Introduction
Lung cancer is the leading cause of mortality
worldwide (1), with ~80% of lung
cancers being non-small-cell lung cancers. Previously, it was
demonstrated that lung cancer, not only has a high incidence of
recurrence, but also poor overall survival (2). However, in spite of substantial
progress made with traditional therapy, there remains a lack of
effective therapeutic strategies. Therefore, novel potential
therapeutic treatments should be developed.
Based on the concept of the tumor immune response
(3–5), development of novel agents is crucial
in order to re-engage the immune system to recognize and kill tumor
cells. Exosomes secreted by immune or tumor cells have drawn
particular attention and have been suggested to have the potential
for exploitation in tumor immunotherapy (6,7).
Exosomes are a type of nano-sized membrane vesicle with a diameter
of 50–90 nm, first identified in the process of reticulocyte
maturation, that discard unwanted membrane proteins such as the
transferrin receptor (8–10). More recently, it has been
demonstrated that exosomes are secreted by a wide range of cell
types such as astrocytes, neurons and epithelial cells, in addition
to tumor and immune cells [reviewed in (11)]. Exosomes contain various types of
proteins that are inherited from their parental cells, such as
tetraspanins and chaperones [Heat shock protein (Hsp) 70 and Hsp90]
(12). Moreover, exosomes derived
from different cell types exhibit selective enrichment of specific
proteins. For example, exosomes secreted from antigen-presenting
cells were enriched in major histocompatibility complex (MHC) class
I and II molecules and co-stimulatory molecules (13). In addition to proteins, functional
RNAs and lipids have also been observed in exosomes (14,15).
The interaction between exosomes and target cells may result in the
direct stimulation of target cells. Thus, exosomes are suggested to
be important in cell-to-cell communication and are considered ideal
agents to engage the immune response (16). Studies have demonstrated that
exosomes derived from immune cells are able to directly activate
CD4+ or CD8+ T cells (17,18).
Exosomes derived from tumor cells containing tumor antigens are
able to transfer tumor antigens to dendritic cells (DCs), eliciting
a specific antitumor effect (6).
Therefore, exosomes derived from tumor cells have been suggested as
a novel type of cancer vaccine.
However, the therapeutic efficacy of tumor
cell-derived exosomes is unsatisfactory. Thus, additional
improvement of exosomes to induce more potent antitumor effects is
of particular importance. Rab27a, one of the Rab family of small
GTPases, was suggested to be important in exosome secretion
(19). Rab proteins are reported
to control vesicular trafficking of different steps, such as
budding, motility and docking, as well as fusion of various
vesicular transport intermediates (20). Rab27a was also suggested to
regulate azurophilic granule exocytosis of neutrophils and NADPH
oxidase activity (21). Defects in
Rab27a have been demonstrated to result in an immunodeficiency
disorder, characterized by impaired cytotoxic T-lymphocytes and
natural killer cell function (22,23).
Knockdown of Rab27a in mice impaired the secretion of
myeloperoxidase stimulated by lipopolysaccharides (LPSs) in
vivo(24). Thus, whether
exosomes from Rab27 expression-modified tumor cells have unique
effects on antitumor immune activation remains unknown.
In the present study, investigation of the antitumor
effect of exosomes derived from Rab27a-overexpressing tumor cells
was conducted. A Rab27a-overexpressing cell line was initially
established via transfection of a Rab27a overexpression vector in
the human non-small-cell lung cancer line, A549. Exosomes were
isolated and the typical exosomal protein markers CD9, CD63, Hsp70
and Hsp90 were identified as highly expressed compared with
exosomes from control tumor cells. Subsequently, exosomes from
Rab27a-overexpressing cells were demonstrated to be capable of
inducing more potent maturation of MHC class II molecules and the
co-stimulatory molecules CD80 and CD86, which were all highly
upregulated in DCs. Furthermore, DCs loaded with exosomes derived
from Rab27-overexpressing cells significantly promoted
CD4+ T-cell proliferation in vitro. In addition,
in vivo immunization with exosomes derived from
Rab27-overexpressing cells inhibited tumor growth in a tumor mouse
model. It was also demonstrated that splenocytes from mice
immunized with exosomes derived from Rab27-overexpressing cells
expressed high levels of type I cytokines, including IL-2 and
IFN-γ, which are important in the regulation of cell-mediated
antitumor immunity. Collectively, it was demonstrated that exosomes
derived from Rab27a-overexpressing cancer cells elicited more
potent antitumor immune effects, which may provide novel insights
for the development of efficient exosome-based cancer vaccines.
Materials and methods
Animals
BALB/c mice (specific pathogen-free grade, five
weeks old, weighing 25–30 g) were obtained from the Medical
Experimental Animal Center (Guangdong, China). The mice were raised
under standard conditions of room temperature, dark-light cycle and
humidity with free access to water. Experiments were conducted
under a protocol approved by the Institutional Animal Care and Use
Committee of the Fourth Military Medical University (Xi'an, China).
All efforts were made to minimize suffering.
