Introduction
Asthma is a common chronic inflammatory disease with
an incidence that has markedly increased over the past two decades.
In addition, the chronic inflammation apparent in a given patient
is often associated with the remodeling of the airway structure,
which may impair lung function (1,2).
These changes include peribronchial fibrosis, fibroblast
proliferation and conversion to myofibroblasts, and smooth muscle
hypertrophy (3). Myofibroblasts
have been suggested to be pivotal factors in the pathogenesis of
peribronchial fibrosis. However, the origin of the myofibroblast,
the primary effector cell of peribronchial fibrosis, has not yet
been elucidated. Three hypotheses have been proposed regarding the
cellular origin of the myofibroblast. The first, and historically
most prevalent, hypothesis postulates that resident peribronchial
fibroblasts respond to a variety of stimuli during fibrogenic
responses and differentiate into myofibroblasts. The second
hypothesis postulates that myofibroblasts are derived from bone
marrow progenitor cells (4), while
the third possible source of fibroblasts and/or myofibroblasts to
be proposed involves epithelial cells, through the process of
epithelial-mesenchymal transition (EMT) (5).
EMT is an orchestrated series of events, in which
differentiated epithelial cells undergo a phenotypic transition to
mesenchymal cells, often fibroblasts and myofibroblasts (5,6).
During EMT, the epithelial cells lose intracellular junctions,
leading to dissociation from the surrounding cells, acquire
mesenchymal-like characteristics and become able to migrate away
from the original location (7).
This important process was initially recognized during embryonic
development and has more recently been identified in tumor
progression and organ fibrosis (8). To date, studies have suggested that
kidney proximal tubule epithelial cells undergo EMT to induce
interstitial fibrosis in progressive renal disease (9). In the fibrotic kidney, ~36% of new
fibroblasts arise from tubular epithelial cells (10). EMT may be triggered by different
signalling molecules, such as transforming growth factor-β1
(TGF-β1), epidermal growth factor, fibroblast growth factor,
hepatocyte growth factor and bone morphogenetic proteins (11).
TGF-β1 is a profibrotic cytokine that has been
indicated to be an important factor promoting the structural
changes of airway remodeling in asthma (12). Snail, a zinc-finger transcription
factor, has been characterized as a key EMT regulator (13). It has been shown that Snail binds
to specific DNA sequences, known as E-boxes, in the promoter of the
E-cadherin gene and represses the transcription of E-cadherin
(14). Therefore, the
downregulation of the cell-cell adhesion protein E-cadherin has
been considered to be characteristic of EMT. Knockout mice
deficient in Snail die at gastrulation due to a failure to undergo
a complete EMT process, which leads to the formation of an abnormal
mesodermal layer that maintains E-cadherin expression. In certain
epithelial tumor cell lines, Snail-regulated EMT promotes cell
motility and invasion (15,16).
An inverse correlation between E-cadherin and Snail expression has
been observed in cultured epithelial lines established from breast
cancer, pancreatic carcinoma and colon cancer. The silencing of
Snail by stable RNA interference in epithelial cells was shown to
attenuate the complete EMT, which was associated with the
upregulation of E-cadherin, the downregulation of mesenchymal
markers and the inhibition of invasion (17).
In the present study, we showed that bronchial
epithelial cell transformation into myofibroblasts was associated
with airway remodeling in asthma and that Snail transcription
factor may be essential in TGF-β1-mediated EMT in bronchial
epithelial cells. These data suggested that bronchial epithelial
cells may be the source of myofibroblasts in peribronchial fibrosis
and may promote airway remodeling in asthma.
Materials and methods
Reagents
The chicken egg ovalbumin (OVA) used in the study
was purchased from Sigma-Aldrich (St. Louis, MO, USA). E-cadherin,
α-smooth muscle actin (α-SMA), vimentin, fibronectin, Snail,
glyceraldehyde 3-phosphate dehydrogenase (GAPDH) and secondary
antibodies were purchased from Santa Cruz Biotechnology, Inc.
(Santa Cruz, CA, USA). TGF-β1 and a TGF-β1 enzyme-linked
immunosorbent assay (ELISA) kit were obtained from R&D Systems
(Minneapolis, MN, USA), while any other laboratory reagents were
purchased from Sigma.
Cell line and culture
The 16HBE human bronchial epithelial cells used in
this study were obtained from the Cancer Research Institute of
Beijing, China. The cells were cultivated in T75 tissue culture
flasks in RPMI-1640 supplemented with 10% fetal bovine serum (FBS),
100 IU/ml penicillin, 100 μg/ml streptomycin, 2 mM L-glutamine and
20 mM hydroxyethyl piperazine ethanesulfonic acid, and incubated in
a humidified incubator containing 5% CO2 at 37°C.
