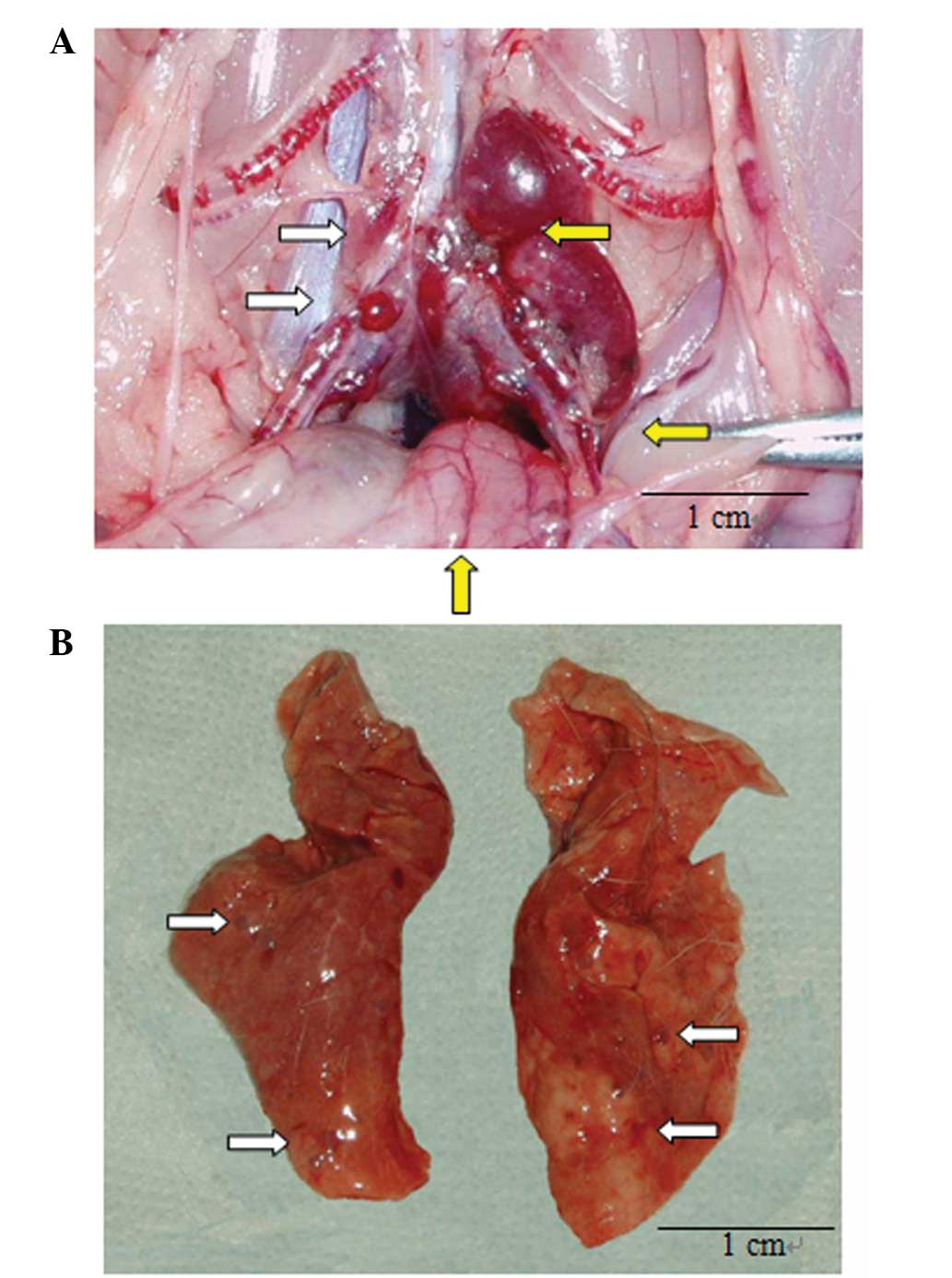
P<0.001; Fig. 3A).
Metastasis of the lungs
For the animals sacrificed on post-VX2 cell
inoculation day 25, macroscopic metastatic lesions were visible at
the edge of the bilateral lungs (Fig.
3B) and microscopic and macroscopic appearances of metastatic
carcinoma were present (Figs. 2B
and 3B). Slight differences were
found in the rates of metastases to the lung and wet weights of the
lungs among the 3 groups, but were not found to be statistically
significant (P=0.055; Table I).
However, a significantly positive correlation was found between the
rate of metastasis to the lung and the wet weight of the lung
(r=0.449; P=0.005).