Cell culture
The human non-small-cell lung cancer cell line, A549
(SunBio Biomedical Technology Co., Ltd., Shanghai, China), was
maintained in RPMI-1640 (Wuhan Boster Biological Technology, Ltd.,
Wuhan, China) supplemented with 10% fetal bovine serum. The cells
were cultured at 37°C in an incubator (Life Technologies,
Baltimore, MD, USA) containing 5% CO2.
Vector construction and transfection
Total RNA was extracted from the cultured cells
using TRIzol reagent (Invitrogen, Carlsbad, CA, USA) according to
the manufacturer's instructions. Up to 5 μg of the total RNA was
reverse-transcribed into cDNA using SMART MMLV reverse
transcriptase (Clontech, Palo Alto, CA, USA). The cDNAs were used
as templates for amplification of Rab27a using two designed primers
(forward: 5′-aaatatgcggccgcatgtctgatggagattatgattacc-3′ and
reverse: 5′-ccgctcgagtcaacagccacatgcccctttctcc-3′) according to the
open reading frame of the Rab27a cDNA sequence (GenBank accession
no. CR536496.1). The cloned Rab27a DNA fragments were subcloned
into pCMV-Sport6 (Invitrogen, Carlsbad, CA, USA) using the
NotI and XhoI restriction sites. The Rab27a DNA
sequences in the recombinant vectors were confirmed by DNA
sequencing (Sangon Biotech, Shanghai, China). For transfection,
cells were seeded in a six-well culture plate (2×105
cells/well) and incubated at 37°C with 5% CO2 until the
cells reached 80% confluence. Experimental procedures were
performed according to the manufacturer's instructions. Briefly,
plasmid DNA (1 μg) was diluted in 500 μl fresh medium with 5 μl
lipofectamine (Invitrogen), mixed and incubated at room temperature
for 15 min. Subsequently, the mixtures were applied to the cells at
a final volume of 3 ml medium. The cells were cultured under the
standard conditions for 24 h.
Exosome isolation
Exosomes were isolated according to previously
reported protocols (14) with
minimal modification. Briefly, cell culture supernatants were
collected at the indicated times by centrifugation (800 × g for 5
min and 12,000 × g for 20 min at 4°C) and the collected
supernatants were filtrated via a 0.22-μm diameter pore filter
(Millipore, Billerica, MA, USA). Subsequently, ultracentrifugation
at 110,000 × g for 3 h at 4°C was performed. Thereafter, the
ultracentrifuged pellets in the bottom of the tube were collected
and resuspended in phosphate-buffered saline (PBS; sterile
filtered), followed by ultracentrifugation at 110,000 × g for 2 h.
Subsequently, the final pellets were collected and resuspended in
sterile-filtered PBS and stored at −80°C for further use. The total
protein concentrations of the exosomes were quantified using a BCA
Protein Assay kit (Pierce, Rockford, IL, USA).
Western blot analysis
A total of 20–30 μg of protein was fractionated by
12% sodium dodecyl sulfate-polyacrylamide gel electrophoresis and
transferred to a nitrocellulose membrane (Amersham Pharmacia
Biotech, Little Chalfont, UK). The membrane was treated by
agitation and blocking at room temperature with 2% non-fat dry milk
in Tris-buffered saline (TBS) for 1 h, followed by incubation with
primary antibodies (Rab27a, CD9, CD63, HSP70 and HSP90 antibodies
from Santa Cruz Biotechnology, Inc., Santa Cruz, CA, USA; CD80,
CD86 and MHC class II antibodies from Abcam, Cambridge, UK) diluted
(1: 10,000) in blocking buffer at 4°C overnight and then washed
three times with TBS and Tween (TBST; 10 mM Tris-HCl, pH 7.5, 150
mM NaCl and 0.05% Tween-20) for 10 min each time at room
temperature. Thereafter, the membrane was incubated in the
corresponding horseradish peroxidase (HRP)-conjugated goat
anti-mouse secondary antibody (Wuhan Boster Biological Technology,
Ltd.; diluted 1:5,000 in the blocking buffer) for 1 h. After being
washed three times with TBST and once with TBS, each for 10 min, 1
ml 4-chloro-1-naphthol as a HRP substrate with 9 ml TBS and 6 μl
H2O2 was used to visualize the target protein
in the dark for 5–30 min.
DC generation and stimulation
Mouse bone marrow-derived DCs were obtained from a
BALB/c mouse as described previously (25). The bone marrow cells were cultured
in RPMI-1640 media with granulocyte-macrophage colony-stimulating
factor (GM-CSF; 20 ng/ml) and IL-4 (10 ng/ml). On day 3, floating
cells were discarded and fresh medium was added. On day 6,
non-adherent and loosely adherent cells with the typical
morphological features of DCs were collected for subsequent use.