Sensitization and antigen challenge
Twenty-four healthy female BABL/c mice, weighing
18–24 g, were randomly divided into the normal control and asthma
groups. The mouse model of asthma was established using OVA. The
mice were sensitized on days 0, 7 and 14 by intraperitoneal
injection of 20 μg OVA emulsified in 1 mg aluminum hydroxide, in a
total volume of 0.2 ml. Seven days subsequent to the final
sensitization, the mice were exposed to 1% OVA aerosol for ≤30 min
every other day for eight weeks. The 1% OVA aerosol was generated
using a compressed air atomizer driven by filling a Perspex
cylinder chamber (diameter 50 cm, height 50 cm) with a nebulized
solution. Saline was used in the control group instead of OVA. The
study protocol was approved by the Medical Ethics and Human
Clinical Trial Committee at the Qingdao University and all the
experiments described were performed in accordance with the
regulations of the Centre of Animal Experiments of Qingdao
University (Qingdao. China).
Tissue samples
Twenty-four hours subsequent to the last antigen
challenge, the mice were sacrificed and the lungs were removed. The
tissues from the left lung were directly obtained from the surgical
suite and immediately fixed in 10% buffered formalin, prior to
being embedded in paraffin. Following this, 5-μm sections were
prepared and stained with hematoxylin and eosin (H&E). The
thickness of the extracellular matrix (ECM) was determined
subsequent to the staining of the tissue sections with H&E. The
average of 10 independent measurements was calculated for each
section and the data were summarized.
Immunohistochemistry
The expression of TGF-β1 was assessed using
semi-quantitative immunohistochemistry. Following
deparaffinization, the sections were incubated in 0.01 mol/l citric
acid buffer (pH 6.0) for 15 min in a microwave for antigen
retrieval. The sections were then cooled and incubated in 3 g/l
H2O2 for 30 min, in order to inactivate
endogenous peroxidase, prior to being blocked with 1:10 normal
horse serum for 30 min. Following this, the supernatant was
discarded. Primary anti-mouse TGF-β1 (1:300 dilution) was added
overnight at 4°C and then biotinylated goat anti-rat secondary
antibody and streptavidin horseradish peroxidase were added to the
slides. The slides were subsequently incubated for 30 min at room
temperature. The staining was completed by incubation with
diaminobenzidine chromogen solution at room temperature. The
stained cells were then mounted and viewed using light
microscopy.
Phase contrast microscopy
The phenotypic changes of the bronchial epithelial
cells were assessed using phase contrast microscopy. The cultured
bronchial epithelial cells were either treated with recombinant
TGF-β1 or left untreated (control) and the morphological changes
were then visualized using phase contrast microscopy. The images
were collected using a Nikon inverted microscope (Nikon Corp.,
Tokyo, Japan).
Small interfering RNA (siRNA)
treatment
Bronchial epithelial cells were grown to 70%
confluence on the culture dishes and the transient transfection was
performed with specific stealth siRNA against Snail or control
siRNA using Lipofectamine 2000, in accordance with the
manufacturer’s instructions. The target sequences for Snail were
5′-GCGAGCTGCAGGACTCTAA-3′ (no. 1), and 5′-GCGAGT GGTTCTTCTGCGCTA-3′
(no. 2); the scrambled control sequence was
5′-CACATGTTCCGATCTCGGC-3′ (all synthesized by Qiagen N.V., Venlo,
The Netherlands). Following 6 h incubation with the RNA-complex,
the medium was replaced and 2 ml fresh medium containing 10% FBS
was added. The cells were treated and harvested at the indicated
times subsequent to the transfection.
Semi-quantitative reverse
transcription-polymerase chain reaction (qPCR)
Total RNA was isolated from the cells using
Trizol® Reagent (Invitrogen Life Technologies, Carlsbad,
CA, USA), according to the manufacturer’s instructions. Total
cellular RNA (1 μg) was then reverse-transcribed into cDNA for the
PCR amplification using a kit from Sigma. The sequences for the PCR
primers used were: Snail sense, 5′-CAAGGAATACCTCAGCCTGG-3′ and
antisense, 5′-ATTCACATCCAGCACATCCA-3′; E-cadherin sense,
5′-TGGGTTATTCCTCCCATCAG-3′ and antisense,
5′-TTTGTCAGGGAGCTCAGGAT-3′; vimentin sense,
5′-CGCTTCGCCAACTACATC-3′ and antisense, 5′-GGTC AGGCTTGGAAACATC-3′;
fibronectin sense, 5′-GCA CCAACTGACCTGAAG-3′ and antisense,
5′-GCCACCATAA GTCCTGATAC-3′; and β-actin sense, 5′-CACCAACTGGGA
CGACAT-3′ and antisense, 5′-ACAG CCTGGATAGCAACG-3′. Amplification
consisted of an initial 5 min incubation at 95°C and then 30 cycles
of amplification with 30 sec of denaturation at 95°C, amplification
for 30 sec at 56°C and extension for 60 sec at 72°C. The final
extension was set for 10 min at 72°C. Data were expressed as the
relative differences between the control and treated cells
following normalization to β-actin expression.