Discussion
The present study has focused on the
characterization of a VX2 rabbit model for the metastasis of
squamous cell carcinoma to the retroperitoneal lymph nodes and
lungs. The pattern of metastasis of the primary tumor from the left
gastrocnemius to the left retroperitoneal lymph nodes and lungs is
comparable with that of patients with metastasizing types of
gynecologic cancer. By days 19, 22 and 25 following inoculation,
the percentages of animals which had developed primary tumors in
the gastrocnemius muscle were 92.3, 100 and 100%, respectively. The
proportion of animals with metastasis to the lungs or
retroperitoneal lymph nodes increased over the duration of the
study. For example, at days 19, 22 and 25, it was found that 58.3,
84.6 and 100% of the animals exhibited metastasis to the left
retroperitoneal lymph node, respectively and 33.3, 38.5 and 76.9%
exhibited lung metastasis, respectively. Similarly, the wet weight
of the lungs and volume of left retroperitoneal lymph nodes
increased with time, consistent with increased mass due to tumor
metastasis. In addition, the number of involved left
retroperitoneal lymph nodes increased in a time-dependent manner.
The rate of metastasis (number of animals with metastasis/day of
sacrifice) to the left retroperitoneal lymph node was found to
positively correlate with lymph node volume and wet lung weight
(r=0.416 and r=0.449, respectively). No metastasis was detected for
the duration of the 25-day study to the right retroperitoneal lymph
nodes and increase in the volume in this tissue was reduced
compared with that of the left retroperitoneal lymph nodes.
Since only approximately 77% of animals exhibited
lung metastasis at 25 days post-inoculation, an increased study
duration may be required to investigate lung metastasis in this
model system. The time-dependent increase in metastasis is likely
to reflect the natural progression of metastasis in this system.
Additional experiments are required to improve the characterization
of this system in order to gain insight into how changes in the
primary tumor and metastasis reflect the development of advanced
cancer in humans.
Lymph node volume was used in the present study as
an indication of metastasis based on the evidence that in the
clinical visual detection of enlarged lymph nodes, radiographic
images are an important indication of the possible metastasis.
However, further microscopic analysis is required for a positive
diagnosis since, as detected in the current study, enlarged lymph
nodes are not always indicative of metastasis. In the current
study, it was assumed for the lungs that the presence of a tumor is
likely to result in an increase in wet lung weight and a
correlation was identified between wet lung weight and
metastasis.
During the current study it was found that the left
popliteal fossa exhibited an enlarged lymph node 12 days
post-inoculation and the proportion of rabbits with metastasis to
this lymph node increased with time. At days 19, 22 and 25 the
percentage of animals with left popliteal fossa metastasis was
16.67, 38.46 and 61.54%, respectively (P=0.072; χ2
test). It is unclear why the rate of metastasis to the left
retroperitoneal lymph node was greater compared with that of the
left popliteal lymph nodes. To address this issue, future studies
are required to investigate the route of metastasis to these
tissues using lymphangiography and other technologies.
The location of transplantation may affect the site
of metastasis. Transplantation of VX2 tumor tissue into the
pyriform sinus submucosa of rabbits resulted in deep cervical lymph
node metastasis at 14 days post-transplantation (17). However, rates of submandibular
lymph node metastasis were found to be 60, 80 and 100% at 14, 21
and 28 days post-inoculation, respectively (17). Additionally, rates of paratracheal
lymph node metastasis were reported to be 0, 80 and 100% at 14, 21
and 28 days following inoculation, respectively (17). Mechanisms responsible for the
observed higher rates of metastases to the deep cervical lymph
nodes compared with local lymph nodes adjacent to orthotopic tumors
remain to be identified.
The VX2 tumor is a transplantable rabbit squamous
carcinoma characterized by rapid growth, stable physiological
characteristics and early metastasis (18). VX2 cells were selected primarily,
since they are the only existing cells to induce squamous cell
carcinoma in rabbits. It is known that adenocarcinoma is the most
common type of endometrial cancer (EC), however, squamous cell
carcinoma is observed in patients with partial EC. Furthermore,
squamous cell carcinoma and adenocarcinoma are epithelial tumors
and the biological characteristics of their retroperitoneal lymph
node metastasis are similar. Although potentially different from
adenocarcinoma, this squamous cell carcinoma is extremely malignant
and may be implanted at almost any site in rabbits. A primary tumor
model involving VX2 may be constructed following transplantation
into rabbit liver, kidney, lung, breast or uterus (8,9,16,19,20).
VX2 rabbit models of cancer have previously been used to
investigate lymph node metastasis (5,7,11–15).