Immature DCs (~5×105) were resuspended in 1 ml complete
culture medium supplemented with GM-CSF (10 ng/ml) and IL-4 (1
ng/ml), and incubated with exosomes at the indicated concentrations
for 24 h. DCs stimulated with PBS or LPS (1 μg/ml) were used as
controls. Cytokine levels in the culture supernatants were analyzed
using the corresponding cytokine ELISA kit (Shanghai BlueGene
Biotech Co., Ltd., Shanghai, China) according to standard
procedures. For the protein expression analysis, cell lysates were
detected by western blot analysis using the indicated
antibodies.
CD4+ T-cell proliferation by
[3H]-thymidine incorporation
CD4+ T cells were collected from the
spleens of BALB/c mice immunized with A549 cells (100 μg cell
lysate per week) at day 6. The cells were purified with
immunomagnetic beads (Miltenyi Biotech, Cologne, Germany) followed
by culturing in complete RPMI-1640 media. Subsequently, DCs pulsed
with exosomes were added (CD4+ T cells/DCs: 10/1) and
incubated for 56 h. Thereafter, 0.5 μCi [3H]-thymidine
per well was added, followed by another 16 h of continuous culture.
Subsequently, [3H]-thymidine uptake was detected by a
MicroBeta counter (Beckmen Coulter, Krefeld, Germany).
Tumor inoculation and exosome challenge
of mice
BALB/c mice were injected with exosomes (10 μg each
time) or PBS (as the control) into the left groin subcutaneously on
day −13, −11, −9 and −7. On day 0, a dose of 5×105 A549
cells was injected into the right leg of each mouse close to the
groin. The tumor volume, indicated by the length and width, was
then determined each day post-tumor inoculation. To investigate the
effect of exosomes on pre-established tumor models, BALB/c mice
were injected with 3×105 A549 cells on day 0. Exosomes
or PBS was injected into the left groin on days +7, +9, +11 and
+13. The tumor volume is presented as length × width2 ×
π/6.
Statistical analysis
Assays were performed in triplicate and data are
presented as the mean ± standard error of the mean. Differences
between groups were analyzed by the Student's t-test. P<0.05 was
considered to indicate a statistically significant difference. Two
asterisks (**) denote P<0.01 and one asterisk
(*) denotes P<0.05.
Results
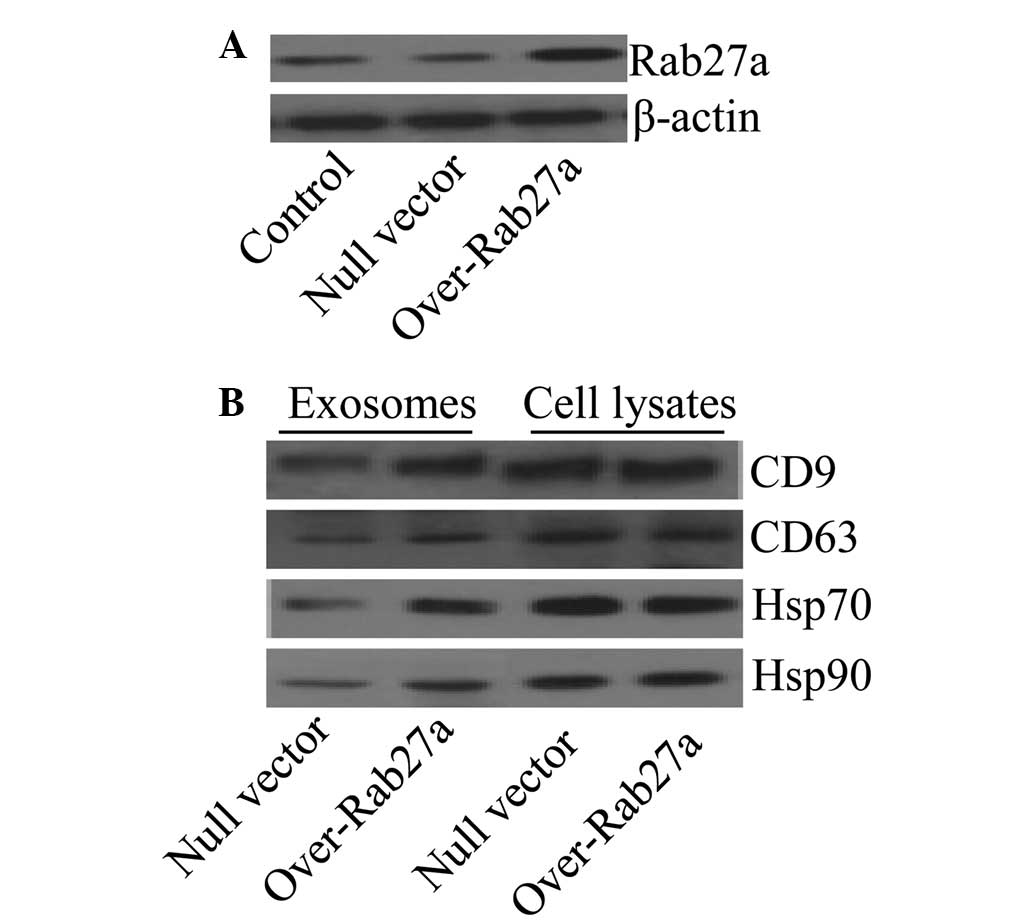
Overexpression of Rab27a increases the
levels of selected exosomal proteins
To assess the effect of Rab27a on exosome secretion,
a Rab27a overexpression vector was transfected into the
non-small-cell lung cancer cell line A549. The expression levels of
Rab27a in the cells were determined by western blot analysis and
the results confirmed that Rab27a was highly expressed in the cells
transfected with the Rab27a overexpression vector compared with the
controls (Fig. 1A). Reportedly,
Rab27a is responsible for exosome secretion (19); thus, in order to determine whether
the overexpression of Rab27a affected the levels of particular
molecules in exosomes, the typical exosomal protein markers CD9,
CD63, Hsp70 and Hsp90 (26) were
screened by western blot analysis. The results showed that exosomes
from Rab27a-overexpressing cells exhibited high levels of CD9,
CD63, Hsp70 and Hsp90 compared with exosomes from the control
cells. In addition, the levels of all these proteins in the
parental cells were not affected (Fig.