Western blot analysis
Total cellular protein was extracted using a lysis
buffer and quantified using protein quantification reagents from
Bio-Rad (Hercules, CA, USA). Following this, 100 μg of the protein
was suspended in 5X reducing sample buffer, boiled for 5 min,
electrophoresed on 10% sodium dodecyl sulfate-polyacrylamide gel
electrophoresis (SDS-PAGE) gels and then transferred to
polyvinylidene difluoride membranes using electroblotting. The
membranes were subsequently blocked in 1% bovine serum albumin
(BSA)/0.05% Tween-20/phosphate-buffered saline (PBS) solution
overnight at 4°C and were then incubated with the primary antibody
for 24 h. A horseradish peroxidase-labeled immunoglobulin G (IgG)
was used as the secondary antibody. Following this, the blots were
developed by incubation in a chemiluminescent substrate and
exposure to X-ray film. The blots were reprobed for GAPDH to ensure
equal protein loading in each lane.
Statistical analysis
Data are expressed as the mean ± standard deviation.
Statistical comparisons of the data from the various groups were
performed using the Student’s t-test. Differences between groups
were considered statistically significant at P<0.05.
Results
Pathological alteration and TGF-β1
expression in lung tissue
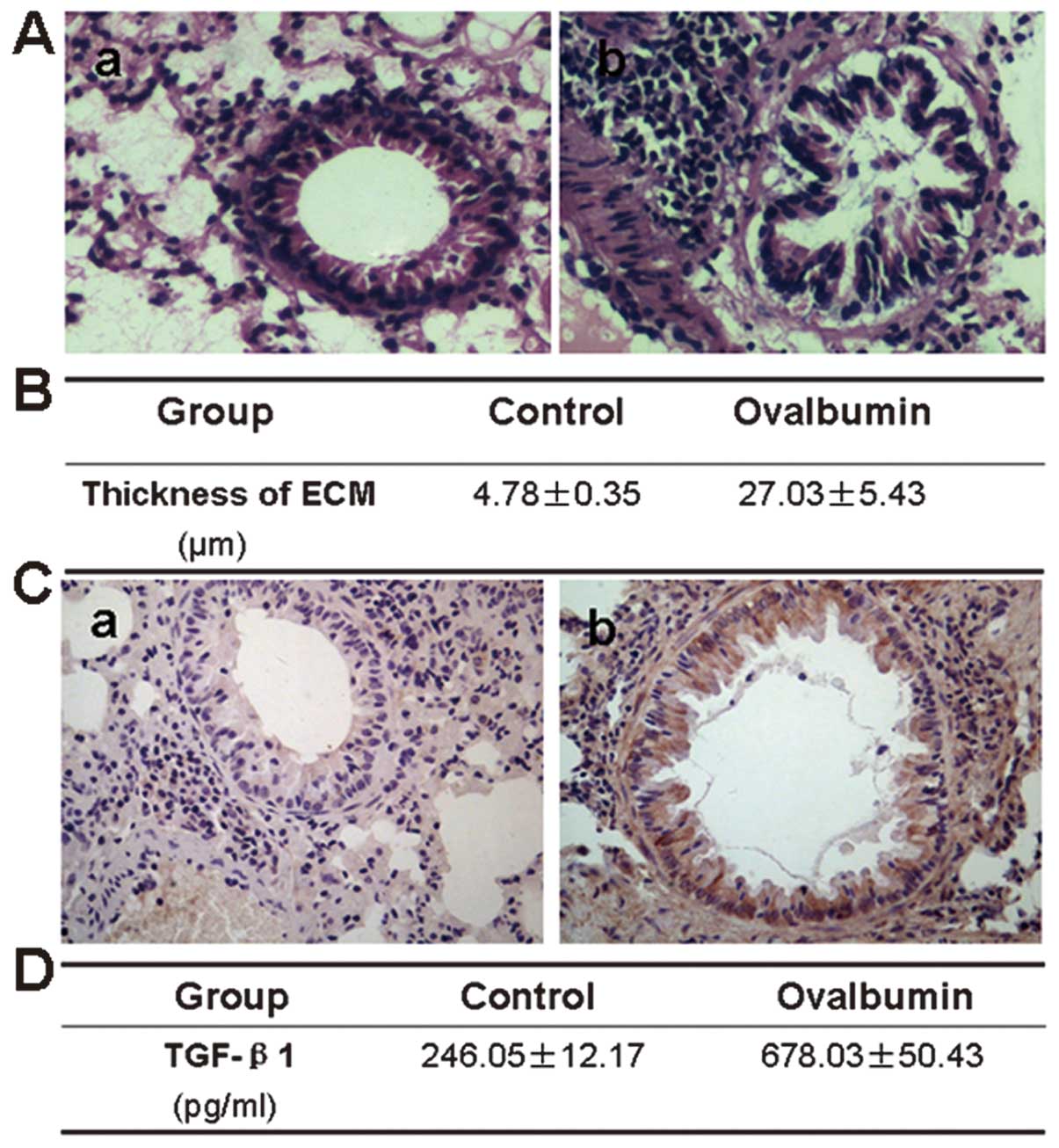
The airway wall in the mouse lung tissue was
stained, prior to undergoing a histological examination. There was
a little collagen deposition in the airway wall of the normal mice,
while in the asthma model mice the deposition increased
significantly, with an extensive distribution in the airway wall.