However, the majority of these studies were imaging studies that
did not specifically characterize retroperitoneal lymph node
metastasis. One previous study characterized a VX2 rabbit model of
retroperitoneal lymphatic metastasis (7). Results of that study differed from
those of the present study as the VX2 tumor grafts were established
by orthotopic embedding of the VX2 cells into the endometrium. The
study found that 100% of the animals developed tumors and
metastasis to the retroperitoneal lymph nodes which occurred within
1 and 3 weeks. The rate of metastasis to the retroperitoneal lymph
nodes was similar to the results of the current study, however, the
endometrium-based model has highlighted inconsistent results
(unpublished data). Additional analyses with regard to the effect
of the location of the primary tumor on metastasis to the
retroperitoneal lymph nodes are required to establish the best VX2
rabbit model.
Metastases to the retroperitoneal lymph nodes and
lungs are a serious challenge to clinicians who treat various types
of gynecologic cancer. In patients with these types of cancer,
retroperitoneal metastasis is present in ≥22% of cases (21,22).
For the majority of types of gynecologic cancer, retroperitoneal
metastasis is a characteristic of the International Federation of
Gynecology and Obstetrics stage III and IV cancer classification
and is an important prognostic factor (1,2,23,24).
In patients with advanced stages of gynecologic cancer, control of
lung metastasis is also essential for patient quality of life and
increased survival rates (25).
Current treatments for retroperitoneal lymphatic and
lung metastases include systemic chemotherapy, reduction in visible
tumor burden and palliative therapy. However, results have
indicated that the treatment of nodal metastasis with chemotherapy
may not control the disease (26).
One study has previously reported that in ovarian cancer patients,
retroperitoneal lymph node involvement was present in 35% (35/100)
of those treated with surgery and 54% (15/28) and 36% (28/77) of
those who also received 3 or 6 courses of chemotherapy,
respectively (26). These
observations indicate that new treatment regimens for these types
of cancer are required.
One limitation of the present study was that a large
majority of ovarian carcinomas are adenocarcinomas, not squamous
carcinomas. Thus, the manner in which this VX2 model reflects the
biology of adenocarcinoma-derived metastases in various types of
gynecologic cancer is unclear. Additional studies are required to
understand how this rabbit model reflects the metastasis of
gynecologic cancer in humans.
In conclusion, the present study determined a unique
rabbit model of the metastasis of squamous carcinoma cells to the
retroperitoneal lymph nodes and lungs. This model is likely to be
useful in understanding how various types of cancer (regardless of
the primary tumor site) metastasize to the retroperitoneal lymph
nodes and lungs.
Acknowledgements
The authors would like to express their sincere
gratitude to Professor Qian Chaonan (State Key Laboratory of
Oncology in South China, Sun Yat-sen University Cancer Center,
Guangzhou, Guangdong, China) for providing valuable comments and
useful suggestions.
Abbreviations:
|
IPTu
|
implanted primary tumor
|
References
|
1
|
Pecorelli S, Benedet JL, Creasman WT and
Shepherd JH: FIGO staging of gynecologic cancer. 1994–1997 FIGO
Committee on Gynecologic Oncology. International Federation of
Gynecology and Obstetrics. Int J Gynaecol Obstet. 64:5–10.
1999.
|
|
2
|
Kanazawa K, Suzuki T and Tokashiki M: The
validity and significance of substage IIIC by node involvement in
epithelial ovarian cancer: impact of nodal metastasis on patient
survival. Gynecol Oncol. 73:2371999. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Szekeres T, Saiko P, Fritzer-Szekeres M,
Djavan B and Jäger W: Chemopreventive effects of resveratrol and
resveratrol derivatives. Ann NY Acad Sci. 1215:89–95. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Trencsenyi G, Kertai P, Bako F, et al:
Renal capsule-parathymic lymph node complex: a new in vivo
metastatic model in rats. Anticancer Res. 29:2121–2126.
2009.PubMed/NCBI
|
|
5
|
Servais EL, Colovos C, Bograd AJ, White J,
Sadelain M and Adusumilli PS: Animal models and molecular imaging
tools to investigate lymph node metastases. J Mol Med (Berl).
89:753–769. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Fu X and Hoffman RM: Human ovarian
carcinoma metastatic models constructed in nude mice by orthotopic
transplantation of histologically-intact patient specimens.
Anticancer Res. 13:283–286. 1993.
|
|
7
|
Chang S, Gu M, Wu F, et al: Establishment
of transplanted endometrial neoplasm model in rabbit and its
biological features. Prog Obstet Gynecol. 9:163–165. 2000.
|
|
8
|
Harima Y, Harima K, Hasegawa T, Shikata N
and Tanaka Y: Histopathological changes in rabbit uterus carcinoma
after transcatheter arterial embolization using cisplatin. Cancer
Chemother Pharmacol. 38:317–322. 1996. View Article : Google Scholar
|
|
9
|
Harima Y, Harima K, Hasegawa T, Shikata N
and Tanaka Y: Transcatheter arterial embolization with cisplatin:
apoptosis in VX2 tumour uterus transplants. Anticancer Res.