1B).
Exosomes derived from
Rab27a-overexpressing cells induced more potent maturation of
DCs
Exosomes derived from cancer cells containing Hsps
have been found to induce more potent immune activation (27). Thus, it was hypothesized that
exosomes derived from Rab27-overexpressing cancer cells that
harbored increased levels of Hsp70 and Hsp90 would induce more
potent immune activation. The effect of the exosomes was analyzed
on DCs, which are the professional antigen-presenting cells in
vivo. The DCs were incubated with the exosomes for 48 h, and
MHC class II molecules as well as the co-stimulatory molecules CD80
and CD86 in the DCs were examined by western blot analysis. The
data showed that the DCs incubated with exosomes derived from
Rab27a-overexpressing cells expressed higher levels of MHC class
II, CD80 and CD86 compared with the controls (Fig. 2A), suggesting that exosomes derived
from Rab27-overexpressing cells had a stronger effect on DC
maturation. Furthermore, exosomes derived from Rab27-overexpressing
cells also induced significantly higher levels of cytokines,
including IL-1β (Fig. 2B), TNF-α
(Fig. 2C) and RANTES, compared
with the controls (Fig. 2D). These
results indicated that exosomes derived from Rab27-overexpressing
cells had a more potent immune-stimulatory activity.
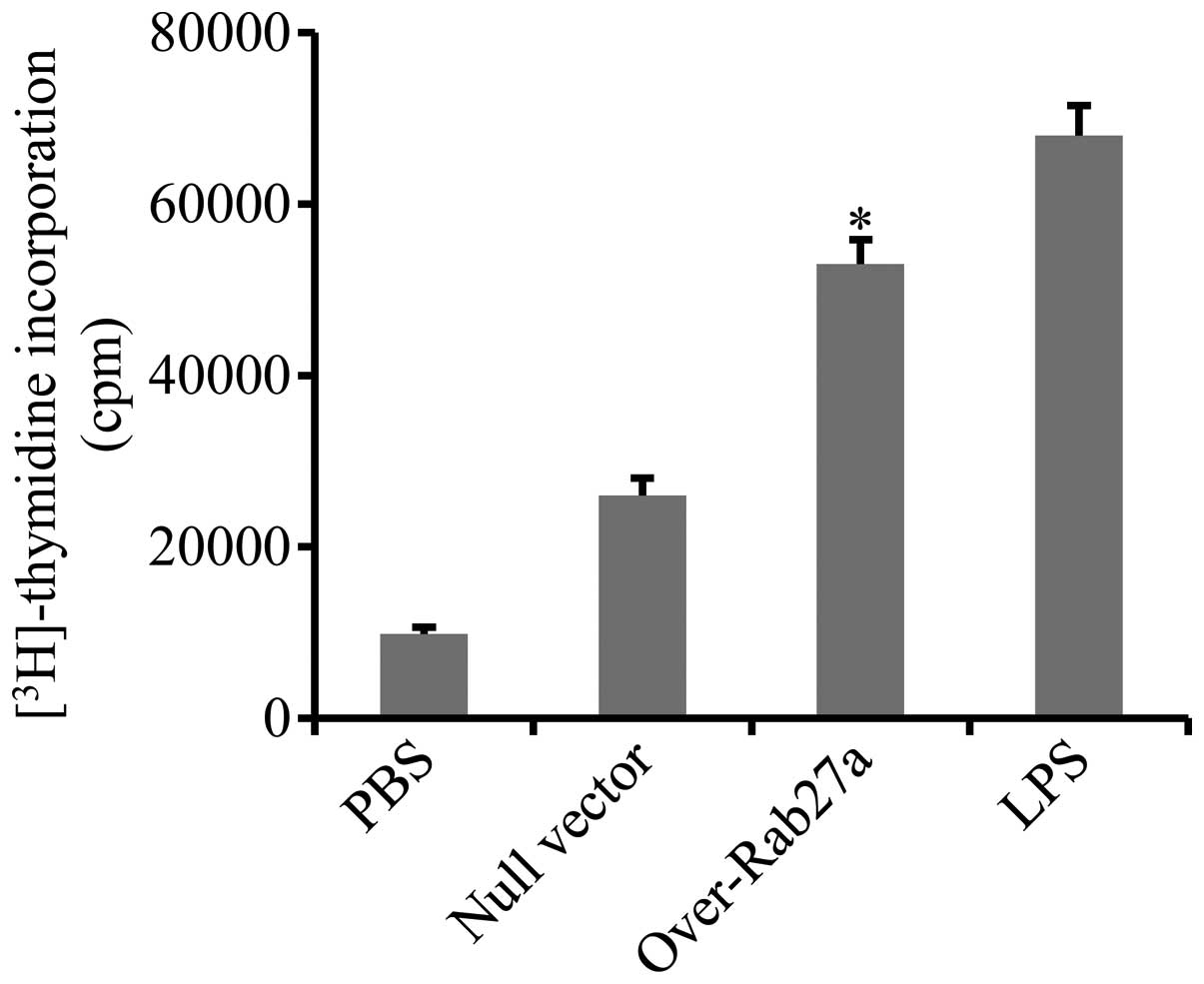
DCs loaded with exosomes derived from
Rab27a-overexpressing cells significantly promoted CD4+
T-cell proliferation