TGF-β1 protein was observed to be expressed in numerous types of
lung cells in the model mice, including airway epithelial cells,
fibroblasts, smooth muscle cells, vascular endothelial cells and
infiltrative inflammatory cells, while there was a low expression
of TGF-β1 protein in the normal mice. TGF-β1 protein levels were
also assayed in the bronchoalveolar lavage fluid, which
demonstrated that the TGF-β1 levels were significantly higher in
the asthmatic mice than those in the control group (Fig. 1).
TGF-β1 induces mesenchymal phenotypic
transformation in bronchial epithelial cells
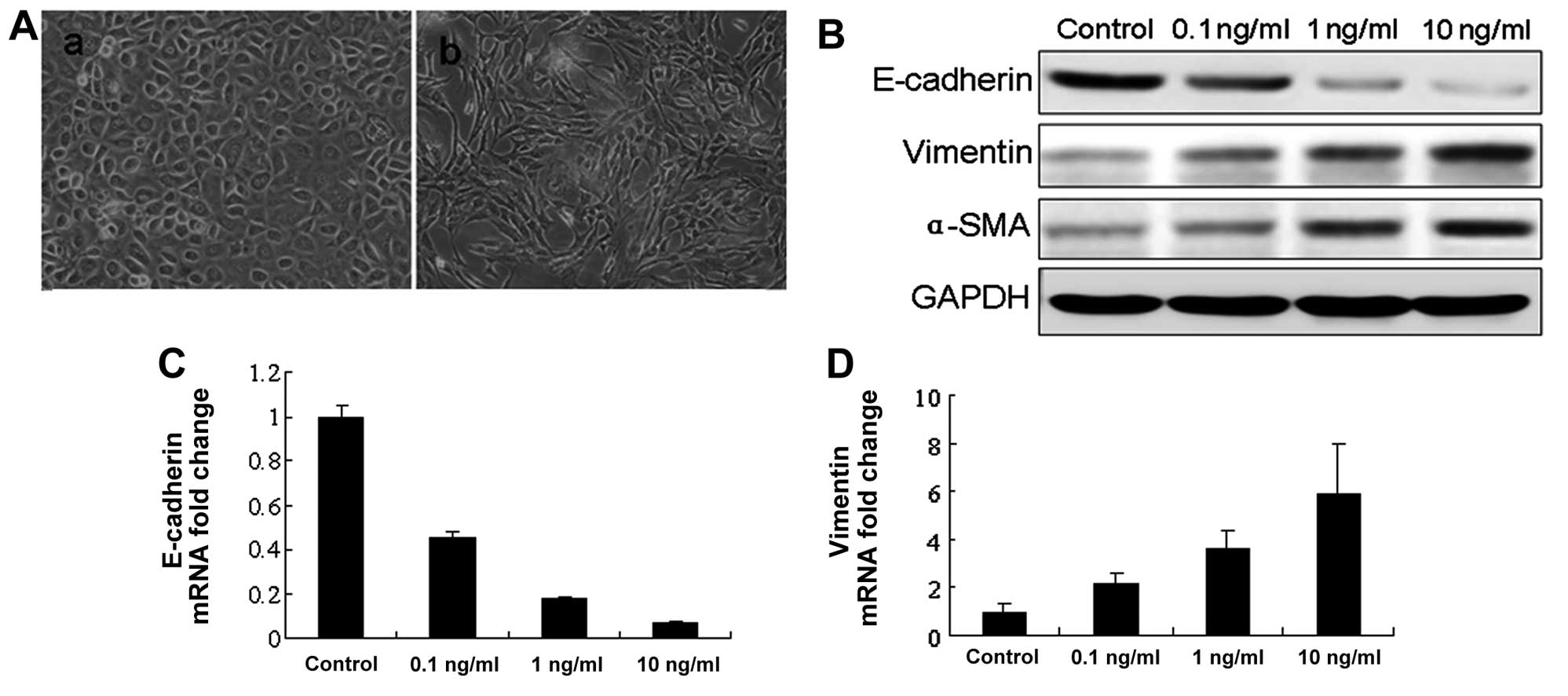
Bronchial epithelial cells were activated with
recombinant TGF-β1 and the morphological changes were observed. The
epithelial cells cultured in serum-free medium (control) showed a
typical polygonal and cobblestone-monolayer morphology. In the
bronchial epithelial cells activated with TGF-β1, phenotypic
changes were observed 48 h subsequent to TGF-β1 activation. When
compared with the control cells, the TGF-β1-activated epithelial
cells exhibited an elongated, spindle-shaped morphology,
characteristic of fibroblasts. These morphological changes were
associated with the loss of epithelial characteristics, such as
E-cadherin, and with the acquisition of certain mesenchymal
characteristics, including an increase in α-SMA and vimentin
expression. α-SMA is considered a marker of myofibroblasts, which
are the cell types present in EMT. The acquisition of a
fibroblastic morphology and mesenchymal markers suggested that the
epithelial cells had undergone EMT following treatment with TGF-β1
(Fig. 2).