16:193–199. 1996.PubMed/NCBI
|
|
10
|
Ishibashi S, Sonoda K, Fujii K, Ishikawa
K, Shiraishi N and Kitano S: A convenient murine model for the
study of intra-abdominal lymph node metastasis. Oncol Rep.
12:115–118. 2004.PubMed/NCBI
|
|
11
|
Choi SH, Kim KH, Moon WK, Kim HC, Cha JH,
Paik JH and Chang KH: Comparison of lymph node metastases
assessment with the use of USPIO-enhanced MR imaging at 1.5 T
versus 3.0 T in a rabbit model. J Magn Reson Imaging. 31:134–141.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Choi SH, Moon WK, Hong JH, et al: Lymph
node metastasis: ultrasmall superparamagnetic iron oxide-enhanced
MR imaging versus PET/CT in a rabbit model. Radiology. 242:137–143.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Herborn CU, Lauenstein TC, Vogt FM,
Lauffer RB, Debatin JF and Ruehm SG: Interstitial MR lymphography
with MS-325: characterization of normal and tumor-invaded lymph
nodes in a rabbit model. AJR Am J Roentgenol. 179:1567–1572. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Wang L, Yao Q, Wang J, et al: MRI and
hybrid PET/CT for monitoring tumour metastasis in a metastatic
breast cancer model in rabbit. Nucl Med Commun. 29:137–143. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Tsuda N, Tsuji T and Kato N: Interstitial
magnetic resonance lymphography using
gadolinium-ethoxybenzyl-diethylenetriamine pentaacetic acid in
rabbits with lymph node metastasis. Invest Radiol. 40:306–312.
2005. View Article : Google Scholar
|
|
16
|
Rhee TK, Ryu RK, Bangash AK, et al: Rabbit
VX2 tumors as an animal model of uterine fibroids and for uterine
artery embolization. J Vasc Interv Radiol. 18:411–418. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Shen N, Wu H, Xu X, Wang J, Hoffman MR,
Rieves AL and Zhou L: Cervical lymph node metastasis model of
pyriform sinus carcinoma. ORL J Otorhinolaryngol Relat Spec.
71:129–134. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Kidd JG and Rous P: A transplantable
rabbit carcinoma originating in a virus induced papilloma and
containing the virus in masked or altered form. J Exp Med.
71:813–838. 1940. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Horkan C, Ahmed M, Liu Z, Gazelle GS,
Solazzo SA, Kruskal JB and Goldberg SN: Radiofrequency ablation:
effect of pharmacologic modulation of hepatic and renal blood flow
on coagulation diameter in a VX2 tumor model. J Vasc Interv Radiol.
15:269–274. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Chen JH, Li Y, Yao Q, Ling R, Wang L, Li
KZ, Wang Z and Chen T: Treatment of rabbits bearing advanced VX2
tumors in the mammary gland with nano-sized liposomal adriamyc in
administered by various routes. Natl Med J China. 85:3039–3042.
2005.PubMed/NCBI
|
|
21
|
Maggioni A, Benedetti Panici P, Dell'Anna
T, et al: Randomised study of systematic lymphadenectomy in
patients with epithelial ovarian cancer macroscopically confined to
the pelvis. Br J Cancer. 95:699–704. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Angioli R, Plotti F, Palaia I, et al:
Update on lymphadenectomy in early and advanced ovarian cancer.
Curr Opin Obstet Gynecol. 20:34–39. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Bolger BS, Dabbas M, Lopes A and Monaghan
JM: Prognostic value of preoperative squamous cell carcinoma
antigen level in patients surgically treated for cervical
carcinoma. Gynecol Oncol. 65:309–313. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Shigematsu N, Ito H, Nishiguchi I, et al:
Prognostic factors of cervical carcinoma treated with postoperative
radiotherapy. Nippon Igaku Hoshasen Gakkai Zasshi. 57:28–33.
1997.(In Japanese).
|
|
25
|
Peiretti M, Zapardiel I, Zanagnolo V,
Landoni F, Morrow CP and Maggioni A: Management of recurrent
cervical cancer: a review of the literature. Surg Oncol.
21:e59–e66. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Morice P, Joulie F, Rey A, et al: Are
nodal metastases in ovarian cancer chemoresistant lesions? Analysis
of nodal involvement in 105 patients treated with preoperative
chemotherapy. Eur J Gynaecol Oncol. 25:169–174. 2004.
|