To confirm that exosomes derived from
Rab27a-overexpressing cells elicit more potent immune activation,
the effect of DCs loaded with exosomes derived from
Rab27a-overexpressing cells on the activation of CD4+ T
cells was determined. CD4+ T cells were purified from
the spleens of mice immunized with A549 cell lysates and
co-incubated with DCs that were pre-pulsed with exosomes. Cell
proliferation was evaluated by measuring the uptake of
[3H]-thymidine. The results showed that the
proliferation of CD4+ T cells was markedly enhanced by
DCs loaded with exosomes derived from Rab27a-overexpressing cells
(Fig. 3).
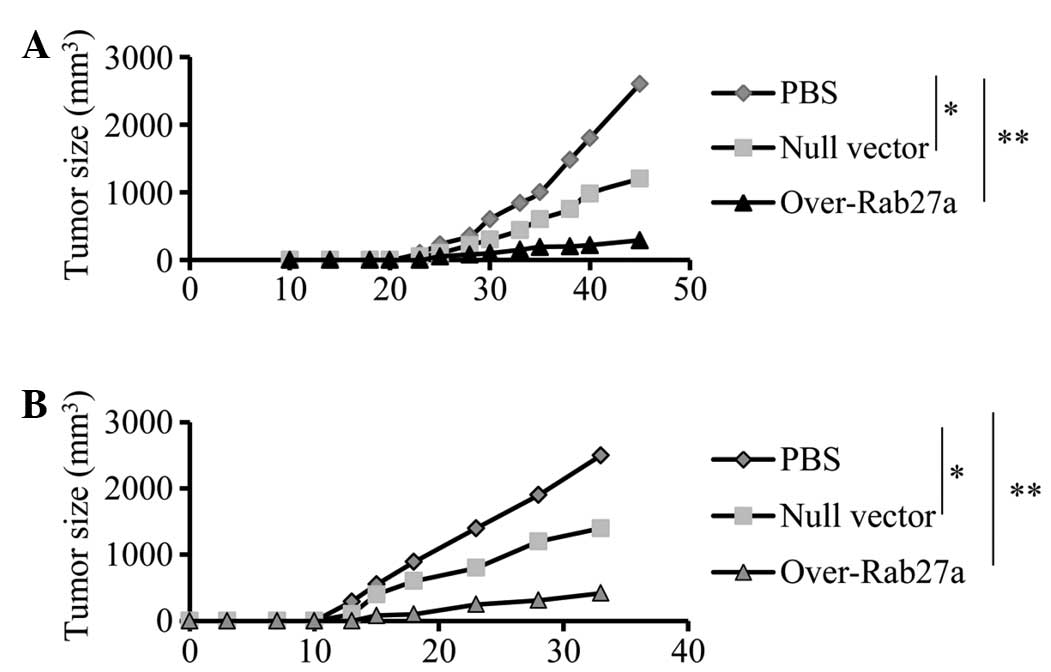
Exosomes derived from
Rab27a-overexpressing cells induce an efficient antitumor
effect
To examine whether exosomes derived from
Rab27a-overexpressing cells induced efficient antitumor effects,
mice were pre-immunized with exosomes and then challenged with A549
cells. The results showed that immunization with exosomes derived
from Rab27a-overexpressing cells significantly inhibited tumor
growth in vivo (Fig. 4A),
compared with exosomes from cells transfected only with the null
vector or treated with PBS (P<0.01). Furthermore, the effect on
tumor growth in a pre-established tumor model was also examined and
similar inhibitory effects on tumor growth were observed (Fig. 4B). The results indicated that
exosomes derived from Rab27a-overexpressing cells induced stronger
antitumor effects than normal exosomes.