TGF-β1 stimulation induces the
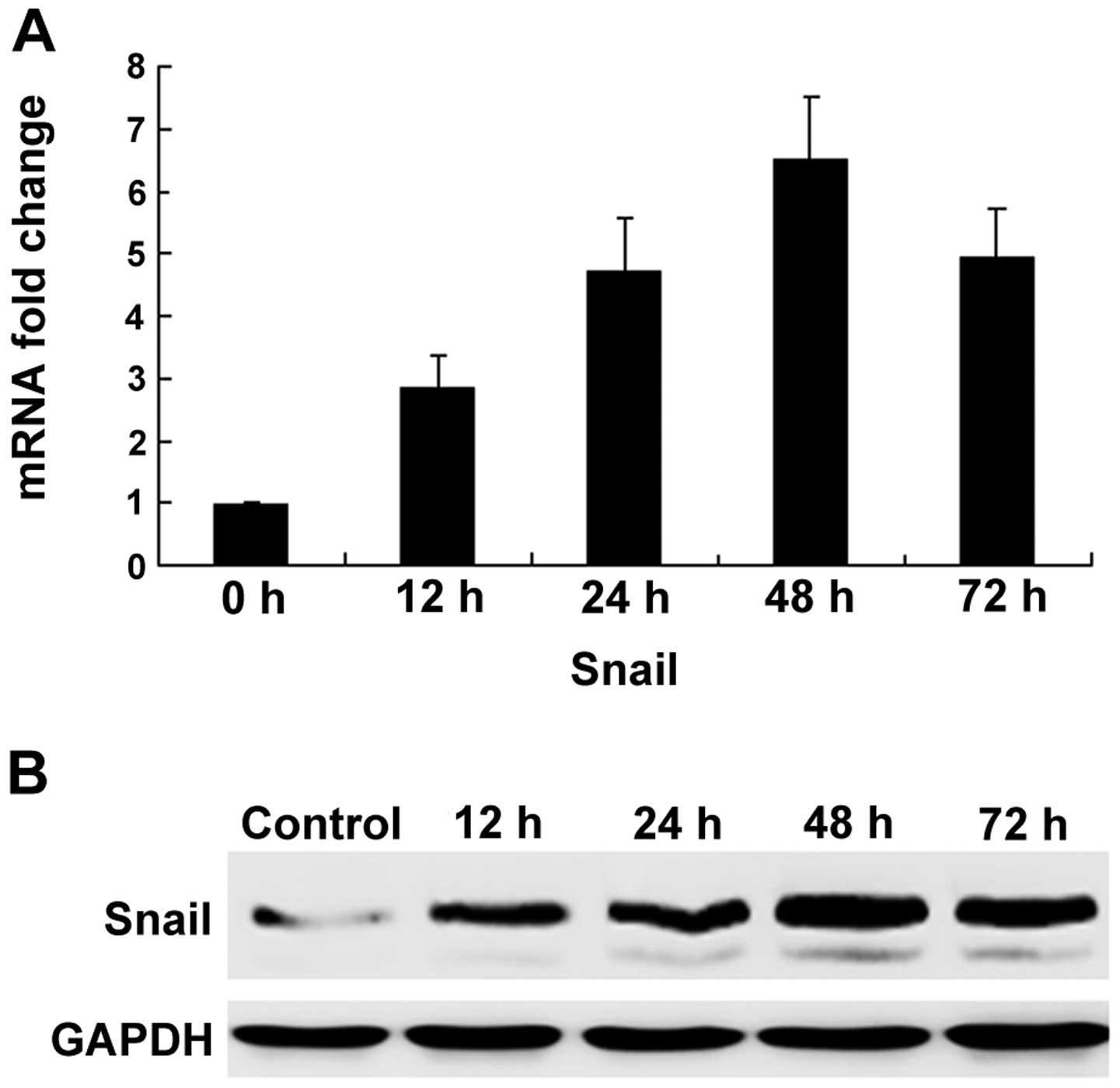
upregulation of Snail
The zinc-finger transcription factor Snail is
critical in the regulation of EMT during tumor progression and
organ fibrosis. As a result of the correlation between Snail and
EMT, we investigated whether the expression of Snail was
upregulated by TGF-β1 in bronchial epithelial cells. Compared with
the untreated cells, TGF-β1 increased Snail mRNA expression 12 h
subsequent to treatment, with the highest level of expression being
reached 48 h subsequent to treatment. The inducing effects of
TGF-β1 on Snail protein levels were further demonstrated using
western blot analysis (Fig.
3).
Silencing Snail blocks TGF-β1-induced
mesenchymal transformation in bronchial epithelial cells
To further elucidate whether Snail was involved in
TGF-β1-mediated EMT in bronchial epithelial cells, siRNAs were used
to knock down the Snail gene in bronchial epithelial cells. As
shown in Fig. 4A, siRNAi-Smad2#1
caused a highly significant knockdown of Snail when compared with
the control siRNA (Fig. 4A).
Following the silencing of Snail in bronchial epithelial cells
using siRNA-Snail, the protein expression levels of E-cadherin and
α-SMA in the total cell lysates were analyzed using western blot
analysis. It was observed that the expression of epithelial markers
was significantly increased, whereas the levels of mesenchymal
markers were decreased (Fig.
4B).
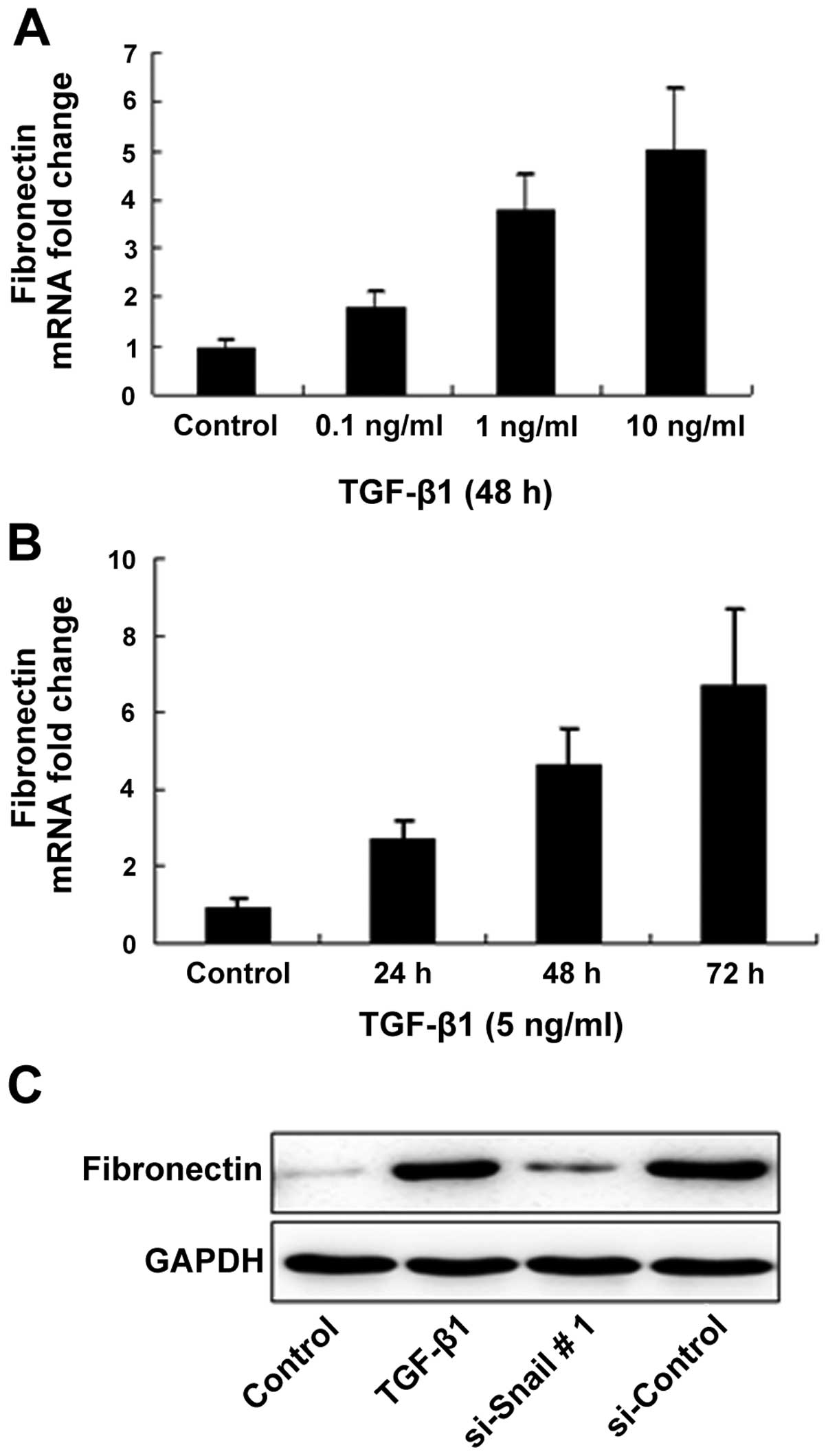
TGF-β1 increases fibronectin expression
in bronchial epithelial cells
To evaluate our hypothesis and investigate whether
the phenotypic changes observed following the treatment of
bronchial epithelial cells with TGF-β1 were due to ECM protein
synthesis, we examined the expression of fibronectin in epithelial
cells activated with TGF-β1 at the transcriptional and
translational levels. As expected, TGF-β1 increased fibronectin
mRNA production by bronchial epithelial cells in a dose- and
time-dependent manner. The treatment of the bronchial epithelial
cells with siRNA-Snail prior to TGF-β1 stimulation significantly
inhibited the expression of fibronectin compared with the control
cells (Fig. 5).