Exosomes derived from
Rab27a-overexpressing cells induced strong production of type I
cytokines
To confirm whether exosomes derived from
Rab27-overexpressing cells induced effective antitumor immunity,
type I cytokines that are critical in cell-mediated antitumor
immunity were screened (27).
Splenocytes derived from exosome-immunized mice were re-stimulated
with A549 cells in vitro. The levels of type I cytokines
IL-2 and IFN-γ were then measured. The results showed that the
concentrations of IL-2 (Fig. 5A)
and IFN-γ (Fig. 5B) were higher in
the splenocytes of mice immunized with exosomes derived from
Rab27a-overexpressing cells than with exosomes derived from normal
cells.
Discussion
Results of the present study have demonstrated that
exosomes derived from Rab27a-overexpressing cancer cells induced
more potent antitumor effects. The improved antitumor efficacy may
be due to their enrichment in molecules that contribute to the
induction of immune activation, such as Hsp70 or Hsp90 and tumor
antigens. However, this theory requires further elucidation.
Exosomes derived from heat-shocked lymphoma cells
have been demonstrated to contain increased amounts of molecules
involved in immunogenicity, including Hsp60, Hsp90, MHC class I and
II, CD40 and CD86, and induce efficient antitumor T-cell immunity
(27). In accordance, similar
effects were obtained in the present study by modification of
Rab27a expression in parental cancer cells. Recently, it has been
reported that exosomes derived from anticancer drug-treated cancer
cells elicit effective natural killer cell antitumor responses
in vitro(28). Thus, it
appears that improvement of exosome-mediated immune activation by
modification of their parental cells is feasible.
Notably, in the present study it was demonstrated
that overexpression of Rab27a increased the levels of certain
molecules in secreted exosomes without affecting the total amounts
in the parental cancer cells. Silencing Rab27a has been
demonstrated to block exosome secretion and downregulate protein
levels of exosomal markers, including HLA-DR, CD63, Tsg101 and
Hsc70, in human HeLa cells (19).
However, the size and morphology of the exosomes was unaffected.
Rab proteins are reported to control vesicular trafficking at
different steps, such as budding, motility and docking, as well as
the fusion of various vesicular transport intermediates (20). Rab27a has also been suggested to
regulate azurophilic granule exocytosis by neutrophils and NADPH
oxidase activity (21). Defects in
Rab27a resulted in an immunodeficiency disorder exhibiting impaired
cytotoxic T-lymphocytes and natural killer cell function (22,23).
Knockdown of Rab27a in mice was shown to impair the secretion of
myeloperoxidase stimulated by LPS in vivo(24). Rab27a has previously been
demonstrated to be involved in the regulation of the secretion of
secretory granules and lysosome-associated organelles in a variety
of cell types (29–31). It is of note that knockdown of
Rab27a blocks exosome secretion in mammary carcinoma cells, which
results in the inhibition of tumor growth as Rab27a-dependent
exosomes are able to modify the tumor microenvironment for the
promotion of tumor growth (32).
Silencing of Rab27a inhibited melanoma exosome secretion as well as
angiogenic growth factors, resulting in the inhibition of tumor
growth and metastasis (33).
Considering these findings, it may be hypothesized that exosomes
secreted in a Rab27a-dependent manner are more tumorigenic.
Overexpression of Rab27 in cancer cells may render secreted
exosomes greater immunogenicity, and thus elicit more potent
antitumor immunity.
The results of the present study showed that
exosomes derived from Rab27a-overexpressing cells induced a
stronger immune response and, compared with the controls, it
markedly enhanced maturation of DCs. Levels of MHC molecules and
the co-stimulatory molecules CD80 and CD86 were found to be highly
increased. The levels of cytokines, including IL-1β, TNF-α and
RANTES, were also highly induced. Hsps have been shown to directly
stimulate the maturation of DCs (34). In the present study, high levels of
Hsp70 and Hsp90 were observed in the exosomes derived from
Rab27a-overexpressing cells, which may be partly responsible for DC
maturation. Moreover, DCs pulsed with exosomes were shown to be
capable of boosting CD4+ T cell proliferation,
suggesting that they are able to induce strong T cell responses.
Thus, it was hypothesized that exosomes derived from
Rab27a-overexpressing cells may induce efficient antitumor immune
effects in vivo. As expected, it was observed that
immunization with exosomes from Rab27a-overexpressing cells
significantly inhibited tumor growth in vivo in a mouse
model. It was also determined that type I cytokines were highly
induced, which are important in tumor suppression via activation of
macrophages and CTL (35).
Exosomes have been proposed to be an ideal source of
tumor antigens and have potential roles in tumor immunotherapy
(36). As expected, cell-free
tumor vaccines based on exosomes have been exploited in a number of
experimental animals and cancer patients. It has been reported that
use of DEX stimulates a specific T-cell response and promotes
natural killer lytic-activity in non-small cell lung
carcinoma-bearing patients (37).