Discussion
In this study, substantially thickened lung tissue
and extensive fibrosis were observed in OVA-sensitized mice, which
was interrelated with TGF-β1 expression in bronchoalveolar lavage
fluid. In vitro experiments demonstrated that bronchial
epithelial cells responded to TGF-β1 by transforming into
myofibroblasts, indicating that these cells are capable of
initiating and modulating the expression of epithelial markers to
mesenchymal markers, in order to undergo EMT. Due to the fact that
bronchial epithelial cells are able to transform into a
fibroblast-like phenotype and secrete ECM compounds in response to
TGF-β1, it is possible that epithelial cells have a function in
peribronchial fibrosis and promote airway remodeling in asthma.
The growth factor TGF-β1 contributes to the
development of fibroblastic foci in the bronchial region, and a
number of studies have focused on the role of TGF-β1 as the key
promoter of peribronchial fibrosis (12,18).
However, the precise mechanisms responsible for the formation of
fibroblastic foci are unknown and the origin of myofibroblasts,
critical elements in the process of fibrosis, is not clearly
understood. It has been suggested that epithelial cells, through
the process of EMT, may be a possible source of myofibroblasts
(5). Our results have shown that
bronchial epithelial cells underwent a transition from a typical
epithelial morphology to fibroblast-like cells following TGF-β1
treatment, which was accompanied by the increased expression of
α-SMA and another mesenchymal marker, vimentin. Of note, TGF-β1 was
unable to induce EMT without disrupting the integrity of cell-cell
contact, indicating the involvement of E-cadherin in
TGF-β1-mediated EMT (19).
E-cadherin is the main component of adhesive junctions and is
important in the assembly of junctional complexes and the
maintenance of epithelial cell morphology. The downregulation of
E-cadherin has been accepted as a characteristic of EMT (20). In the present study, the data
demonstrated that bronchial epithelial cells underwent a transition
from an epithelial to a mesenchymal phenotype following activation
with TGF-β1, which was characterized by a decrease in E-cadherin
expression and an increase in vimentin and α-SMA expression.
Furthermore, TGF-β1 treatment caused a significant change in
bronchial epithelial cell morphology, with a transition from a
typical epithelial morphology to a mesenchymal spindle-shaped
morphology.
The E-cadherin suppressor, Snail, has been shown to
be a key regulator of EMT in normal development and tumor
progression (21). To the best of
our knowledge, we have demonstrated for the first time that the
Snail transcription factor was upregulated and that, subsequently,
E-cadherin was downregulated during the EMT of bronchial epithelial
cells. The increasing expression levels of the mesenchymal markers
vimentin and α-SMA were consistent with the TGF-β1-induced
upregulation of Snail. Our interest in Snail was further enhanced
by the results from the knockdown experiments, due to the fact that
the downregulation of Snail by a siRNA-expressing vector reversed
the EMT of bronchial epithelial cells in response to TGF-β1
treatment. These results demonstrated that the role of TGF-β1 as an
inducer of EMT was, at least in part, dependent on the functions of
Snail.
Airway remodeling is one of the pathophysiological
characteristics of asthma, and its main pathological changes
include subepithelial fibrosis formation and increased collagen
deposition on the airway wall (22). In order to confirm the effect of
EMT on peribronchial fibrosis, we showed that TGF-β1 increased
fibronectin mRNA production by bronchial epithelial cells in a
dose- and time-dependent manner; however, Snail siRNA transfection
suppressed fibronectin protein expression in TGF-β1-treated
bronchial epithelial cells. These data further indicated that EMT
was a key factor in peribronchial fibrosis and promoted airway
remodeling in asthma.
Results of the present study showed that the
concentration of TGF-β1 in the bronchoalveolar lavage fluid was
lower than that used in vitro to treat bronchial epithelial
cells. This may have been due to the natural differences between
in vivo and in vitro experiments, with the latter
being acute and artificial. In addition, a number of other factors
may contribute to the effect.
In conclusion, the present study showed that
bronchial epithelial cells stimulated by TGF-β1 exhibited
phenotypic and molecular alterations, characteristic of EMT,
through the upregulated expression of Snail, a transcription factor
involved in EMT. We also showed that myofibroblasts originating
from bronchial epithelial cells may contribute to peribronchial
fibrosis in asthma. These results may enhance our understanding of
the molecular mechanisms underlying the pathogenesis of
peribronchial fibrosis, which may provide opportunities to prevent
and treat asthma effectively.
Acknowledgements
This study was supported by the Natural Science
Foundation of Shandong Province (no. Y2007C113) and the Science and
Technique Foundation of Shandong Province (no. 2010GWZ20216).