A method for ovarian cancer treatment via combination of
tumor-associated ascites-derived exosomes and a Toll-like receptor
3 agonist has also been described and evaluated (7). Ascites-derived exosomes in
combination with GM-CSF for immunotherapy of colorectal cancer was
shown to induce a beneficial tumor-specific antitumor cytotoxic
T-lymphocyte (CTL) response with well-tolerated side effects
(38). Therefore, efforts have
been made to improve the efficacy of exosome-based tumor vaccines.
Exosomes derived from DCs pulsed with lymphocytic leukemia cell
antigen in combination with cyclophosphamide and a sodium salt of
polyinosinic-polycytidylic acid markedly induced spleen cell
proliferation and cytotoxic effects in vitro and suppressed
tumor growth in vivo(39).
Notably, a study showed that intradermal immunization with exosomes
induced more effective CTL than subcutaneous administration
(40). It is possible that the
efficiency of exosomes in antitumor immunity is not only associated
with the processing protocol but also the source cell type, as a
study has demonstrated that exosomes derived from ovalbumin
(OVA)-pulsed DCs elicited more efficient antitumor immunity than
exosomes derived from tumor cells expressing OVA (41).
In conclusion, results of the present study have
demonstrated that exosomes derived from Rab27a-overexpressing
cancer cells elicit effective antitumor immunity, providing novel
insights for the development of efficient exosome-based cancer
vaccines. However, more studies are required to confirm these
findings.
Abbreviations:
|
NSCLC
|
non-small-cell lung cancer
|
|
HSP
|
heat shock protein
|
|
DCs
|
dendritic cells
|
|
MHC
|
major histocompatibility complex
|
References
|
1
|
Jemal A, Siegel R, Ward E, et al: Cancer
statistics, 2007. CA Cancer J Clin. 57:43–66. 2007. View Article : Google Scholar
|
|
2
|
Schiller JH, Harrington D, Belani CP, et
al: Comparison of four chemotherapy regimens for advanced
non-small-cell lung cancer. N Engl J Med. 346:92–98. 2002.
View Article : Google Scholar
|
|
3
|
Thomas L: On immunosurveillance in human
cancer. Yale J Biol Med. 55:329–333. 1982.PubMed/NCBI
|
|
4
|
Kaplan DH, Shankaran V, Dighe AS, et al:
Demonstration of an interferon gamma-dependent tumor surveillance
system in immunocompetent mice. Proc Natl Acad Sci USA.
95:7556–7561. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Shankaran V, Ikeda H, Bruce AT, et al:
IFNgamma and lymphocytes prevent primary tumour development and
shape tumour immunogenicity. Nature. 410:1107–1111. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Wolfers J, Lozier A, Raposo G, et al:
Tumor-derived exosomes are a source of shared tumor rejection
antigens for CTL cross-priming. Nat Med. 7:297–303. 2001.PubMed/NCBI
|
|
7
|
Navabi H, Croston D, Hobot J, et al:
Preparation of human ovarian cancer ascites-derived exosomes for a
clinical trial. Blood Cells Mol Dis. 35:149–152. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Pan BT and Johnstone RM: Fate of the
transferrin receptor during maturation of sheep reticulocytes in
vitro: selective externalization of the receptor. Cell. 33:967–978.
1983. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Harding C, Heuser J and Stahl P:
Receptor-mediated endocytosis of transferrin and recycling of the
transferrin receptor in rat reticulocytes. J Cell Biol. 97:329–339.
1983. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Pan BT, Teng K, Wu C, Adam M and Johnstone
RM: Electron microscopic evidence for externalization of the
transferrin receptor in vesicular form in sheep reticulocytes. J
Cell Biol. 101:942–948. 1985. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Yang C and Robbins PD: The roles of
tumor-derived exosomes in cancer pathogenesis. Clin Dev Immunol.
2011:8428492011. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Stoorvogel W, Kleijmeer MJ, Geuze HJ and
Raposo G: The biogenesis and functions of exosomes. Traffic.
3:321–330. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Mignot G, Roux S, Thery C, Ségura E and
Zitvogel L: Prospects for exosomes in immunotherapy of cancer. J
Cell Mol Med. 10:376–388. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Valadi H, Ekström K, Bossios A, et al:
Exosome-mediated transfer of mRNAs and microRNAs is a novel
mechanism of genetic exchange between cells. Nat Cell Biol.
9:654–659. 2007. View
Article : Google Scholar : PubMed/NCBI
|
|
15
|
Subra C, Laulagnier K, Perret B and Record
M: Exosome lipidomics unravels lipid sorting at the level of
multivesicular bodies. Biochimie. 89:205–212. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Ratajczak J, Wysoczynski M, Hayek F,
Janowska-Wieczorek A and Ratajczak MZ: Membrane-derived
microvesicles: important and underappreciated mediators of
cell-to-cell communication. Leukemia. 20:1487–1495. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Zitvogel L, Regnault A, Lozier A, et al:
Eradication of established murine tumors using a novel cell-free
vaccine: dendritic cell-derived exosomes. Nat Med. 4:594–600. 1998.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Raposo G, Nijman HW, Stoorvogel W, et al:
B lymphocytes secrete antigen-presenting vesicles. J Exp Med.
183:1161–1172. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Ostrowski M, Carmo NB, Krumeich S, et al:
Rab27a and Rab27b control different steps of the exosome secretion
pathway. Nat Cell Biol. 12:19–30, sup pp. 11–13. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Zerial M and McBride H: Rab proteins as
membrane organizers. Nat Rev Mol Cell Biol. 2:107–117. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Johnson JL, Brzezinska AA, Tolmachova T,
et al: Rab27a and Rab27b regulate neutrophil azurophilic granule
exocytosis and NADPH oxidase activity by independent mechanisms.
Traffic. 11:533–547. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Griscelli C, Durandy A, Guy-Grand D, et
al: A syndrome associating partial albinism and immunodeficiency.
Am J Med. 65:691–702. 1978. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Klein C, Philippe N, Le Deist F, et al:
Partial albinism with immunodeficiency (Griscelli syndrome). J
Pediatr. 125:886–895. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Munafó DB, Johnson JL, Ellis BA, et al:
Rab27a is a key component of the secretory machinery of azurophilic
granules in granulocytes. Biochem J. 402:229–239. 2007.PubMed/NCBI
|
|
25
|
Guo J, Wang B, Zhang M, et al:
Macrophage-derived chemokine gene transfer results in tumor
regression in murine lung carcinoma model through efficient
induction of antitumor immunity. Gene Ther. 9:793–803. 2002.
View Article : Google Scholar
|
|
26
|
Chaput N, Taïeb J, Schartz NE, et al:
Exosome-based immunotherapy. Cancer Immunol Immunother. 53:234–239.
2004. View Article : Google Scholar
|
|
27
|
Chen W, Wang J, Shao C, et al: Efficient
induction of antitumor T cell immunity by exosomes derived from
heat-shocked lymphoma cells. Eur J Immunol. 36:1598–1607. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Lv LH, Wan YL, Lin Y, et al: Anticancer
drugs cause release of exosomes with heat shock proteins from human
hepatocellular carcinoma cells that elicit effective natural killer
cell antitumor responses in vitro. J Biol Chem. 287:15874–15885.
2012. View Article : Google Scholar
|
|
29
|
Desnos C, Schonn JS, Huet S, et al: Rab27A
and its effector MyRIP link secretory granules to F-actin and
control their motion towards release sites. J Cell Biol.
163:559–570. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Stinchcombe JC, Barral DC, Mules EH, et
al: Rab27a is required for regulated secretion in cytotoxic T
lymphocytes. J Cell Biol. 152:825–834. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Barral DC, Ramalho JS, Anders R, et al:
Functional redundancy of Rab27 proteins and the pathogenesis of
Griscelli syndrome. J Clin Invest. 110:247–257. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Bobrie A, Krumeich S, Reyal F, et al:
Rab27a supports exosome-dependent and -independent mechanisms that
modify the tumor microenvironment and can promote tumor
progression. Cancer Res. 72:4920–4930. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Peinado H, Alečković M, Lavotshkin S, et
al: Melanoma exosomes educate bone marrow progenitor cells toward a
pro-metastatic phenotype through MET. Nat Med. 18:883–891. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Cho BK, Palliser D, Guillen E, et al: A
proposed mechanism for the induction of cytotoxic T lymphocyte
production by heat shock fusion proteins. Immunity. 12:263–272.
2000. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Fowler DW, Copier J, Wilson N, Dalgleish
AG and Bodman-Smith MD: Mycobacteria activate γδ T-cell anti-tumour
responses via cytokines from type 1 myeloid dendritic cells: a
mechanism of action for cancer immunotherapy. Cancer Immunol
Immunother. 61:535–547. 2012.
|
|
36
|
Tan A, De La Peña H and Seifalian AM: The
application of exosomes as a nanoscale cancer vaccine. Int J
Nanomedicine. 5:889–900. 2010.PubMed/NCBI
|
|
37
|
Morse MA, Garst J, Osada T, et al: A phase
I study of dexosome immunotherapy in patients with advanced
non-small cell lung cancer. J Transl Med. 3:92005. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Dai S, Wei D, Wu Z, et al: Phase I
clinical trial of autologous ascites-derived exosomes combined with
GM-CSF for colorectal cancer. Mol Ther. 16:782–790. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Guo F, Chang CK, Fan HH, et al:
Anti-tumour effects of exosomes in combination with
cyclophosphamide and polyinosinic-polycytidylic acid. J Int Med
Res. 36:1342–1353. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Hao S, Ye Z, Yang J, Bai O and Xiang J:
Intradermal vaccination of dendritic cell-derived exosomes is
superior to a subcutaneous one in the induction of antitumor
immunity. Cancer Biother Radiopharm. 21:146–154. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Hao S, Bai O, Yuan J, Qureshi M and Xiang
J: Dendritic cell-derived exosomes stimulate stronger CD8+ CTL
responses and antitumor immunity than tumor cell-derived exosomes.
Cell Mol Immunol. 3:205–211. 2006.
|