References
|
1
|
Schlender A, Alperin PE, Grossman HL and
Sutherland ER: Modeling the impact of increased adherence to asthma
therapy. PLoS One. 7:e511392012. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Di Giampaolo L, Quecchia C, Schiavone C,
Cavallucci E, Renzetti A, Braga M and Di Gioacchino M:
Environmental pollution and asthma. Int J Immunopathol Pharmacol.
24(Suppl 1): S31–S38. 2011.
|
|
3
|
Yamauchi K and Inoue H: Airway remodeling
in asthma and irreversible airflow limitation - ECM deposition in
airway and possible therapy for remodeling. Allergol Int.
56:321–329. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Epperly MW, Guo H, Gretton JE and
Greenberger JS: Bone marrow origin of myofibroblasts in irradiation
pulmonary fibrosis. Am J Respir Cell Mol Biol. 29:213–224. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Yanez-Mo M, Lara-Pezzi E, Selgas R, et al:
Peritoneal dialysis and epithelial-to-mesenchymal transition of
mesothelial cells. N Engl J Med. 348:403–413. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Lv ZD, Na D, Ma XY, Zhao C, Zhao WJ and Xu
HM: Human peritoneal mesothelial cell transformation into
myofibroblasts in response to TGF-β1 in vitro. Int J Mol
Med. 27:187–193. 2011.PubMed/NCBI
|
|
7
|
Lv ZD, Kong B, Li JG, Qu HL, Wang XG, Cao
WH, Liu XY, Wang Y, Yang ZC, Xu HM and Wang HB: Transforming growth
factor-β1 enhances the invasiveness of breast cancer cells by
inducing a Smad2-dependent epithelial-to-mesenchymal transition.
Oncol Rep. 29:219–225. 2013.
|
|
8
|
Weber CE, Li NY, Wai PY and Kuo PC:
Epithelial-mesenchymal transition, TGF-β, and osteopontin in wound
healing and tissue remodeling after injury. J Burn Care Res.
33:311–318. 2012.
|
|
9
|
Carew RM, Wang B and Kantharidis P: The
role of EMT in renal fibrosis. Cell Tissue Res. 347:103–116. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Grgic I, Duffield JS and Humphreys BD: The
origin of interstitial myofibroblasts in chronic kidney disease.
Pediatr Nephrol. 27:183–193. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Chapman HA: Epithelial-mesenchymal
interactions in pulmonary fibrosis. Annu Rev Physiol. 73:413–435.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Qu ZH, Yang ZC, Chen L, Lv ZD, Yi MJ and
Ran N: Inhibition airway remodeling and transforming growth
factor-β1/Smad signaling pathway by astragalus extract in asthmatic
mice. Int J Mol Med. 29:564–568. 2012.
|
|
13
|
Reinke LM, Xu Y and Cheng C: Snail
represses the splicing regulator epithelial splicing regulatory
protein 1 to promote epithelial-mesenchymal transition. J Biol
Chem. 287:36435–36442. 2012. View Article : Google Scholar
|
|
14
|
Lander R, Nordin K and LaBonne C: The
F-box protein Ppa is a common regulator of core EMT factors Twist,
Snail, Slug, and Sip1. J Cell Biol. 194:17–25. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Yu LX, Zhou L, Li M, Li ZW, Wang DS and
Zhang SG: The Notch1/cyclooxygenase-2/Snail/E-cadherin pathway is
associated with hypoxia-induced hepatocellular carcinoma cell
invasion and migration. Oncol Rep. 29:362–370. 2013.PubMed/NCBI
|
|
16
|
Myong NH: Loss of E-cadherin and
acquisition of vimentin in epithelial-mesenchymal transition are
noble indicators of uterine cervix cancer progression. Korean J
Pathol. 46:341–348. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Li H, Wang H, Wang F, Gu Q and Xu X: Snail
involves in the transforming growth factor β1-mediated
epithelial-mesenchymal transition of retinal pigment epithelial
cells. PLoS One. 6:e233222011.
|
|
18
|
Qin XJ, Zhang GS, Zhang X, Qiu ZW, Wang
PL, Li YW, Li W, Xie QM, Ke YH, Lee JJ and Shen HH: Protein
tyrosine phosphatase SHP2 regulates TGF-β1 production in airway
epithelia and asthmatic airway remodeling in mice. Allergy.
67:1547–1556. 2012.
|
|
19
|
Yook JI, Li XY, Ota I, Fearon ER and Weiss
SJ: Wnt-dependent regulation of the E-cadherin repressor snail. J
Biol Chem. 280:11740–11748. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Franz M, Spiegel K, Umbreit C, et al:
Expression of Snail is associated with myofibroblast phenotype
development in oral squamous cell carcinoma. Histochem Cell Biol.
131:651–660. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Ohkubo T and Ozawa M: The transcription
factor Snail downregulates the tight junction components
independently of E-cadherin downregulation. J Cell Sci.
117:1675–1685. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Zhang WX and Li CC: Airway remodeling: a
potential therapeutic target in asthma. World J Pediatr. 7:124–128.
2011. View Article : Google Scholar : PubMed/NCBI
|